Abstract
Although staphylococci are identified by phenotypic analysis in many clinical laboratories, these results are often incorrect because of phenotypic variation. Genetic analysis is necessary for definitive species identification. In the present study, we developed a simple multiplex-PCR (M-PCR) for species identification of human-associated staphylococci, which were as follows: Staphylococcus aureus, S. capitis, S. caprae, S. epidermidis, S. haemolyticus, S. hominis, S. lugdunensis, S. saprophyticus, and S. warneri. This method was designed on the basis of nucleotide sequences of the thermonuclease (nuc) genes that were universally conserved in staphylococci except the S. sciuri group and showed moderate sequence diversity. In order to validate this assay, 361 staphylococcal strains were studied, which had been identified at the species levels by sequence analysis of the hsp60 genes. In consequence, M-PCR demonstrated a sensitivity of 100% and a specificity of 100%. By virtue of simplicity and accuracy, this method will be useful in clinical research.
INTRODUCTION
Staphylococci are normal inhabitants of human skin and mucous membranes. Staphylococcus aureus is a common cause of invasive and life-threatening infections, but coagulase-negative staphylococci (CNS) cause mainly nosocomial infections, such as catheter-related bloodstream infections, prosthetic valve endocarditis, central nervous system shunt infections and prosthetic joint infections (17, 31, 42). Unlike other CNS, however, S. lugdunensis is highly pathogenic and can cause aggressive skin and soft tissue infections, bone and joint infections, and native valve endocarditis (13, 31, 42). In addition, the oxacillin MIC breakpoints to determine methicillin resistance differ among staphylococcal species. Therefore, it is important to identify staphylococci at the species levels.
A variety of methods have been reported for species identification of staphylococci. Although conventional biochemical assays are employed in many clinical laboratories, they are frequently imprecise due to phenotypic variation (5, 16, 19, 26). Real-time PCR has been developed, but some staphylococcal species are indistinguishable, and/or interpretation of results is intricate (12, 37). Sequence analyses of several target genes are the most reliable methods (10, 25, 28, 36). However, these are expensive, laborious, and time-consuming. Thus, a simple and reliable assay is needed for identification of staphylococcal species.
The purpose of the present study was to develop a rapid and accurate multiplex-PCR (M-PCR) for species identification of human-associated staphylococci, which contained the following: S. aureus, S. capitis, S. caprae, S. epidermidis, S. haemolyticus, S. hominis, S. lugdunensis, S. saprophyticus, and S. warneri. This method was based on nucleotide sequences of the thermonuclease (nuc) genes that had been used for staphylococcal identification (4, 35).
MATERIALS AND METHODS
Bacterial strains and species identification.
A total of 24 staphylococcal strains, which included 5 species with whole-genome sequences (14, 23, 24, 32, 40), were used for phylogenetic analysis of the nuc genes (Table 1). A total of 361 staphylococcal strains were studied to assess the utility of M-PCR for species identification of human-associated staphylococci, which were as follows: S. aureus (n = 60), S. epidermidis (n = 104), S. lugdunensis (n = 26), S. haemolyticus (n = 24), S. saprophyticus (n = 23), S. warneri (n = 23), S. hominis subsp. hominis (n = 8), S. hominis subsp. novobiosepticus (n = 14), S. capitis subsp. capitis (n = 7), S. capitis subsp. ureolyticus (n = 24), S. caprae (n = 16), S. auricularis (n = 1), S. arlettae (n = 1), S. carnosus (n = 1), S. chromogenes (n = 1), S. cohnii subsp. cohnii (n = 1), S. cohnii subsp. urealyticum (n = 1), S. croceolyticus (n = 1), S. delphini (n = 1), S. devriesei (n = 1), S. equorum (n = 1), S. felis (n = 1), S. fleurettii (n = 1), S. gallinarum (n = 1), S. hyicus (n = 1), S. intermedius (n = 1), S. kloosii (n = 1), S. lentus (n = 1), S. lutrae (n = 1), S. nepalensis (n = 1), S. muscae (n = 1), S. pasteuri (n = 1), S. pettenkoferi (n = 1), S. piscifermentans (n = 1), S. pseudintermedius (n = 1), S. saccharolyticus (n = 1), S. schleiferi (n = 1), S. sciuri (n = 1), S. simiae (n = 1), S. simulans (n = 1), S. succinus (n = 1), S. vitulinus (n = 1), S. xylosus (n = 1).
