Abstract
The case of a 25-year-old Japanese male who had cerebral schistosomiasis caused by Schistosoma haematobium is reported here. Although serum antibody tests showed a cross-reaction with other helminths and no ova were excreted in urine or feces, the existence of Schistosoma haematobium in the brain was confirmed by PCR analysis.
CASE REPORT
In October 2009, a 25-year-old Japanese man was admitted to a local community hospital in Japan with a 1-week history of mild headache and sporadic paraphasia. He had worked as an agricultural consultant in the Republic of Malawi from April 2007 to June 2009. During his stay, he lived with local residents, consumed water from a well, and had swum in a lake at least twice. He had been in excellent health until October 2009, except for a Giardia lamblia infection in 2008. At the community hospital, a computed tomography (CT) scan of the patient's head showed four hyperdense and edematous lesions in the left parietal lobe, and these lesions were suspected to be related to tropical infectious diseases due to the fact that the onset of his symptoms appeared soon after his return from the Republic of Malawi. Subsequently, the patient was referred to our institution for further workup.
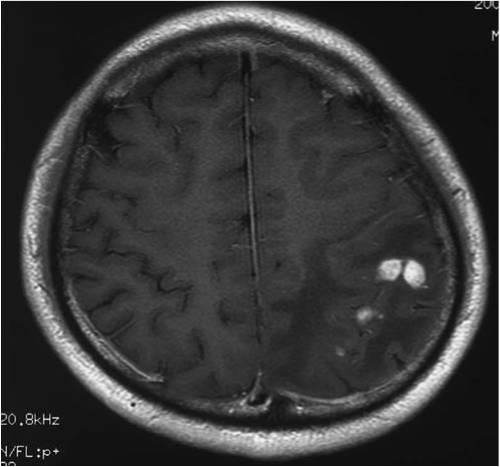
Upon presentation to our institute, the patient's temperature was 36.8°C, his pulse was 60 beats per minute (bpm), and his blood pressure was 120/70 mm Hg. Although the patient was alert and appropriate at a glance, verbal paraphasia was occasionally observed. Laboratory evaluation revealed the following: white blood cell count, 8,780/μl (67.5% neutrophils, 25.0% lymphocytes, 1.5% eosinophils); serum C-reactive protein, 0.03 mg/dl; IgE, 18 U/ml; HIV antibody negative; toxoplasma IgM and IgG negative; and Entamoeba antibody negative. A magnetic resonance imaging (MRI) scan of the brain with gadolinium enhancement showed a couple of ill-defined, heterogeneously enhancing lesions. They were each approximately 10 mm in diameter, in the left parietal lobe, with increased intensity of the signal on the T1-weighted image (Fig. 1). A lumbar puncture was not performed. The patient's headache and nausea worsened rapidly, and we were obliged to relieve his symptoms as soon as possible. Based on the clinical presentation and characteristic imaging finding, we clinically concluded that the cerebral lesions were neurocysticercosis. Albendazole (15 mg/kg of body weight per day) was administered with dexamethasone (0.1 mg/kg per day) for a total of 8 days. The patient's headache and nausea then subsided, and the verbal paraphasia disappeared. The findings from an MRI scan of the brain were improved but still remained.
Fig. 1.
T1-weighted MRI scan with enhancement, obtained at admission, showed tumor-like lesions in the left parietal lobe with the presence of edema.
One week after the initiation of treatment, the results of the commercially available serum enzyme-linked immunosorbent assay (ELISA; SRL, Tokyo, Japan), which can detect IgG antibody for 12 helminthic diseases as a screening (22), were reported: Spirometra erinacei (also known as Spirometra mansoni) antibody on admission was positive, whereas Taenia solium antibody was negative. Schistosoma species are not included in this screening ELISA. Repeated microscopic examination of urine and stool specimens disclosed no ova or parasites. An enhanced CT scan from the neck to the pelvis was unremarkable, without evidence of subcutaneous nodules. From these findings, cerebral sparganosis, which is due to Spirometra species, was strongly suspected as the cause of the cerebral lesions. Cerebral sparganosis responds best to surgical excision of the parasite, because praziquantel has limited success or no effect on adult worms (14, 17). Eleven days after admission, subtotal excision of the nodules at left parietal lobe was achieved by a craniotomy. No live worms or degenerative worms were observed in the surgical field. Pathological examination of the specimen revealed gliosis and multiple necrotizing granulomas scattered within the parenchyma of the brain, with deposits of helminth ova in the center of these granulomas (Fig. 2a). Granulomas had multinucleated giant cells around these ova, which seem to have a prominent terminal spine (Fig. 2b). These morphological characteristics suggested that the helminth ova were eggs of the Schistosoma species, particularly S. haematobium. To identify Schistosoma species, we performed serological tests in our laboratory in the Section of Environmental Parasitology, Tokyo Medical and Dental University (Tokyo, Japan). The result of ELISA revealed increases in serum IgG antibodies against the ova of S. haematobium and Schistosoma mansoni (S. mansoni) and against the larvae of Spirometra erinacei. The serum IgG antibodies against S. haematobium and S. mansoni increased to a level higher than those against S. erinacei. For the treatment for the residual lesion, oral praziquantel was commenced at a dose of 20 mg/kg twice a day for a total of 3 days. An MRI scan of the brain with gadolinium enhancement 3 months after the excision and the chemotherapy showed a significant reduction in the high signal change. The patient remained in stable condition without clinical complications 4 months after completion of the therapy.
