Abstract
The risk factors for relapse of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia after vancomycin treatment are unknown. Diversilab typing was used to classify recurrent bacteremia as relapse or reinfection. Bacteremia for >7 days and staphylococcal cassette chromosome mec element (SCCmec) type II were independently associated with relapse of MRSA bacteremia after vancomycin treatment.
TEXT
Vancomycin is recommended as the initial treatment of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. However, a number of studies report high rates of vancomycin failure defined as persistent bacteremia (13, 14, 19, 22, 23, 31, 33). While certain investigations have used recurrence as a component of their definition of vancomycin treatment failure (13), few have specifically explored this outcome. Prior studies of recurrent S. aureus bacteremia included both methicillin-susceptible and -resistant infections, used a variety of drug regimens, and did not always differentiate between relapse and reinfection (3, 4, 6, 9, 15–17, 21, 26, 27, 30).
The purpose of this study was to determine the microbial virulence determinants and patient characteristics that predict relapse of MRSA bacteremia after treatment with vancomycin. We performed a retrospective review of patients with MRSA bacteremia from August 2005 to May 2007 hospitalized at Memorial Hermann Hospital, a 700-bed tertiary care hospital. Patients who were ≥18 years old and who received >5 days of vancomycin as initial therapy for MRSA bacteremia were selected. Clinical characteristics, including age, gender, onset and source of infection, comorbidities (prior antibiotics, previous hospital and nursing home contact, immunosuppression, diabetes, liver disease, dialysis, mechanical ventilation, cardiovascular disease, and chronic obstructive pulmonary disease [COPD]), and vancomycin treatment initiation and duration, were extracted from patient medical records. Recurrence of MRSA bacteremia was defined as the return of MRSA bacteremia 2 weeks after documented negative blood cultures. The recurrence was considered a relapse if the Diversilab (DL) (bioMérieux, Durham, NC) typing results of sequential isolates were identical, defined as 95% similarity and no band differences (29). Persistent bacteremia was defined as bacteremia for >7 days. This study was approved by the Institutional Review Board for the University of Texas Health Science Center at Houston (HSC-MS-09-0076).
MICs were performed in duplicate using vancomycin, daptomycin, or linezolid Etest strips (bioMérieux) according to the manufacturer's instructions. Screening for the heterogeneously vancomycin-intermediate S. aureus (hVISA) phenotype was performed using the Etest macromethod (32, 33), with confirmation by population analysis profile-area under the curve (28). Testing for the agr type, the staphylococcal cassette chromosome mec element (SCCmec), and the Panton-Valentine leukocidin (PVL) gene was performed as previously described (11, 12, 33, 34). Data management and analysis were performed using SAS version 9.1.3 (SAS, Cary, NC). Bivariate analysis was conducted by the χ2 test or Fisher's exact test for categorical variables. Variables with an individual effect (P value < 0.15) were tested in a multivariate logistic regression model, with assessment for multicollinearity as previously described (1).
A total of 113 adult patients with MRSA bacteremia treated with vancomycin for >5 days were identified. Twelve of these patients had recurrent MRSA bacteremia. DL typing determined that recurrent isolates were identical to the primary bloodstream isolates in 11/12 (91.7%) of the patients; these patients were thus considered to have had a relapse of MRSA bacteremia. Detailed clinical characteristics of the patients who had a relapse of MRSA bacteremia are shown in Table 1. Patient clinical characteristics, comorbidities, times until vancomycin treatment administration, and durations of vancomycin therapy did not differ between patients with and without relapse (data not shown).
Table 1.
