Abstract
A DNA recombining protein has been partly purified from cell lines derived from patients suffering from the hereditary disease, Bloom's syndrome. The protein induces the formation of displacement loops in phi X174 RFI DNA molecules after the addition of single-stranded DNA fragments. A filter binding method and electron microscopy were used to determine the reaction. The recombinogenic protein is dependent on divalent cations and ATP for activity.
Full text
PDF




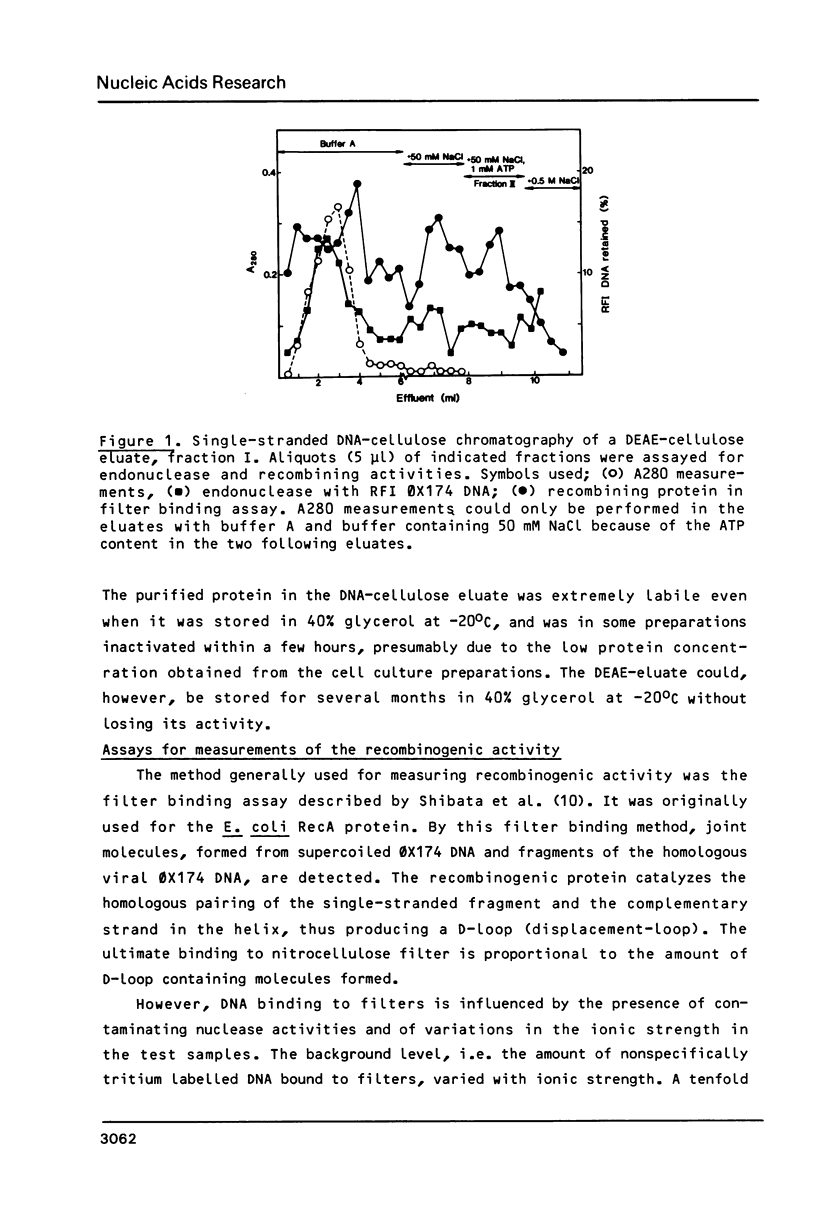
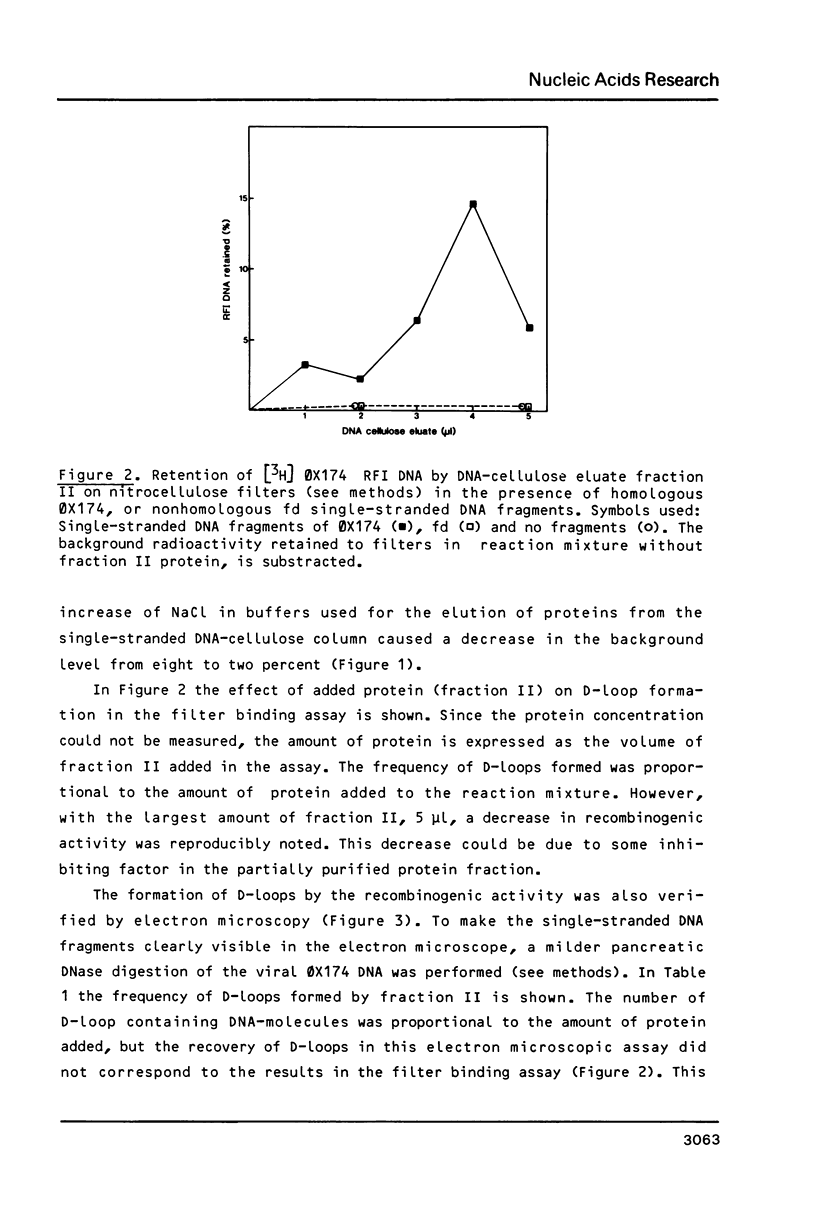
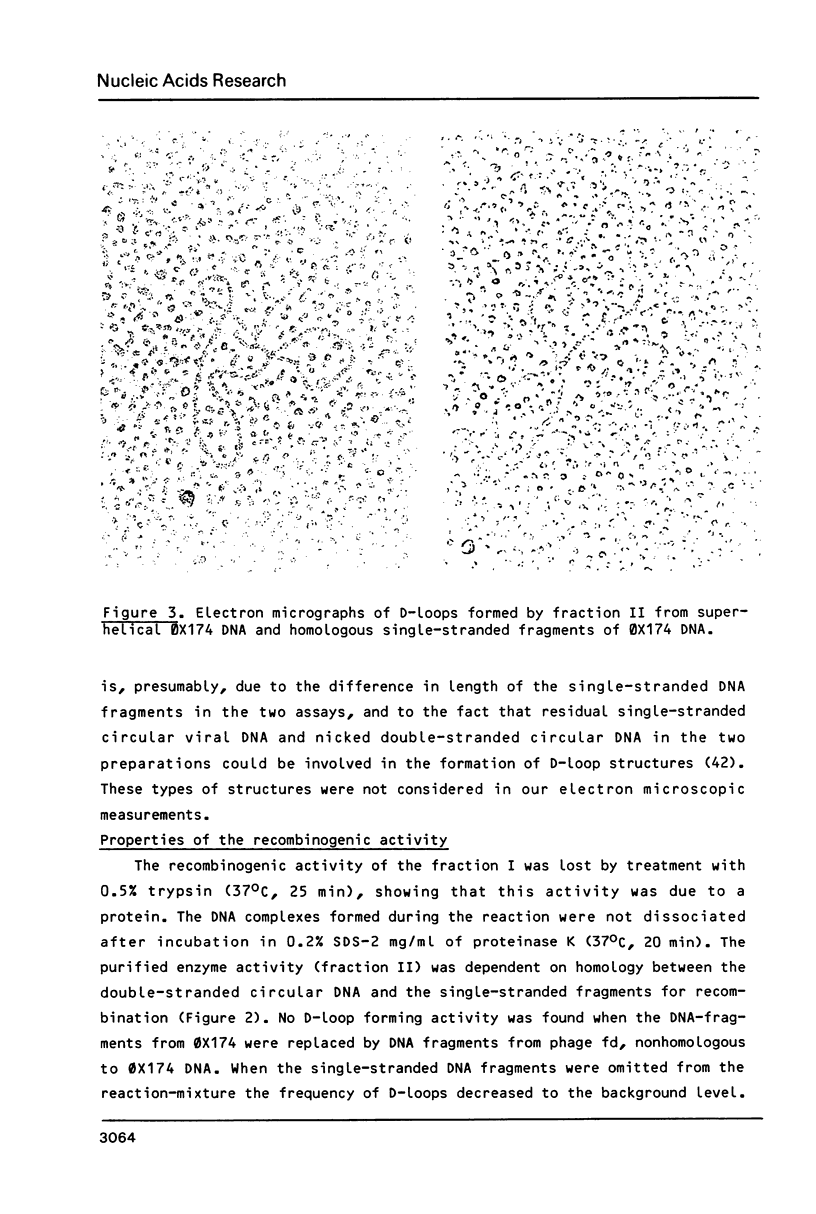



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto E., West S. C., Howard-Flanders P. Can recA protein promote homologous pairing between duplex regions of DNA? EMBO J. 1982;1(7):821–825. doi: 10.1002/j.1460-2075.1982.tb01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cox M. M., McEntee K., Lehman I. R. A simple and rapid procedure for the large scale purification of the recA protein of Escherichia coli. J Biol Chem. 1981 May 10;256(9):4676–4678. [PubMed] [Google Scholar]
- DasGupta C., Radding C. M. Lower fidelity of RecA protein catalysed homologous pairing with a superhelical substrate. Nature. 1982 Jan 7;295(5844):71–73. doi: 10.1038/295071a0. [DOI] [PubMed] [Google Scholar]
- DasGupta C., Radding C. M. Lower fidelity of RecA protein catalysed homologous pairing with a superhelical substrate. Nature. 1982 Jan 7;295(5844):71–73. doi: 10.1038/295071a0. [DOI] [PubMed] [Google Scholar]
- Eitner G., Adler B., Lanzov V. A., Hofemeister J. Interspecies recA protein substitution in Escherichia coli and Proteus mirabilis. Mol Gen Genet. 1982;185(3):481–486. doi: 10.1007/BF00334144. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kano Y., Tatsumi M., Paul P. Effects of a tumor promoter and an anti-promoter on spontaneous and UV-induced 6-thioguanine-resistant mutations and sister-chromatid exchanges in V79 Chinese hamster cells. Mutat Res. 1980 Jul;71(2):243–251. doi: 10.1016/0027-5107(80)90076-7. [DOI] [PubMed] [Google Scholar]
