Abstract
Trypanosoma cruzi, the agent of Chagas' disease, infects a variety of mammalian cells in a process that includes multiple cycles of intracellular division and differentiation starting with host receptor recognition by a parasite ligand(s). Earlier work in our laboratory showed that the neurotrophin-3 (NT-3) receptor TrkC is activated by T. cruzi surface trans-sialidase, also known as parasite-derived neurotrophic factor (PDNF). However, it has remained unclear whether TrkC is used by T. cruzi to enter host cells. Here, we show that a neuronal cell line (PC12-NNR5) relatively resistant to T. cruzi became highly susceptible to infection when overexpressing human TrkC but not human TrkB. Furthermore, trkC transfection conferred an ∼3.0-fold intracellular growth advantage. Sialylation-deficient Chinese hamster ovarian (CHO) epithelial cell lines Lec1 and Lec2 also became much more permissive to T. cruzi after transfection with the trkC gene. Additionally, NT-3 specifically blocked T. cruzi infection of the TrkC-NNR5 transfectants and of naturally permissive TrkC-bearing Schwann cells and astrocytes, as did recombinant PDNF. Two specific inhibitors of Trk autophosphorylation (K252a and AG879) and inhibitors of Trk-induced MAPK/Erk (U0126) and Akt kinase (LY294002) signaling, but not an inhibitor of insulin-like growth factor 1 receptor, abrogated TrkC-mediated cell invasion. Antibody to TrkC blocked T. cruzi infection of the TrkC-NNR5 transfectants and of cells that naturally express TrkC. The TrkC antibody also significantly and specifically reduced cutaneous infection in a mouse model of acute Chagas' disease. TrkC is ubiquitously expressed in the peripheral and central nervous systems, and in nonneural cells infected by T. cruzi, including cardiac and gastrointestinal muscle cells. Thus, TrkC is implicated as a functional PDNF receptor in cell entry, independently of sialic acid recognition, mediating broad T. cruzi infection both in vitro and in vivo.
INTRODUCTION
Trypanosoma cruzi is the infectious agent of Chagas' disease, a chronic, incurable, debilitating condition widespread in Latin America and increasingly prevalent in the United States, Western Europe, and Australia (13, 29). T. cruzi is an obligate intracellular parasite and can invade muscle cells, epithelial cells, macrophages and most other types of nucleated cells. It preferentially invades astrocytes in the central nervous system (CNS), Schwann cells in the peripheral nervous system (PNS), and enteric glial cells in the enteric nervous system (ENS) (11, 30, 36). Intracellular parasite growth during acute Chagas' disease can lead to partial destruction of the PNS and ENS and cause megacolon and megaesophagus (megaviscera) in patients with chronic Chagas' disease (3, 22, 29). Therefore, it is critical to understand the molecular mechanisms underlying T. cruzi recognition of host cell receptors that drive entry into cell hosts, a process required for completion of the life cycle in humans and other mammalian hosts.
Recently, it was shown in our lab that T. cruzi uses the tyrosine receptor kinase TrkA to invade neural cells (12). However, TrkA expression in the nervous system is restricted to a subset of neurons, particularly in the CNS, where it is located only in the forebrain (17, 23). Notably, TrkA is not expressed in highly permissive Schwann cells and astrocytes, which abound in the PNS and CNS, respectively (42). On the other hand, Schwann cells and astrocytes express the other Trk family receptors TrkB and TrkC (42).
The receptor tyrosine kinases TrkA, TrkB, and TrkC are expressed in the nervous system, where they primarily regulate activity, survival, and proliferation of neural cells (2, 18). They are also expressed in nonneural cells, but their function in those cells is not yet clear. Trk receptors are transmembrane glycoproteins with multiple extracellular sites of glycosylation/sialylation, and they share sequence homology, structure, and ligands (2, 18, 39). Previous work in our lab showed that T. cruzi, through its trans-sialidase, also known as parasite-derived neurotrophic factor (PDNF), which is located on the T. cruzi trypomastigote surface through a glycosylphosphatidylinositol (GPI) anchor, binds and activates TrkA and TrkC but not TrkB (9, 41). As such, PDNF mimics naturally occurring Trk neurotrophin ligands in mammalian hosts in their distinct, yet overlapping specificity for Trk receptor engagement. The neurotrophins nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3) bind primarily to TrkA, TrkB, and TrkC, respectively. However, NT-3 also binds TrkA and TrkB, albeit with an affinity 102 to 104 times lower than that to TrkC (Kd = 1011) (18).
Our results presented here define TrkC as a T. cruzi entry receptor in neural and nonneural cells and indicate that TrkC-mediated cell entry is important for proper T. cruzi infection in vivo.