Table 1.
Strains used for sequence analysis of the nuc genes
| Species | Strain | Source | Size of ORF (bp) | GenBank accession no. for sequence of nuc | Reference |
|---|---|---|---|---|---|
| S. aureus | N315 | Human | 534 | BA000018 | 23 |
| S. epidermidis | RP62A | Human | 537 | CP000029 | 14 |
| S. haemolyticus | JCSC 1435 | Human | 537 | AP006716 | 40 |
| S. saprophyticus | ATCC 15305T | Human | 531 | AP008934 | 24 |
| S. lugdunensis | ATCC 43809T | Human | 543 | AB598384 | This study |
| S. warneri | ATCC 27836T | Human | 531 | AB598385 | This study |
| S. caprae | CCM 3573T | Goat | 537 | AB598386 | This study |
| S. capitis subsp. capitis | ATCC 27840T | Human | 537 | AB598387 | This study |
| S. capitis subsp. ureolyticus | JCSC 4868 | Human | 537 | AB598388 | This study |
| S. hominis subsp. hominis | ATCC 27844T | Human | 546 | AB598389 | This study |
| S. hominis subsp. novobiosepticus | JCSC 3392 | Human | 546 | AB598390 | This study |
| S. pseudintermedius | LMG 22219T | Cat | 507 | AB327164 | 34 |
| S. delphini group A | LMG 22190T | Dolphin | 516 | AB327167 | 34 |
| S. delphini group B | P-27B | Pigeon | 507 | AB327166 | 34 |
| S. intermedius | ATCC 29663T | Pigeon | 507 | AB327165 | 34 |
| S. schleiferi subsp. schleiferi | P-45C | Pigeon | 504 | None | 35 |
| S. schleiferi subsp. coagulans | JCM 7470T | Dog | 504 | AB465334 | 35 |
| S. hyicus | JCM 2423T | Pig | 510 | AB465332 | 35 |
| S. felis | JCM 7469T | Cat | 507 | AB465335 | 35 |
| S. chromogenes | P-29B | Pigeon | 507 | AB465333 | 35 |
| S. xylosus | h-2B | Horse | 537 | AB465329 | This study |
| S. kloosii | P-40 | Pigeon | 543 | AB465330 | This study |
| S. simulans | JCM2424T | Human | 534 | AB465331 | This study |
| S. carnosus | TM300 | Dry sausage | 543 | AM295250 | 32 |
All strains used in this study were identified at the species levels by sequence analysis of the hsp60 genes (25), which were determined by high sequence similarity (>95%).
DNA extraction.
DNA was extracted by using the procedure described previously (35). A single colony was suspended to a 1.0 McFarland standard in 100 μl of TE buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) with 10 U of achromopeptidase (Wako Chemical, Co. Ltd.). The suspension was heated at 55°C for at least 10 min until it became transparent. The supernatant was used as template DNA for PCR.
Sequence analysis of the nuc genes.