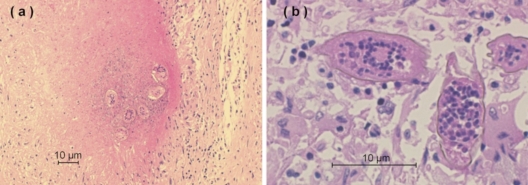
Fig. 2.
(a) Photomicrograph showing nodular granulomas within the parenchyma of the brain containing deposits of S. haematobium ova in the center of the granulomas (hematoxylin-eosin stained; magnification, ×100). (b) Ova of S. haematobium with a characteristic prominent terminal spine (hematoxylin-eosin stained; magnification, ×400).
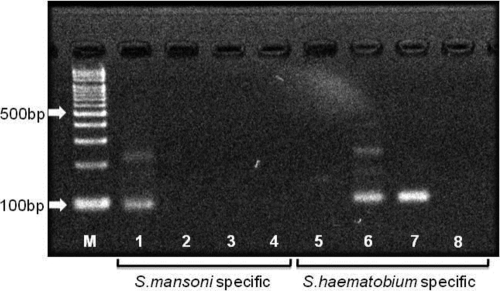
In order to make the definitive diagnosis, the brain specimen was tested by PCR assays. DNA extraction from a paraffin-embedded section of the brain specimen was carried out by using a PCR template preparation kit (TaKaRa DEXPAT Easy; TaKaRa, Shiga, Japan). DNA was amplified with two PCR assays utilizing distinct primer pairs. The first primer targeted to the 97-bp repeated DNA sequence, DraI, which is specific to S. haematobium (10). The second primer targeted to the 121-bp tandem repeated DNA sequence, which is specific to S. mansoni (11, 25). These specific DNA sequences are not contained in the DNA of S. japonicum. As shown in Fig. 3, the PCR amplification using the brain specimen showed an intense band of S. haematobium DNA; however, there was no band specific to S. mansoni DNA. Therefore, we finally diagnosed the patient's cerebral lesions as cerebral schistosomiasis due to S. haematobium.
Fig. 3.
PCR assay for Schistosoma haematobium and S. mansoni. M, marker; lanes 1 and 5, S. mansoni DNA; lanes 2 and 6, S. haematobium DNA; lanes 3 and 7, patient DNA; lanes 4 and 8, no DNA; lanes 1 to 4, S. mansoni-specific 121-bp tandem repeat sequence; lanes 5 to 8, S. haematobium-specific DraI sequence.
Diagnosis of a focal lesion in the brain of patients with a recent history of a stay in sub-Saharan Africa is usually challenging. Since there were no systemic findings characteristic of a specific tropical disease in this patient, we were obliged to rely on epidemiologic and brain MRI features to establish the provisional diagnosis. The patient's final diagnosis was made by PCR assay using a brain specimen from the surgical excision. Although the prevalence of central nervous system (CNS) invasion in schistosomal infection has been considered to be low (20), CNS involvement in S. haematobium infection may be underdiagnosed. An autopsy study in Africa showed that over half of patients infected with S. haematobium in the bladder had brain lesions (8). Another pathological study in Africa has found scattered ova of S. haematobium or S. mansoni in the brain at autopsy in over a quarter of 150 unselected cadavers (1).