- GERMAN J. CYTOLOGICAL EVIDENCE FOR CROSSING-OVER IN VITRO IN HUMAN LYMPHOID CELLS. Science. 1964 Apr 17;144(3616):298–301. doi: 10.1126/science.144.3616.298. [DOI] [PubMed] [Google Scholar]
- Holloman W. K., Wiegand R., Hoessli C., Radding C. M. Uptake of homologous single-stranded fragments by superhelical DNA: a possible mechanism for initiation of genetic recombination. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2394–2398. doi: 10.1073/pnas.72.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Inhibition of carcinogen-induced chromosomal aberrations by an anticarcinogenic protease inhibitor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3544–3547. doi: 10.1073/pnas.77.6.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., van Beelen P., Schilperoort R. A. Isolation of a recombination deficient Agrobacterium tumefaciens mutant. Mol Gen Genet. 1979 Jun 7;173(2):171–175. doi: 10.1007/BF00330307. [DOI] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Setlow J. K. Similarity in properties and mapping of three Rec mutants of Haemophilus influenzae. J Bacteriol. 1976 Jul;127(1):327–333. doi: 10.1128/jb.127.1.327-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Loveday K. S., Latt S. A. The effect of a tumor promotor, 12-O-tetradecanoyl-phorbol-13-acetate (TPA), on sister-chromatid exchange formation in cultured Chinese hamster cells. Mutat Res. 1979 Aug;67(4):343–348. doi: 10.1016/0165-1218(79)90030-2. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Recombination-deficient mutants of colicinogenic Salmonella typhimurium detected by their failure to produce colicin. J Bacteriol. 