The nuc genes were sequenced by using the procedure described previously (35). Primers Nuc-alF1 (5′-CCNAAYACNCCNGTNCARCCN-3′) and Nuc-alR (5′-NADCCANACRTANGCNARNGT-3′) were used to amplify the conserved regions of the nuc genes. PCR products were cloned into plasmid pCR-4 I-TOPO (Invitrogen, Life Technologies, Carlsbad, CA) and were transformed into Escherichia coli TOP10 cells (Invitrogen). Insert DNA of the recombinant plasmid was sequenced by using a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Foster City, CA) with an ABI Prism 3100 genetic analyzer (Applied Biosystems). The 5′ and 3′ regions were obtained by inverse PCR, and the complete sequences of the nuc genes were determined. For the species for which a degenerate PCR of the nuc genes was inadequate, degenerate primers Nuc-AsdF (5′-WRNCKRTTCATNARRTAYTT-3′) and AsdR (5′-ACNTAYMGNGARATGMGNGAR-3′) were used to amplify the conserved regions of the aspartate kinase genes, which were located about 2 to 8 kbp upstream of the nuc genes. Downstream sequences were analyzed by inverse PCR in order to determine the complete sequences of the nuc genes. Multiple alignments were carried out by using the CLUSTAL W program (41). Construction of the phylogenetic tree was performed by the neighbor-joining method (33).
M-PCR for species identification of human-associated staphylococci.
As previously described (35), the primer pairs for M-PCR were designed on the basis of nucleotide sequences of the nuc and adjacent genes, and they were specific for each species (Table 2). For M-PCR, the reaction mixture contained 2 μl template DNA, 0.2 μM each primer, 200 μM each deoxynucleotide triphosphate, Ex Taq buffer, and 2 U Ex Taq polymerase (Takara Co., Ltd., Kyoto, Japan), in a final volume of 50 μl. A Takara PCR thermal cycler was used for amplification, with an initial denaturation step (95°C, 5 min); 30 cycles of denaturation (95°C, 30 s), annealing (58°C, 30 s), and extension (72°C, 70 s); and a final elongation step at 72°C for 2 min. PCR products were visualized by electrophoresis in 1× Tris-acetate-EDTA on a 1% agarose gel stained with ethidium bromide.
Table 2.
Oligonucleotide primers for M-PCR for species identification of human-associated staphylococci
| primer | Sequence (5′–3′) | Size of PCR product (bp) | Species | Reference |
|---|---|---|---|---|
| hom-F | TACAGGGCCATTTAAAGACG | 177 | S. hominis | This study |
| hom-R | GTTTCTGGTGTATCAACACC | |||
| epi-F | TTGTAAACCATTCTGGACCG | 251 | S. epidermidis | This study |
| epi-R | ATGCGTGAGATACTTCTTCG | |||
| aur-F | TCGCTTGCTATGATTGTGG | 359 | S. aureus | 35 |
| aur-R | GCCAATGTTCTACCATAGC | |||
| hae-F | TAGTGGTAGGCGTATTAGCC | 434 | S. haemolyticus | This study |
| hae-R | ACGATATTTGCCATTCGGTG | |||
| cap-F | ACTACGCCTATGATTATTGC | 525 | S. capitis | This study |
| cap-R | GAYGCTTCTTTACCATAGGG | |||
| lug-F | TCCAATGATGGTAACGAGGC | 695 | S. lugdunensis | This study |
| lug-R | TTTTGCGCCTCGTTTTGTGC | |||
| sap-F | TTTTGGATGCGATAGATTGG | 843 | S. saprophyticus | This study |
| sap-R | TCTTCAGACTTTTCAAAGGC | |||
| war-F | CGTTTGTAGCAAAACAGGGC | 999 | S. warneri | This study |
| war-R | GCAACGAGTAACCTTGCCAC | |||
| rae-F | TTGTTCTWGCACTYATTGCG | 1,227 | S. caprae | This study |
| rae-R | TTTTATAGAACAGGGTCGAC |
Nucleotide sequence accession numbers.
GenBank accession numbers for the sequence of the nuc genes determined in this study are as follows: AB598384 for S. lugdunensis, AB598385 for S. warneri, AB598386 for S. caprae, AB598387 for S. capitis subsp. capitis, AB598388 for S. capitis subsp. ureolyticus, AB598389 for S. hominis subsp. hominis, AB598390 for S. hominis subsp. novobiosepticus, AB465329 for S. xylosus, AB465330 for S. kloosii, and AB465331 for S. simulans.