Among 22 previous cases of cerebral schistosomiasis due to S. haematobium, 7 cases were diagnosed by ovum excretion in urine or feces (15, 23), and 15 cases were diagnosed by immunological testing (18). However, the detailed mechanism of egg deposition in the brain remains unknown. The presence of egg deposits may reflect either an aberrant migration of worms or the embolization of eggs from a remote location (19, 27). In this case, no worms were detected in the brain specimen, and portal hypertension or liver cirrhosis, which could increase the possibility of worm migration to the CNS (26), was not observed. These findings suggest that cerebral schistosomiasis in this case was caused by translocated eggs from a remote location rather than by eggs from ectopically parasitizing adult worms in the brain.
Diagnosing cerebral schistosomiasis can be difficult, since neurological symptoms and radiological findings are nonspecific. In some reported cases of neuroschistosomiasis, brain tumors, such as meningioma and glioma, had been suspected initially (2, 13, 23). Moreover, as in the present case, patients with cerebral schistosomiasis may have no clinical evidence of systemic disease (21, 28). The presence of parasite ova in the urine and/or stool can be detected in only 40 to 50% of neuroschistosomiasis patients (7). Antibody-based assays are quite sensitive but cannot distinguish a history of exposure from acute infection; they can also cross-react with other helminths (3, 9), such as T. solium (12). In our patient, the elevated IgG antibody of Spirometra erinacei screened by the commercial ELISA led to a presumptive diagnosis of cerebral sparganosis, which in radiological findings mimics cerebral schistosomiasis. The further ELISA performed after histopathological analysis of the brain specimen revealed that IgG antibody titers of S. haematobium, S. mansoni, and Spirometra erinacei were increased. An elaborate examination to screen the infectious focus outside the brain could not detect any abnormality. We then tried to detect the parasite DNA directly from the brain specimen and successfully amplified S. haematobium DNA by PCR assay (Fig. 3). These results strongly suggest that PCR assays are helpful means of confirming the results of serum ELISAs for schistosomiasis. Identification and differentiation of human schistosomiasis by PCR in the laboratory setting (16) and in clinical specimens (24, 26) have been reported.
Praziquantel, a pyrazinoisoquinoline derivative, is the mainstay of the treatment for human schistosomiasis. While the standard regimen for chronic systemic schistosomiasis is 40 to 60 mg/kg of praziquantel in divided doses for 1 day, as described in the Centers for Disease Control and Prevention database (http://www.cdc.gov/parasites/schistosomiasis/health_professionals/index.html), the length of the treatment for cerebral schistosomiasis has not been clearly established (4). To reduce the severity of the inflammatory reaction in the brain parenchyma, corticosteroids are commonly used for CNS invasion. Repeated courses of praziquantel and corticosteroids may be required to reduce neurological symptoms in a severe case (13). Our patient responded well to 40 mg/kg of praziquantel in two divided doses for a total of 3 days after surgical excision of the nodules (5, 6).
In conclusion, we report the first case of cerebral schistosomiasis due to S. haematobium that was diagnosed by molecular methods. We successfully treated the patient with surgical excision and oral praziquantel. PCR assay is a promising method for definitive diagnosis and species identification of cerebral schistosomiasis when Schistosoma ova in urine and/or stool are absent.
Acknowledgments
We are grateful to Nobuaki Akao for support of the diagnosis, and Yukari Horie, Naoki Oyaizu, and Haruo Onoda for their excellent technical assistance. We thank Kei Ouchi for his comments on drafts of the manuscript.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Alves W. 1958. The distribution of schistosoma eggs in human tissues. Bull. World Health Organ. 18:1092–1097 [PMC free article] [PubMed] [Google Scholar]
- 2. Braga B. P., da Costa L. B., Junior, Lambertucci J. R. 2003. Magnetic resonance imaging of cerebellar schistosomiasis mansoni. Rev. Soc. Bras Med. Trop. 36:635–636 [DOI] [PubMed] [Google Scholar]
- 3. Carod-Artal F. J. 2008. Neurological complications of Schistosoma infection. Trans. R. Soc. Trop. Med. Hyg. 102:107–116 [DOI] [PubMed] [Google Scholar]