MATERIALS AND METHODS
Parasites.
All in vitro and in vivo studies were performed with Silvio X-10/4 (28) and Tulahuen (33) strains, respectively. Both of these strains of trypomastigotes were grown in Vero cells. The parasites were harvested by centrifugation at 500 × g for 5 min to remove host cells and cell debris and washed two times with Dulbecco's modified Eagle's medium (DMEM) at 1,200 × g for 10 min.
Cell lines and primary cultures.
PC12-NNR5 cells were gifts from Lloyd Green (College of Physicians and Surgeons, Columbia University, NY). CHO Lec1 and Lec2 cells were gifts from Pamela Stanley (34). CHO cell mutants and Trk receptor-deficient PC12 cell mutant NNR5 was cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS; Gemini Bio Products), 100 U/ml penicillin-streptomycin (Gibco), 2 mM l-glutamine (Gibco), 1 mM nonessential amino acids (Gibco), and 1 mM sodium pyruvate (Gibco). Human Schwann cells (permanent cell line) (7) were maintained in DMEM supplemented with 10% FBS (Gemini Bio Products) and 100 U/ml penicillin-streptomycin (Gibco). Primary cortical astrocytes were isolated from newborn C57BL/6 mouse pups, as described before (15). In short, pups were sacrificed by decapitation, and the whole brain was removed from the skull. Cleaned cortices were minced, and dissociated cell suspension was created by prolonged vortexing in complete medium (DMEM supplemented with 20% horse serum). The cell suspension was sequentially filtered through 70- and 10-mm mesh and plated on Falcon Primeria dishes in complete medium. Cultures were washed 3 days after isolation, and the medium was changed every 3 to 4 days thereafter. Astrocytes were used after at least 2, and at most 6, weeks in culture.
Mice.
Female C57BL/6 mice, aged between 6 to 8 weeks, were purchased from The Jackson Laboratory. All murine experiments were approved by the Institutional Animal Care and Use Committees (IACUC) of Tufts University-Tufts Medical Center.
Purification of recombinant PDNF.
A small version of PDNF devoid of C-terminal tandem repeat (sPDNF) (GenBank accession number AJ002174; derived from Silvio X-10/4) (8) was expressed in BL21(DE3) bacterial cells at the Gastroenterology Research on Absorptive and Secretory Processes center (Tufts University Medical School-Tufts Medical Center). The protein was purified using a metal chelate column as previously described (5).
Cloning and transfection.
As described previously (41), TrkB and TrkC were directionally cloned from human RNA (RNA was a gift from Tugba Bagci, Neuroscience Department, Tufts Medical School, Tufts University) via reverse transcription-PCR into the pIRES-dsRed mammalian expression vector (Clontech). TrkA was directionally cloned from plasmid 15002 (Addgene) that contained human TrkA cDNA with the same strategy using forward primer ACTCGAGATGCTGCGAGGCGGAC and reverse primer CCTAGCCCAGGACATCCAGGTAGA.
Infection in vitro.
Except for astrocytes (see below), cells (3,000/well) were plated in 96-well plates (Falcon), incubated for 1 to 2 days in growth medium, and then incubated with trypomastigotes (30,000/well) in 0.1% bovine serum albumin (BSA)/DMEM (2 h, 37°C). After infection, cells were washed 5 times with prewarmed DMEM to remove uninvaded parasites and were grown in 1% fetal calf serum (FCS)/DMEM for 2 days. Cell monolayers were analyzed by phase-contrast microscopy, as follows. Cells were fixed with methanol for 2 min and stained with acid-base staining reagents (Diff Quick; Baxter) by following the manufacturer's instructions. Infected and noninfected cells (>200 cells/well) were counted in triplicate (12). To determine whether TrkC ligands or specific antibodies inhibit infection of TrkC-expressing cells, the specified cell lines were preincubated for 30 min at 37°C with the indicated concentration of ligands (on the graph abscissa): sPDNF (purified in the lab as described above, NGF (Sigma-Aldrich), NT-3 (R&D Systems), BDNF (R&D systems), anti-TrkC (α-TrkC) (Upstate, 07-226), and α-TrkB (Upstate, 07-225). After treatment with ligands, cell monolayers were washed once with DMEM. To test whether invasion is dependent on TrkC signaling, cells were preincubated for 1 h at 37°C with the Trk-selective inhibitors K252a (500 nM; 50% inhibitory concentration [IC50], 3 nM) (Calbiochem) and AG879 (50 μM; IC50, 10 μM), and with negative-control insulin-like growth factor 1 receptor (IGF-1R) inhibitor (125 nM to 2 mM; IC50, 1 nM), and then incubated with parasites as described above. To block the TrkC-binding site on the parasite surface, trypomastigotes were pretreated with specified quantities of TrkCECD-Fc, FGFR-Fc, or other Trk-Fc receptors (R&D Systems) for 30 min at 37°C in a total volume of 1 ml (0.25, 0.5, 0.75, and 1.0 μg/ml), and they were subsequently incubated with host cells as described previously (12).