RESULTS
Development of M-PCR for species identification of human-associated staphylococci.
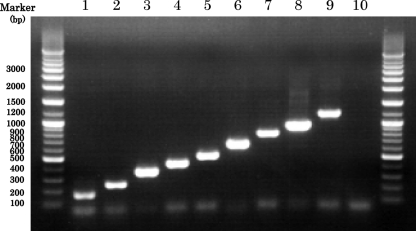
M-PCR successfully amplified the DNA fragments corresponding in size to each species as follows: S. hominis, 177 bp; S. epidermidis, 251 bp; S. aureus, 359 bp; S. haemolyticus, 434 bp; S. capitis, 525 bp; S. lugdunensis, 695 bp; S. saprophyticus, 843 bp; S. warneri, 999 bp; and S. caprae, 1,227 bp (Fig. 1). In order to validate this assay, 361 staphylococcal strains, which had been identified at the species levels by sequence analysis of the hsp60 genes, were studied. For nine human-associated staphylococcal species, the sizes of the amplified DNA fragments matched those predicted for each species, and others showed negative results. The 177-bp fragments contained S. hominis subsp. hominis and S. hominis subsp. novobiosepticus, the 525-bp fragments contained S. capitis subsp. capitis and S. capitis subsp. ureolyticus, and the 843-bp fragments contained S. saprophyticus subsp. saprophyticus and S. saprophyticus subsp. bovis, so this method could not identify these three species at the subspecies levels. Consequently, M-PCR had a sensitivity of 100% and a specificity of 100% in the identification of human-associated staphylococci at the species levels.
Fig. 1.
Electrophoresis after multiplex PCR for species identification of human-associated staphylococci on a 1.0% agarose gel. Lanes: 1, S. hominis; 2, S. epidermidis; 3, S. aureus; 4, S. haemolyticus; 5, S. capitis; 6, S. lugdunensis; 7, S. saprophyticus; 8, S. warneri; 9, S. caprae; 10, water (negative control).
Phylogenetic analysis of the nuc genes of staphylococci.
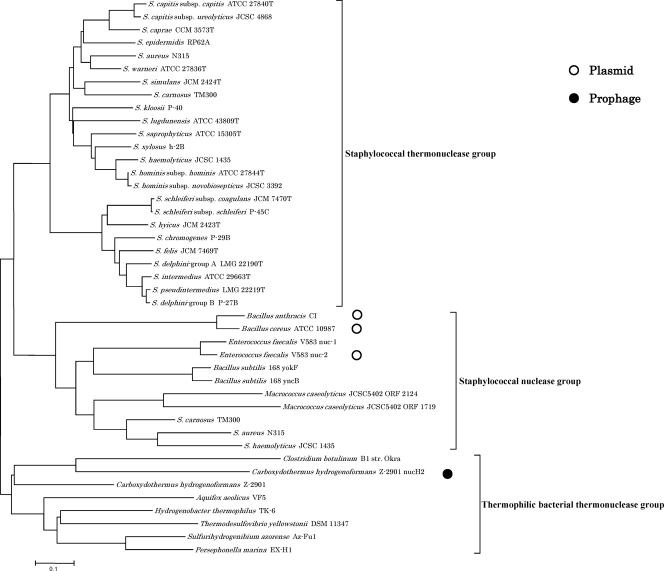
We constructed the phylogenetic tree based on amino acid sequences of the nuc genes of 24 staphylococcal strains (staphylococcal thermonuclease group), staphylococcal nuclease family proteins of Gram-positive bacteria (staphylococcal nuclease group), and thermonuclease family proteins of thermophilic bacteria (thermophilic bacterial thermonuclease group) (Fig. 2).