Clinical characteristics of the patients who experienced relapse of MRSA bacteremia after vancomycin treatment
| Case | Bacteremia no. | Patient age (yr) | Sourcea | TEE performeda,b | Treatment length (days)a | Days to relapse | Days of bacteremia/outcome | MIC (μg/ml)d |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Vanc | Dapto | Linezolid | ||||||||
| 1 | 1 | 62 | Unknown | Yes | 14 | 8 | 1.5 | 0.5 | 1.5 | |
| 2 | 14 | 1/death | 1.5 | 0.5 | 0.75 | |||||
| 2 | 1 | 64 | Skin | Yesc | 17 | 16 | 0.75 | 0.75 | 1 | |
| 2 | 16 | 6/death | 0.5 | 0.5 | 0.5 | |||||
| 3 | 1 | 51 | Unknown | Yes | 13 | 1 | 1.5 | 0.38 | 0.38 | |
| 2 | 102 | 15 | 2 | 0.5 | 0.38 | |||||
| 4 | 1 | 58 | Catheter | Yes | 28 | 14 | 2 | 0.75 | 0.5 | |
| 2 | 20 | 22/death | 3 | 1 | 0.75 | |||||
| 5 | 1 | 62 | Skin | No | 17 | 5 | 1.5 | 0.19 | 1 | |
| 2 | 120 | 6 | 3 | 1 | 0.75 | |||||
| 6 | 1 | 67 | Unknown | Yes | 30 | 19 | 1.5 | 0.38 | 0.75 | |
| 2 | 14 | 10 | 1.5 | 0.25 | 0.75 | |||||
| 7 | 1 | 20 | Unknown | Yes | 14 | 1 | 1.5 | 0.38 | 0.75 | |
| 2 | 36 | 3 | 1.5 | 0.25 | 0.5 | |||||
| 8 | 1 | 27 | Respiratory | Yes | 14 | 10 | 1.5 | 0.38 | 1 | |
| 2 | 23 | 20 | 1.5 | 0.38 | 0.75 | |||||
| 9 | 1 | 32 | Unknown | Yes | 18 | 4 | 1.5 | 0.38 | 0.75 | |
| 2 | 41 | 5 | 1.5 | 0.25 | 0.5 | |||||
| 10 | 1 | 18 | Unknown | No | 19 | 2 | 1.5 | 0.25 | 0.75 | |
| 2 | 45 | 5 | 1.5 | 0.38 | 0.75 | |||||
| 3 | 35 | 4 | 2 | 0.38 | 0.75 | |||||
| 11 | 1 | 66 | Multiple sites | Yes | 22 | 14 | 1.5 | 0.38 | 0.75 | |
| 2 | 79 | 16/death | 1.5 | 0.5 | 2 | |||||
Refers to the initial bacteremic episode.
TEE, transesophageal echocardiogram.
Endocarditis was diagnosed on the second episode of bacteremia for case 2. No other cases of endocarditis were detected.
Vanc, vancomycin; Dapto, daptomycin.
The microbiologic characteristics of isolates from relapsed MRSA bacteremia compared to those from patients with a single episode are shown in Table 2. Factors significantly associated with relapse included agr type II (P = 0.0006) and SCCmec type II (P = 0.0002). The vancomycin MIC of the isolate was not associated with relapse of MRSA bacteremia. SCCmec II was present in 10/13 isolates with vancomycin MICs of >1.5 μg/ml, compared to 22/100 isolates with MICs of ≤1.5 μg/ml (P = 0.0002). PVL was present in 63 isolates (55.6%) and was not associated with relapse of bacteremia. The hVISA phenotype was significantly associated with relapse of MRSA bacteremia (P = 0.009) on bivariate analysis. Persistent bacteremia in the first bacteremic episode of patients who later had a relapse of bacteremia was significantly more likely (P = 0.004) than such bacteremia in patients with a single episode.
Table 2.
Microbiologic characteristics of isolates from patients with multiple episodes of MRSA bacteremia compared to those from patients with a single episodea
| Variable | No. (%) of patients with: |
P value | |
|---|---|---|---|
| Relapse (n = 11) | Single episode (n = 102) | ||
| agr type II | 10 (90.9) | 37 (36.3) | 0.0006* |
| SCCmec type II | 9 (81.8) | 23 (22.6) | 0.0002* |
| PVL | 6 (54.6) | 57 (55.9) | 1.00 |
| Vancomycin MIC of >1.5 | 3 (27.3) | 10 (9.8) | 0.11 |
| hVISA | 2 (18.2) | 0 (0.0) | 0.009* |
| Persistent bacteremia | 6 (54.5) | 14 (13.7) | 0.004* |
*, statistically significant.