To ascertain parasite replication levels, infection was performed as described above with the following changes: cells were fixed 1 or 2 days postinfection; after fixation and staining, the number of amastigotes per cell was counted for at least 40 infected cells per well.
For experiments using primary astrocytes, 2,000 cells/well were plated on poly-l-lysine-coated 96-well plates in 10% horse serum and allowed to attach overnight. The following day, cells were rinsed twice with DMEM and preincubated with different doses of ligands (NGF, 60 ng/ml; BDNF, 60 ng/ml; NT-3, 7.5 to 120 ng/ml; sPDNF, 7.5 to 120 ng/ml) in 0.1% BSA/DMEM for 30 min. Without rinsing away the blocking agent, parasites were added (2 × 105 cells/well), and plates were centrifuged (1,000 rpm, 5 min) and left to infect for 3 h at 37°C. Cells were rinsed 3 times and left in 1% horse serum for 3 days and then fixed and stained by Diff Quick. For inhibition of infection, astrocytes were pretreated with various inhibitors (K252a [100 nM], AG879 [50 μM], LY294002 [Akt kinase inhibitor, 10 μM], U0126 [MAPK/Erk signaling inhibitor, 10 μM], and IGF-1R inhibitor [IGF-1RI, 250 nM]) in DMEM containing 0.1% horse serum for 1 h at 37°C.
Murine model of acute T. cruzi infection.
Trypomastigotes (Tulahuen strain) were harvested from Vero cell cultures and washed 1 time in DMEM and 2 times in phosphate-buffered saline (PBS). Parasites (5,000 in 30 μl of PBS) were injected subcutaneously into the mouse footpad. When noted in Results, specific antibodies or BSA were injected into the footpad simultaneously with the parasites. Mice were sacrificed 3 days later, and the footpad site where parasites had been injected was removed. The footpad without the digits was cut into small pieces, and DNA was isolated using a DNeasy tissue kit (Qiagen) according to the manufacturer's protocol. DNA was then used to perform real-time PCR, as previously described (10). Each reaction mixture contained 50 ng of total DNA, samples were analyzed with primers specific for T. cruzi DNA, and a one-copy mouse gene, the tnf gene, was used as an internal control for each sample. To determine the number of parasites, a standard curve was calculated using uninfected tissue spiked with known quantities of parasites; the standard curve was run with each plate. The forward and reverse T. cruzi primers used to detect T. cruzi minicircle DNA were GCTCTTGCCCACAMGGGTGC and CCAAGCAGCGGATAGTTCAGG, respectively. The forward and reverse tnf primers used to detect the murine tnf gene were TCCCTCTCATCAGTTCTATGGCCCA and CAGCAAGCATCTATGCACTTAGACCCC, respectively.
RESULTS
TrkC mediates cellular invasion in neuronal, glial, and epithelial cell lines.
Our previous work showed that T. cruzi binds and activates TrkC and TrkA but not TrkB, the third Trk family receptor member (9, 12, 41). Given the importance of cellular invasion in the life cycle of the intracellular parasite T. cruzi, we undertook experiments to determine whether TrkC mediates invasion.