Fig. 2.
The phylogenetic tree based on amino acid sequences of the nuc genes of staphylococci (staphylococcal thermonuclease group), staphylococcal nuclease family proteins of Gram-positive bacteria (staphylococcal nuclease group), and thermonuclease family proteins of thermophilic bacteria (thermophilic bacterial thermonuclease group). The tree was constructed by the neighbor-joining method using CLUSTAL W. The genes on plasmids and a prophage are indicated by open circles and a filled circle, respectively.
There were the nuc genes at specific gene loci in 24 staphylococcal strains, which were located about 2 to 8 kbp downstream of the aspartate kinase genes (Table 1). We could not detect this gene in any S. sciuri group (S. sciuri, S. lentus, and S. vitulinus) strains. The staphylococcal thermonuclease group formed two main clusters (Fig. 2). One included S. aureus, and the other included S. intermedius. Phylogenetic relationships of the nuc genes among staphylococci were analogous mostly to those of the hsp60 genes (25). The sequence identity of the nuc genes among staphylococci ranged from 59.6 to 99.6% (mean, 67.2%). The sequence similarity of these genes was 99.6% between two subspecies of S. schleiferi, 98.4% between two subspecies of S. hominis, 89.9% between two subspecies of S. capitis, and 79.5% between S. delphini group A and S. delphini group B. It was 95.9% between S. delphini group B and S. pseudintermedius and 87.7% between S. delphini group B and S. intermedius, so the nuc gene sequence of S. delphini group B was more similar to that of S. pseudintermedius and S. intermedius than that of S. delphini group A (34, 35).
There were staphylococcal nuclease genes in Gram-positive bacteria (2, 21–23, 27, 29, 32, 40) and nuc genes in thermophilic bacteria (1, 9, 30, 38, 43) whose complete genome sequences had been determined. Each group formed a distinct cluster from the staphylococcal thermonuclease group, and some genes were on plasmids or a prophage (Fig. 2).
DISCUSSION
Staphylococci are identified by phenotypic analysis in many clinical laboratories. Although many phenotypic identification methods are commercially available, diagnostic accuracy has been reported to be 36.7 to 93.6% (5, 16, 19, 26). This inaccuracy is problematic in clinical practice. Because CNS are closely related to prosthetic device infections, CNS isolated from blood cultures of previously healthy individuals may be regarded as contaminants. However, S. lugdunensis can cause native valve endocarditis, and community-acquired S. lugdunensis bacteremia has been associated with endocarditis (7, 44). Failure to identify S. lugdunensis might lead to delayed or inadequate treatment with an increase in morbidity and mortality.
Currently, the breakpoint for oxacillin resistance is ≥4 μg/ml in S. aureus and S. lugdunensis, whereas it is ≥0.5 μg/ml in other staphylococci (8). If other staphylococcal strains are misidentified as S. aureus or S. lugdunensis, those showing oxacillin MICs of 0.5 to 2 μg/ml are incorrectly considered to be sensitive, which will result in usage of ineffective antimicrobial agents. If S. aureus or S. lugdunensis strains are misidentified as other staphylococci, those showing oxacillin MICs of 0.5 to 2 μg/ml are falsely considered to be resistant. This indicates that antibacterial agents for methicillin-resistant S. aureus, such as vancomycin, can be used for treatment of methicillin-sensitive S. aureus infection. This is also problematic since vancomycin was previously reported to be inferior to beta-lactam antibiotics in the treatment of methicillin-sensitive S. aureus bacteremia (6, 20, 39). In addition, it has been reported that isolates with oxacillin MICs of 0.5 to 2 μg/ml are mecA negative, particularly in S. saprophyticus and S. warneri (15, 18). This can lead to inappropriate use of antibiotics, such as vancomycin. For these species, the new oxacillin MIC breakpoints need to be determined for methicillin resistance. As a result, it is necessary to precisely identify staphylococci at the species levels.