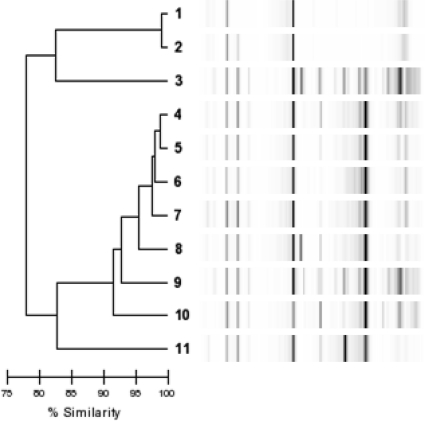
Multivariate analysis was performed to determine independent predictors of relapse of MRSA bacteremia after treatment with vancomycin. Persistent bacteremia was significantly associated with relapse (odds ratio [OR] = 10.1; 95% confidence interval [CI] = 2.0 to 49.6). SCCmec II was also associated with relapse of bacteremia (OR = 19.1; 95% CI = 3.3 to 110.0). DL typing of the first isolate from patients with relapsed MRSA bacteremia is shown in Fig. 1. There were at least seven different clones among the 11 patients with a relapse of bacteremia. Thus, a common clone expressing the virulence determinants identified in this study is not responsible for causing relapse of MRSA bacteremia.
Fig. 1.
Dendrogram of the first isolate from each patient with relapsed MRSA bacteremia.
Recurrence of MRSA bacteremia occurred in roughly 10% of patients in this study, consistent with other reports in the literature (4). The majority of recurrent episodes of MRSA bacteremia after vancomycin treatment in our institution were due to relapse (91.7%), as described by other investigations (4, 7, 10). However, the possibility of reinfection with the same clone cannot be excluded. Multivariate analysis identified persistent bacteremia as an independent risk factor for later relapse of MRSA bacteremia following vancomycin treatment. It is possible that persistence of bacteremia enables the establishment of occult foci of infection. Indeed, persistence of MRSA bacteremia during treatment with vancomycin is associated with metastatic infections (23). It should be noted that all patients in this study were afebrile and clinically well prior to hospital discharge, with no evidence of metastatic infection. Persistent MRSA bacteremia despite adequate vancomycin treatment is associated with isolates with higher vancomycin MIC values, even those considered “susceptible” (13, 14, 23, 31), as well as agr type II (18).
This investigation additionally identified SCCmec type II as a predictor for relapse of MRSA bacteremia after treatment with vancomycin. Other studies have linked SCCmec II with mortality from S. aureus bacteremia (5, 8). It has been reported that isolates with SCCmec II may have reduced vancomycin susceptibility compared to organisms harboring other SCCmec types (20). Indeed, SCCmec II was associated with elevated vancomycin MICs in this investigation. The precise mechanism by which isolates with SCCmec II predispose to relapse following vancomycin treatment is unknown. In addition to having reduced vancomycin susceptibility, such isolates may have variable expression of proteins that enable persistence following antimicrobial therapy (25).