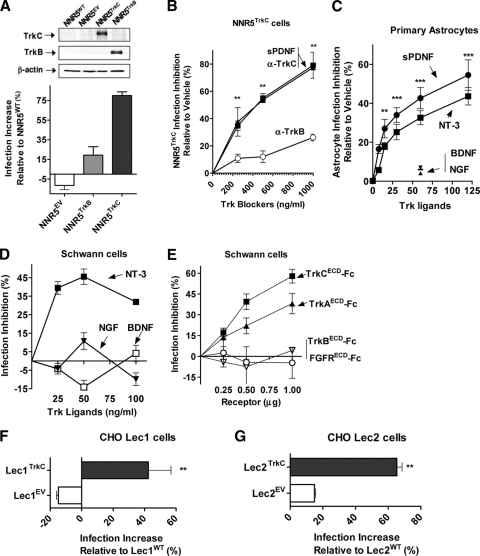
To start, we used a rat pheochromocytoma neuronal cell line that lacks Trk receptors (PC12-NNR5) to determine whether forced TrkC expression specifically increases susceptibility to invasion. For this purpose, we generated stable clones of PC12-NNR5 cells (NNR5WT) by transfection with the full-length human trkC (NNR5TrkC) or trkB (NNR5TrkB) gene or empty vector (NNR5EV) (41). Preliminary experiments showed, on the basis of Western blots probed with Trk-specific antibodies, that the trk gene products were correctly expressed in the transfected stable clones (Fig. 1 A, inset). We then seeded the clones in 96-well plates in triplicate, incubated them with T. cruzi for 2 h, removed uninvaded parasites by rinsing, and grew the cells for 2 days to allow for intracellular parasite differentiation and division. We found that invasion increased 85% in NNR5TrkC cells compared to results for NNR5EV or NNR5TrkB transfectants (Fig. 1A), suggesting that TrkC, but not TrkB, mediates invasion in neuronal PC12 cells. This conclusion was supported by inhibition experiments with two types of TrkC blockers, antibodies and T. cruzi PDNF: preincubation of NNR5TrkC transfectants with α-TrkC antibodies effectively inhibited invasion in a dose-dependent manner (79% ± 2.3 at 1 μg/ml), whereas α-TrkB antibodies were much less effective (26% ± 1.3 at 1 μg/ml) (Fig. 1B). T. cruzi PDNF, a TrkC-binding molecule (41), was also effective in blocking T. cruzi invasion of NNR5TrkC (Fig. 1B), suggesting that PDNF is the parasite molecule responsible for TrkC-mediated invasion (Fig. 1A).
Fig. 1.
TrkC mediates T. cruzi infection in neuronal PC12 cells, glial Schwann cells, and astrocytes and in epithelial CHO Lec1 and Lec2 cell lines. Cells were plated in 96-well plates in triplicate, infected with T. cruzi trypomastigotes (2 h), and washed to remove uninvaded parasites, and invaded parasites were allowed to differentiate and multiply for 2 days. (A) NNR5 cells transfected with human trkC (NNR5TrkC), but not with human trkB (NNR5TrkB) or an empty vector (NNR5EV), become more permissive to invasion. (Inset) Western blot of transfected cell lines probed with antibodies to TrkC, TrkB, and β-actin, showing correct transfection with the trkC and trkB genes. (B) TrkC blockers sPDNF (a small, N-terminal version of PDNF that contains Trk-binding sites) (9) and TrkC antibody (α-TrkC) inhibit T. cruzi infection of PC12-NNR5 cells stably expressing human trkC (NNR5TrkC) cells. NNR5TrkC cells were preincubated with sPDNF, TrkC, or TrkB antibodies for 1 h prior to infection with T. cruzi; **, P < 0.01. (C) TrkC blockers specifically inhibit T. cruzi infection of primary cultures of astrocytes, using an infection protocol similar to that for panel A; **, P < 0.01, ***, P < 0.001 relative to vehicle. (D) TrkC blocker NT-3, but not TrkC nonblockers BDNF and NGF, inhibits invasion in human Schwann cells. Schwann cells were incubated with TrkC ligand NT-3, TrkB ligand BDNF, and TrkA ligand NGF for 1 h prior to infection with T. cruzi. (E) sPDNF blockers TrkAECD-Fc and TrkCECD-Fc (the extracellular domain [ECD] of TrkA and TrkC coupled to Fc fragment) but not ECD of TrkB and fibroblast growth factor receptor (FGFR) blocks infection in Schwann cells. Trypomastigotes were preincubated with the Trk receptors (to block parasite-bound PDNF) or control FGFRECD-Fc and then used to infect cells. Error bars represent the standard errors of the means (SEM) between at least three independent experiments. (F) CHO Lec1 cells, which contain terminal N-linked oligomannosyl structures without sialylated lactosamine chains, become more permissive to T. cruzi invasion after transfection with the human trkC gene; **, P < 0.001. (G) CHO Lec2 cells, which express unsialylated N-linked lactosamine residues, become more permissive to invasion by T. cruzi after transfection with the human trkC gene; **, P < 0.01.
We used primary cultures of mouse astrocytes and an immortalized human Schwann cell line (9, 42) to determine whether TrkC mediates invasion in glial cells, which endogenously express TrkC (15, 42) and are highly permissive to T. cruzi (9, 11, 36). We found that TrkC-binding ligands (NT-3 and PDNF) specifically and effectively blocked invasion of astrocytes in a dose-dependent manner, whereas non-TrkC-binding ligands (NGF and BDNF) did not (Fig. 1C). Likewise, NT-3, but not NGF and BDNF, inhibited infection of Schwann cells (Fig. 1D). These results suggest that TrkC is used by T. cruzi to invade glial cells, an inference supported by the results obtained by preincubating the parasites with Trk extracellular domain (ECD) linked to Fc fragment (TrkCECD-Fc and TrkAECD-Fc) (Fig. 1E). This inhibition was specific because TrkBECD-Fc and FGFRECD-Fc were ineffective in inhibiting invasion (Fig. 1E). TrkAECD-Fc was an infection inhibitor because it also binds to PDNF whether soluble or bound to the T. cruzi surface (12).