1970 Oct;104(1):345–350. doi: 10.1128/jb.104.1.345-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H., Little J. B. Effect of tumor promoters, protease inhibitors, and repair processes on x-ray-induced sister chromatid exchanges in mouse cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1943–1947. doi: 10.1073/pnas.76.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierré A., Paoletti C. Purification and characterization of recA protein from salmonella typhimurium. J Biol Chem. 1983 Mar 10;258(5):2870–2874. [PubMed] [Google Scholar]
- Radding C. M. Genetic recombination: strand transfer and mismatch repair. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Grossman L. Purification and properties of the human placental apurinic/apyrimidinic endonuclease. Methods Enzymol. 1980;65(1):216–224. doi: 10.1016/s0076-6879(80)65030-7. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa K., Sawamura M., Matsushima T., Sugimura T. Inhibition of chemically induced sister chromatid exchanges by elastatinal. Chem Biol Interact. 1979 Jan;24(1):107–110. doi: 10.1016/0009-2797(79)90106-6. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Countryman J. K., Howard-Flanders P. Purification and properties of the recA protein of Proteus mirabilis. Comparison with Escherichia coli recA protein; specificity of interaction with single strand binding protein. J Biol Chem. 1983 Apr 10;258(7):4648–4654. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Kahn R., DasGupta C., Radding C. M. Formation of nascent heteroduplex structures by RecA protein and DNA. Cell. 1982 Aug;30(1):37–44. doi: 10.1016/0092-8674(82)90009-5. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980 Aug;143(2):966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]