Little is known about the ecology of CNS despite commensal bacteria. It is thought that S. capitis is seen mostly on the head and that S. saprophyticus is a member of the normal rectal or urogenital flora of some women (31, 42). However, isolates have been identified not genotypically but phenotypically. Although matrix-assisted laser desorption ionization–time of flight mass spectrometry, which is the novel phenotypic method, appears to be sensitive and specific (11), the results are unreliable without the protein extraction step (3). Genetic analysis is needed for definitive species identification. Our assay can also be helpful to elucidate the ecology of CNS in humans.
Sequence analyses of several target genes have been reported for staphylococcal identification, and the mean sequence similarity of 16S rRNA, rpoB, hsp60, sodA, and dnaJ genes was 97%, 86%, 82%, 81%, and 77%, respectively (10, 25, 28, 36). Since the nuc genes showed moderate sequence diversity (mean similarity, 67%) and were generally found in staphylococci except the S. sciuri group, we could develop an M-PCR for species identification of staphylococci based on nucleotide sequences of the nuc and adjacent genes.
The nuc genes were present at specific gene loci in 24 staphylococcal strains but absent in the S. sciuri group. In whole-genome analysis of a Macrococcus caseolyticus strain (2), which is a species closely related to the genus Staphylococcus (25), this strain also did not have the nuc gene. Additionally, there were nuc genes in thermophilic bacteria, and one was on a prophage. These results suggest that the nuc genes might have been derived from thermophilic bacteria and might have been acquired by the common ancestor of staphylococci after divergence from the S. sciuri group.
In summary, our M-PCR has been proved to be a rapid and accurate method for species identification of human-associated staphylococci. This is simple and easy to interpret. This will be useful mainly in clinical research and help to elucidate the ecology of human-associated staphylococci.
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid (S0991013) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) for the Foundation of Strategic Research Projects in Private Universities.
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Arai H., Kanbe H., Ishii M., Igarashi Y. 2010. Complete genome sequence of the thermophilic, obligately chemolithoautotrophic hydrogen-oxidizing bacterium Hydrogenobacter thermophilus TK-6. J. Bacteriol. 192:2651–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baba T., et al. 2009. Complete genome sequence of Macrococcus caseolyticus strain JCSCS5402, reflecting the ancestral genome of the human-pathogenic staphylococci. J. Bacteriol. 191:1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bizzini A., Durussel C., Bille J., Greub G., Prod'hom G. 2010. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 48:1549–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brakstad O. G., Aasbakk K., Maeland J. A. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30:1654–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carretto E., et al. 2005. Identification of coagulase-negative staphylococci other than Staphylococcus epidermidis by automated ribotyping. Clin. Microbiol. Infect. 11:177–184 [DOI] [PubMed] [Google Scholar]
- 6. Chang F. Y., et al. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine 82:333–339 [DOI] [PubMed] [Google Scholar]
- 7. Choi S. H., et al. 2010. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J. Clin. Microbiol. 48:3346–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Deckert G., et al. 1998. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392:353–358 [DOI] [PubMed] [Google Scholar]
- 10. Drancourt M., Raoult D. 2002. rpoB gene sequence-based identification of Staphylococcus species. J. Clin. Microbiol. 40:1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubois D., et al. 2010. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards K. J., Kaufmann M. E., Saunders N. A. 2001. Rapid and accurate identification of coagulase-negative staphylococci by real-time PCR. J. Clin. Microbiol. 39:3047–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank K. L., Del Pozo J. L., Patel R. 2008. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin. Microbiol. Rev. 21:111–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill S. R., et al. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gradelski E., Valera L., Aleksunes L., Bonner D., Fung-Tomc J. 2001. Correlation between genotype and phenotypic categorization of staphylococci based on methicillin susceptibility and resistance. J. Clin. Microbiol. 39:2961–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heikens E., Fleer A., Paauw A., Florijn A., Fluit A. C. 2005. Comparison of genotypic and phenotypic methods for species-level identification of clinical isolates of coagulase-negative staphylococci. J. Clin. Microbiol. 43:2286–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huebner J., Goldmann D. A. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223–236 [DOI] [PubMed] [Google Scholar]