Previous studies implicated host-cell sialyl residues in T. cruzi invasion (26, 31). Given that PDNF is a sialic acid-binding protein, we determined whether PDNF interaction with sialyl residues on the host cell surface plays a role in TrkC-mediated invasion. For this, we used two sialic acid-deficient Chinese hamster ovarian (CHO) cell lines, Lec1, which contains terminal N-linked oligomannosyl structures without sialylated lactosamine chains, and Lec2, which expresses unsialylated N-linked lactosamine residues (34). We found that transfection of both Lec1 and Lec2 cell lines with the trkC gene (Lec1TrkC and Lec2TrkC) significantly increased T. cruzi invasion compared to transfection with an empty vector (Lec1EV and Lec2EV) (Fig. 1F and G). Thus, T. cruzi invasion of cells via TrkC is independent of TrkC sialyl residues.
TrkC signaling is required for TrkC-mediated invasion.
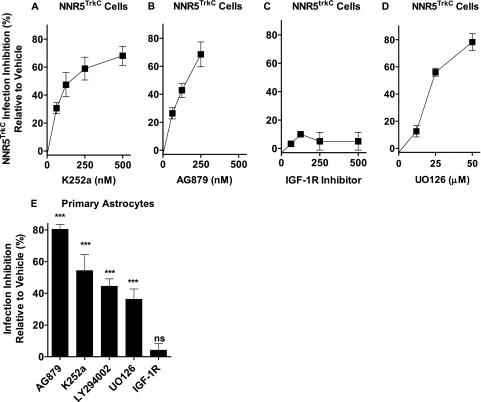
To determine whether TrkC signaling is required for cellular invasion, NNR5TrkC cells were seeded in 96-well plates and preincubated with K252a and AG879, two distinct inhibitors of Trk autophosphorylation (1, 27), followed by T. cruzi. The results showed that both K252a and AG879 blocked invasion in a dose-dependent manner (Fig. 2 A and B). An inhibitor of cell surface IGF-1R (14) was ineffective in blocking T. cruzi invasion of NNR5TrkC cells (Fig. 2C). Trk autophosphorylation leads to activation of Erk1/2 kinase, triggering cytoskeletal rearrangement and other features relevant to neurotrophin response. UO126, a selective inhibitor of Erk phosphorylation, inhibited T. cruzi invasion of NNR5TrkC cells (Fig. 2D). Similar results were obtained with infection inhibition using primary astrocytes as host cells (Fig. 2E). In addition, results with primary astrocytes showed that TrkC-mediated invasion is blocked by LY294002, an inhibitor of Akt kinase signaling also activated by Trk signaling (43) (Fig. 2E). These results suggest that TrkC activation and TrkC-initiated Erk and Akt signaling is required for TrkC-mediated invasion.
Fig. 2.
TrkC signaling is required for T. cruzi invasion of NNR5TrkC cells and astrocytes. (A to D) NNR5TrkC cells were plated in 96-well plates in triplicate and preincubated with vehicle or the indicated concentrations of signaling inhibitors for 1 h before infection with T. cruzi, as in Fig. 1. Error bars represent the SEM between three independent experiments. (E) Primary cultures of astrocytes (in triplicate) were seeded in 96-well plates and preincubated with vehicle medium or K252a (100 nM), AG879 (50 μM), LY294002 (10 μM), U0126 (10 μM), and IGF-1R inhibitor (IGF-1RI, 250 nM); ***, P < 0.001; ns, not significant. Similar results were obtained in three separate experiments; P < 0.001.
Invasion through TrkA and TrkC increases intracellular replication.
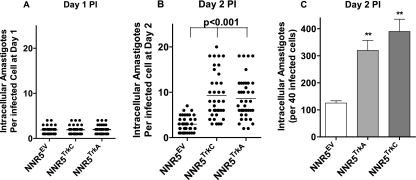
The cell infection results presented in Fig. 1 and 2 are based on the percentage of cells containing intracellular amastigotes, regardless of the number of intracellular parasites. Given that Trk receptor activation leads to increased cell survival, metabolism, glucose transport, and other trophic activities (2, 18, 19), it may be that T. cruzi entry via TrkC and TrkA receptors results in a growth advantage to the intracellular parasite. Indeed, if we measured the number of amastigotes 1 day after infection, we found that NNR5EV cells had a mean (± standard deviation [SD]) of 1.9 ± 0.8 amastigotes/infected cell, results similar to the 1.9 ± 0.8 and 2. 0 ± 0.8 obtained for NNR5TrkC and NNR5TrkA transfectants, respectively (Fig. 3 A). This indicates that the three cell lines bore the same number of intracellular amastigotes at the start of infection and that parasite replication starts 1 day after infection. However, at 2 days after infection, the mean number of amastigotes/infected NNR5EV cells increased to 2.9 ± 1.6, whereas the mean number of amastigotes for NNR5TrkC and NNR5TrkA transfectants jumped to 9.3 ± 4.9 and 8.6 ± 4.4, respectively (Fig. 3B), indicating a preferential T. cruzi growth in the Trk transfectants. The preferential advantage in T. cruzi growth when entering cells via TrkC or TrkA is also evident when calculating the number of intracellular parasites/40 infected cells (Fig. 3C).