- 4. Carod-Artal F. J. 2010. Neuroschistosomiasis. Expert Rev. Anti Infect. Ther. 8:1307–1318 [DOI] [PubMed] [Google Scholar]
- 5. Chen A. W., Alam M. H., Williamson J. M., Brawn L. A. 2006. An unusually late presentation of neuroschistosomiasis. J. Infect. 53:e155–e158 [DOI] [PubMed] [Google Scholar]
- 6. Doherty J. F., Moody A. H., Wright S. G. 1996. Katayama fever: an acute manifestation of schistosomiasis. BMJ 313:1071–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrari T. C., Moreira P. R., Cunha A. S. 2004. Spinal cord schistosomiasis: a prospective study of 63 cases emphasizing clinical and therapeutic aspects. J. Clin. Neurosci. 11:246–253 [DOI] [PubMed] [Google Scholar]
- 8. Gelfand M. 1950. Schistosomiasis in South Central Africa, p. 194–202 Post Graduate Press, Capetown, South Africa [Google Scholar]
- 9. Gryseels B., Polman K., Clerinx J., Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118 [DOI] [PubMed] [Google Scholar]
- 10. Hamburger J., Na He, Abbasi I., Ramzy R. M., Jourdane J., Ruppel A. 2001. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am. J. Trop. Med. Hyg. 65:907–911 [DOI] [PubMed] [Google Scholar]
- 11. Hamburger J., Xu Y. X., Ramzy R. M., Jourdane J., Ruppel A. 1998. Development and laboratory evaluation of a polymerase chain reaction for monitoring Schistosoma mansoni infestation of water. Am. J. Trop. Med. Hyg. 59:468–473 [DOI] [PubMed] [Google Scholar]
- 12. Handali S., et al. 2010. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin. Vaccine Immunol. 17:68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houston S., et al. 2004. First report of Schistosoma mekongi infection with brain involvement. Clin. Infect. Dis. 38:e1–e6 [DOI] [PubMed] [Google Scholar]
- 14. Ishii H., et al. 2001. A rare case of eosinophilic pleuritis due to sparganosis. Intern. Med. 40:783–785 [DOI] [PubMed] [Google Scholar]
- 15. Jaureguiberry S., et al. 2007. Acute neuroschistosomiasis: two cases associated with cerebral vasculitis. Am. J. Trop. Med. Hyg. 76:964–966 [PubMed] [Google Scholar]
- 16. Kato-Hayashi N., et al. 2010. Identification and differentiation of human schistosomes by polymerase chain reaction. Exp. Parasitol. 124:325–329 [DOI] [PubMed] [Google Scholar]
- 17. Kim D. G., et al. 1996. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J. Neurosurg. 85:1066–1071 [DOI] [PubMed] [Google Scholar]
- 18. Liu H. Q., Feng X. Y., Yao Z. W., Sun H. P. 2006. Characteristic magnetic resonance enhancement pattern in cerebral schistosomiasis. Chin. Med. Sci. J. 21:223–227 [PubMed] [Google Scholar]
- 19. Liu L. X. 1993. Spinal and cerebral schistosomiasis. Semin. Neurol. 13:189–200 [DOI] [PubMed] [Google Scholar]
- 20. Maguire J. H. 2010. Trematodes (schistosomes and other flukes), p. 3595–3605 In Mandell G. L., Bennett J. E., Dolin R. (ed.), Mandell, Douglas, and Bennett's principle and practice of infectious diseases, 7th ed., vol. 2 Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 21. Massachusetts General Hospital 2001. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 21-2001. A 31-year-old man with an apparent seizure and a mass in the right parietal lobe. N. Engl. J. Med. 345:126–131 [DOI] [PubMed] [Google Scholar]
- 22. Nawa Y. 1997. Histopathological and immunological diagnosis for parasitic zoonoses, p. 39–52 In Ishikura H. (ed.), Host response to international parasitic zoonoses. Springer, Tokyo, Japan [Google Scholar]
- 23. Pollner J. H., Schwartz A., Kobrine A., Parenti D. M. 1994. Cerebral schistosomiasis caused by Schistosoma haematobium: case report. Clin. Infect. Dis. 18:354–357 [DOI] [PubMed] [Google Scholar]
- 24. Sandoval N., et al. 2006. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology 133:581–587 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki T., et al. 2006. Early detection of Schistosoma mansoni infection by touchdown PCR in a mouse model. Parasitol. Int. 55:213–218 [DOI] [PubMed] [Google Scholar]
- 26. van Dijk K., et al. 2010. The potential of molecular diagnosis of cutaneous ectopic schistosomiasis. Am. J. Trop. Med. Hyg. 83:958–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang P., et al. 2010. Research development of the pathogenesis pathways for neuroschistosomiasis. Neurosci. Bull. 26:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou J., et al. 2009. Cerebral schistosomiasis japonica without gastrointestinal system involvement. Surg. Neurol. 71:481–486 [DOI] [PubMed] [Google Scholar]