- 18. Hussain Z., et al. 2000. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J. Clin. Microbiol. 38:752–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim M., et al. 2008. Comparison of the MicroScan, VITEK 2, and Crystal GP with 16S rRNA sequencing and MicroSeq 500 v2.0 analysis for coagulase-negative staphylococci. BMC Microbiol. 8:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S. H., et al. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 52:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klee S. R., et al. 2010. The genome of a Bacillus isolate causing anthrax in chimpanzees combines chromosomal properties of B. cereus with B. anthracis virulence plasmids. PLoS One 5:e10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kunst F., et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256 [DOI] [PubMed] [Google Scholar]
- 23. Kuroda M., et al. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225–1240 [DOI] [PubMed] [Google Scholar]
- 24. Kuroda M., et al. 2005. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc. Natl. Acad. Sci. U. S. A. 102:13272–13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwok A. Y., Chow A. W. 2003. Phylogenetic study of Staphylococcus and Macrococcus species based on partial hsp60 gene sequences. Int. J. Syst. Evol. Microbiol. 53:87–92 [DOI] [PubMed] [Google Scholar]
- 26. Layer F., Ghebremedhin B., Moder K. A., Konig W., Konig B. 2006. Comparative study using various methods for identification of Staphylococcus species in clinical specimens. J. Clin. Microbiol. 44:2824–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paulsen I. T., et al. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071–2074 [DOI] [PubMed] [Google Scholar]
- 28. Poyart C., Quesne G., Boumaila C., Trieu-Cuot P. 2001. Rapid and accurate species-level identification of coagulase-negative staphylococci by using the sodA gene as a target. J. Clin. Microbiol. 39:4296–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rasko D. A., et al. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reysenbach A. L., et al. 2009. Complete and draft genome sequences of six members of the Aquificales. J. Bacteriol. 191:1992–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogers K. L., Fey P. D., Rupp M. E. 2009. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. North Am. 23:73–98 [DOI] [PubMed] [Google Scholar]
- 32. Rosenstein R., et al. 2009. Genome analysis of the meat starter culture bacterium Staphylococcus carnosus TM300. Appl. Environ. Microbiol. 75:811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 34. Sasaki T., et al. 2007. Reclassification of phenotypically identified Staphylococcus intermedius strains. J. Clin. Microbiol. 45:2770–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sasaki T., et al. 2010. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 48:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah M. M., et al. 2007. dnaJ gene sequence-based assay for species identification and phylogenetic grouping in the genus Staphylococcus. Int. J. Syst. Evol. Microbiol. 57:25–30 [DOI] [PubMed] [Google Scholar]
- 37. Skow A., et al. 2005. Species-level identification of staphylococcal isolates by real-time PCR and melt curve analysis. J. Clin. Microbiol. 43:2876–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith T. J., et al. 2007. Analysis of the neurotoxin complex genes in Clostridium botulinum A1–A4 and B1 strains: BoNT/A3, /Ba4 and /B1 clusters are located within plasmids. PLoS One 2:e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stryjewski M. E., et al. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44:190–196 [DOI] [PubMed] [Google Scholar]
- 40. Takeuchi F., et al. 2005. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J. Bacteriol. 187:7292–7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Eiff C., Peters G., Heilmann C. 2002. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2:677–685 [DOI] [PubMed] [Google Scholar]
- 43. Wu M., et al. 2005. Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z-2901. PLoS Genet. 1:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zinkernagel A. S., et al. 2008. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 36:314–321 [DOI] [PubMed] [Google Scholar]