Fig. 3.
TrkA- and TrkC-mediated invasion of PC12-NNR5 cells increases the rate of T. cruzi intracellular replication. NNR5EV, NNR5TrkA, and NNR5TrkC cells were plated in 96-well plates in triplicate and infected with T. cruzi for 2 h, uninvaded parasites were washed away. Invaded parasites were allowed to replicate for 1 day (A) and 2 days (B), and the number of intracellular amastigotes were counted for 40 infected cells (from three separate wells). Each point on the graph represents the number of amastigotes per infected cell. The graph shows a representative experiment of three independent experiments. (C) The bar graph shows the total number of amastigotes in 40 infected cells 2 days after infection; error bars represent the SEM between three experiments. **, P = <0.001 for TrkA and TrkC compared to EV.
TrkA and TrkC mediate infection in mice.
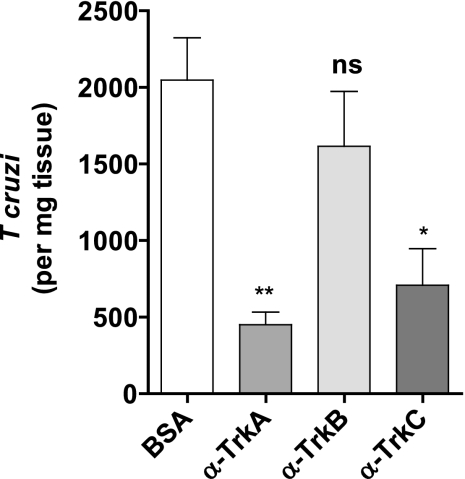
To determine whether TrkC and/or TrkA mediate infection in vivo, we infected mice subcutaneously in the footpad with 5,000 T. cruzi trypomastigotes mixed with 2 μg of BSA or α-TrkA, α-TrkB, or α-TrkC antibodies (to block Trk receptors in the subcutaneous infection site). After allowing for local cellular invasion and replication, mice were sacrificed 3 days after parasite inoculation, and parasitism in the footpad was quantified by quantitative PCR (qPCR). The experiments were done four times with at least 15 mice in each group. We found that α-TrkA and α-TrkC antibodies, but not α-TrkB antibodies, significantly blocked infection (Fig. 4).
Fig. 4.
TrkC and TrkA, but not TrkB, antibodies block T. cruzi infection in vivo. Mice (5/group) were infected subcutaneously in the footpad with 5 × 103 T. cruzi trypomastigotes (Tulahuen strain) mixed with 2 μg of antibodies to TrkA (α-TrkA), TrkB (α-TrkB), TrkC (α-TrkC), or BSA, and footpad parasitism was quantified 3 days later by qPCR. Error bars represent SD for mice from three independent experiments, or 15 mice per group; * and **, P = 0.011 and 0.009, respectively, compared to BSA.
DISCUSSION
Our results demonstrate that T. cruzi, via PDNF, engages the neurotrophin receptor TrkC to invade neuronal, glial, and epithelial cells. TrkC is expressed by most neurons in the CNS, particularly those in the brain cortex, hippocampus, and cerebellum (24, 32). It is also widely expressed by neurons in the PNS and the ENS (4, 32, 35). TrkC is expressed in endothelial cells, dendritic cells, glial Schwann cells, and astrocytes (16, 20, 21, 37, 38), in addition to neurons. The expression of TrkC is in contrast to that of TrkA, which earlier studies showed to be a T. cruzi cellular invader (12). TrkA has limited expression in the CNS and ENS and is not normally expressed by glial cells. Thus, our data showing that T. cruzi invades cells via TrkC receptor greatly expands the molecular basis underlying cellular invasion in mammalian hosts.
TrkC is a trophic receptor whose signaling pathways, which start with Trk ligation and receptor autophosphorylation, promote differentiation and survival of both neural and nonneural tissues (18). T. cruzi/PDNF activation of TrkC signaling promotes survival of cultured neuronal and glial cells, mimicking the protective effects of endogenous Trk ligands (41). Using small-molecule inhibitors to block specific TrkC signaling pathways, we show that parasite invasion is dependent on such protective signaling pathways, specifically TrkC phosphorylation and Erk1/2 and Akt phosphorylation events (Fig. 2A to E).
T. cruzi interactions with TrkC provide a mechanism to gain access to the intracellular environment that T. cruzi needs to replicate and complete its life cycle. Additionally, invasion through TrkC and TrkA triggers an environment that promotes increased parasite replication relative to that in Trk-independent pathways (Fig. 3). Given that Trk receptor activation and signaling leads to increased cell survival, metabolism, and glucose transport, it may not be surprising that Trk receptor signaling confers an intracellular growth advantage to the parasite. However, the mechanism responsible for such growth advantage remains to be determined.
In contrast to the requirement of TrkC signaling for cell entry, our results with CHO-Lec mutants demonstrate that T. cruzi invasion via TrkC does not depend on sialyl residues on TrkA and TrkC receptors (40), as invasion of CHO Lec1 and Lec2 cells was significantly enhanced upon transfection with the trkC gene. Transgenic TrkC and TrkA, other transgenic glycoproteins, and endogenous glycoconjugates in Lec1 and Lec2 CHO cells lack sialyl residues, which in the Lec2 type is due to the absence of CMP-sialic acid transport from the cytosol to the Golgi (34). This conclusion is consistent with other biological properties of trans-sialidase/PDNF, such as direct binding and activation of cytosolic Akt kinase, which is independent of the sialic acid-binding property of the enzyme (6), as sialo-glycoproteins are absent from the cytosol.
TrkC-T. cruzi interaction may be used for invasion of mammalian tissues, as judged from our neutralization experiments using Trk-specific antibodies with mice. The results showed that α-TrkC and α-TrkA antibodies blocked cutaneous infection in an acute model of Chagas' disease, whereas α-TrkB and BSA were ineffective, in agreement with the results obtained using pan-anti-Trk human antibodies (25). These results raise the possibility of using a Trk receptor-targeted molecular approach to block parasite invasion and decrease the parasite burden during infection.
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Berg M. M., Sternberg D. W., Parada L. F., Chao M. V. 1992. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J. Biol. Chem. 267:13–16 [PubMed] [Google Scholar]
- 2. Bibel M., Barde Y. A. 2000. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14:2919–2937 [DOI] [PubMed] [Google Scholar]
- 3. Brener Z. 1972. A new aspect of Trypanosoma cruzi life-cycle in the invertebrate host. J. Protozool. 19:23–27 [DOI] [PubMed] [Google Scholar]
- 4. Chalazonitis A. 2004. Neurotrophin-3 in the development of the enteric nervous system. Prog. Brain Res. 146:243–263 [DOI] [PubMed] [Google Scholar]
- 5. Chuenkova M., Pereira M., Taylor G. 1999. trans-sialidase of Trypanosoma cruzi: location of galactose-binding site(s). Biochem. Biophys. Res. Commun. 262:549–556 [DOI] [PubMed] [Google Scholar]
- 6. Chuenkova M. V., PereiraPerrin M. 2009. Trypanosoma cruzi targets Akt in host cells as an intracellular antiapoptotic strategy. Sci. Signal. 2:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chuenkova M. V., Furnari F. B., Cavenee W. K., Pereira M. A. 2001. Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. U. S. A. 98:9936–9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chuenkova M. V., Pereira M. A. 2000. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell 11:1487–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chuenkova M. V., PereiraPerrin M. 2004. Chagas' disease parasite promotes neuron survival and differentiation through TrkA nerve growth factor receptor. J. Neurochem. 91:385–394 [DOI] [PubMed] [Google Scholar]
- 10. Cummings K. L., Tarleton R. L. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 129:53–59 [DOI] [PubMed] [Google Scholar]
- 11. Da Mata J. R., Camargos M. R., Chiari E., Machado C. R. 2000. Trypanosoma cruzi infection and the rat central nervous system: proliferation of parasites in astrocytes and the brain reaction to parasitism. Brain Res. Bull. 53:153–162 [DOI] [PubMed] [Google Scholar]
- 12. de Melo-Jorge M., PereiraPerrin M. 2007. The Chagas' disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe 1:251–261 [DOI] [PubMed] [Google Scholar]
- 13. Dorn P. L., et al. 2007. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg. Infect. Dis. 13:605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girnita A., et al. 2004. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 64:236–242 [DOI] [PubMed] [Google Scholar]
- 15. Hertz L., Peng L., Lai J. C. 1998. Functional studies in cultured astrocytes. Methods 16:293–310 [DOI] [PubMed] [Google Scholar]
- 16. Hess D. M., et al. 2007. Localization of TrkC to Schwann cells and effects of neurotrophin-3 signaling at neuromuscular synapses. J. Comp. Neurol. 501:465–482 [DOI] [PubMed] [Google Scholar]
- 17. Holtzman D. M., et al. 1995. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J. Neurosci. 15:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang E. J., Reichardt L. F. 2003. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72:609–642 [DOI] [PubMed] [Google Scholar]
- 19. Jacobs S. R., et al. 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180:4476–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawaguchi-Manabe H., et al. 2007. A novel cardiac hypertrophic factor, neurotrophin-3, is paradoxically downregulated in cardiac hypertrophy. Life Sci. 81:385–392 [DOI] [PubMed] [Google Scholar]
- 21. Klein R., et al. 1994. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368:249–251 [DOI] [PubMed] [Google Scholar]
- 22. Koberle F. 1968. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 6:63–116 [DOI] [PubMed] [Google Scholar]
- 23. Lad S. P., Neet K. E., Mufson E. J. 2003. Nerve growth factor: structure, function and therapeutic implications for Alzheimer's disease. Curr. Drug Targets CNS Neurol. Disord. 2:315–334 [DOI] [PubMed] [Google Scholar]
- 24. Lamballe F., Klein R., Barbacid M. 1991. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 66:967–979 [DOI] [PubMed] [Google Scholar]
- 25. Lu B., Alroy J., Luquetti A. O., PereiraPerrin M. 2008. Human autoantibodies specific for neurotrophin receptors TrkA, TrkB, and TrkC protect against lethal Trypanosoma cruzi infection in mice. Am. J. Pathol. 173:1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ming M., Chuenkova M., Ortega-Barria E., Pereira M. E. 1993. Mediation of Trypanosoma cruzi invasion by sialic acid on the host cell and trans-sialidase on the trypanosome. Mol. Biochem. Parasitol. 59:243–252 [DOI] [PubMed] [Google Scholar]
- 27. Ohmichi M., et al. 1993. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry 32:4650–4658 [DOI] [PubMed] [Google Scholar]
- 28. Postan M., Dvorak J. A., McDaniel J. P. 1983. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am. J. Trop. Med. Hyg. 32:497–506 [DOI] [PubMed] [Google Scholar]
- 29. Rassi A., Jr., Rassi A., Marin-Neto J. A. 2010. Chagas disease. Lancet 375:1388–1402 [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg I., Prioli R. P., Ortega-Barria E., Pereira M. E. 1991. Stage-specific phospholipase C-mediated release of Trypanosoma cruzi neuraminidase. Mol. Biochem. Parasitol. 46:303–305 [DOI] [PubMed] [Google Scholar]
- 31. Schenkman R. P., Vandekerckhove F., Schenkman S. 1993. Mammalian cell sialic acid enhances invasion by Trypanosoma cruzi. Infect. Immun. 61:898–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shelton D. L., et al. 1995. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J. Neurosci. 15:477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silva J. S., Twardzik D. R., Reed S. G. 1991. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J. Exp. Med. 174:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanley P. 1989. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell Biol. 9:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sternini C., et al. 1996. Cellular localization of Pan-trk immunoreactivity and trkC mRNA in the enteric nervous system. J. Comp. Neurol. 368:597–607 [DOI] [PubMed] [Google Scholar]
- 36. Tafuri W. L. 1970. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas' disease: light and electron microscope studies. Am. J. Trop. Med. Hyg. 19:405–417 [DOI] [PubMed] [Google Scholar]
- 37. Takeo C., et al. 2003. Rat cerebral endothelial cells express trk C and are regulated by neurotrophin-3. Biochem. Biophys. Res. Commun. 305:400–406 [DOI] [PubMed] [Google Scholar]
- 38. Tessarollo L., et al. 1997. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. U. S. A. 94:14776–14781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urfer R., et al. 1995. An immunoglobulin-like domain determines the specificity of neurotrophin receptors. EMBO J. 14:2795–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watson F. L., Porcionatto M. A., Bhattacharyya A., Stiles C. D., Segal R. A. 1999. TrkA glycosylation regulates receptor localization and activity. J. Neurobiol. 39:323–336 [DOI] [PubMed] [Google Scholar]
- 41. Weinkauf C., Pereiraperrin M. 2009. Trypanosoma cruzi promotes neuronal and glial cell survival through the neurotrophic receptor TrkC. Infect. Immun. 77:1368–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamauchi J., Chan J. R., Shooter E. M. 2003. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. U. S. A. 100:14421–14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng W. H., Quirion R. 2006. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]