This investigation has important limitations, including its retrospective design and the focus of a single clinical site. The number of patients that experienced relapse of bacteremia was small. Thus, it is possible that some predictors of relapse were not identified; however, the identified factors associated with relapse are likely powerful predictors. DL typing used to classify recurrence as relapse or reinfection may have less discriminatory power than pulsed-field gel electrophoresis (2). Furthermore, we did not assess the impact of vancomycin dosing and serum concentrations, which are difficult to evaluate due to their high variability throughout the treatment course (24). Nonetheless, nearly 10% of patients treated with vancomycin for MRSA bacteremia experienced a relapse of bacteremia. Patients with persistent bacteremia or isolates with certain microbiologic characteristics such as SCCmec II should be monitored for relapse if vancomycin is used as the primary agent.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Allison P. D. 2003. Logistic regression using the SAS system. SAS Institute, Cary, NC [Google Scholar]
- 2. Babouee B., Frei R., Schultheiss E., Widmer A. F., Goldenberger D. 2011. Comparison of the DiversiLab repetitive element PCR system with spa typing and pulsed-field gel electrophoresis for clonal characterization of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 49:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambers H. F., Miller R. T., Newman M. D. 1988. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann. Intern. Med. 109:619–624 [DOI] [PubMed] [Google Scholar]
- 4. Chang F. Y., et al. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore, MD) 82:333–339 [DOI] [PubMed] [Google Scholar]
- 5. Davis S. L., Rybak M. J., Amjad M., Kaatz G. W., McKinnon P. S. 2006. Characteristics of patients with healthcare-associated infection due to SCCmec type IV methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 27:1025–1031 [DOI] [PubMed] [Google Scholar]
- 6. Ehni W. F., Reller L. B. 1989. Short-course therapy for catheter-associated Staphylococcus aureus bacteremia. Arch. Intern. Med. 149:533–536 [PubMed] [Google Scholar]
- 7. Fowler V. G., Jr., et al. 1999. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J. Infect. Dis. 179:1157–1161 [DOI] [PubMed] [Google Scholar]
- 8. Ganga R., et al. 2009. Role of SCCmec type in outcome of Staphylococcus aureus bacteremia in a single medical center. J. Clin. Microbiol. 47:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korzeniowski O., Sande M. A. 1982. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann. Intern. Med. 97:496–503 [DOI] [PubMed] [Google Scholar]
- 10. Liao C. H., Lai C. C., Chen S. Y., Huang Y. T., Hsueh P. R. 2010. Strain relatedness of meticillin-resistant Staphylococcus aureus isolates recovered from patients with repeated bacteraemia. Clin. Microbiol. Infect. 16:463–469 [DOI] [PubMed] [Google Scholar]
- 11. Lina G., et al. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lina G., et al. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 13. Lodise T. P., et al. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maclayton D. O., Suda K. J., Coval K. A., York C. B., Garey K. W. 2006. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Ther. 28:1208–1216 [DOI] [PubMed] [Google Scholar]
- 15. Malanoski G. J., Samore M. H., Pefanis A., Karchmer A. W. 1995. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch. Intern. Med. 155:1161–1166 [DOI] [PubMed] [Google Scholar]
- 16. Markowitz N., Quinn E. L., Saravolatz L. D. 1992. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann. Intern. Med. 117:390–398 [DOI] [PubMed] [Google Scholar]
- 17. Mayhall C. G., Medoff G., Marr J. J. 1976. Variation in the susceptibility of strains of Staphylococcus aureus to oxacillin, cephalothin, and gentamicin. Antimicrob. Agents Chemother. 10:707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCalla C., et al. 2008. Microbiological and genotypic analysis of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 52:3441–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moise P. A., Sakoulas G., Forrest A., Schentag J. J. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moise P. A., et al. 2009. Genotypic and phenotypic relationships among methicillin-resistant Staphylococcus aureus from three multicentre bacteraemia studies. J. Antimicrob. Chemother. 63:873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Musher D. M., Fletcher T. 1982. Tolerant Staphylococcus aureus causing vertebral osteomyelitis. Arch. Intern. Med. 142:632–634 [PubMed] [Google Scholar]
- 22. Neoh H. M., et al. 2007. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann. Clin. Microbiol. Antimicrob. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neuner E. A., Casabar E., Reichley R., McKinnon P. S. 2010. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 67:228–233 [DOI] [PubMed] [Google Scholar]
- 24. Patel N., et al. 2011. Vancomycin: we can't get there from here. Clin. Infect. Dis. 52:969–974 [DOI] [PubMed] [Google Scholar]
- 25. Peacock S. J., et al. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raad I. I., Sabbagh M. F. 1992. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin. Infect. Dis. 14:75–82 [DOI] [PubMed] [Google Scholar]
- 27. Rahal J. J., Jr., Chan Y. K., Johnson G. 1986. Relationship of staphylococcal tolerance, teichoic acid antibody, and serum bactericidal activity to therapeutic outcome in Staphylococcus aureus bacteremia. Am. J. Med. 81:43–52 [DOI] [PubMed] [Google Scholar]
- 28. Satola S. W., Farley M. M., Anderson K. F., Patel J. B. 2011. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. J. Clin. Microbiol. 49:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shutt C. K., Pounder J. I., Page S. R., Schaecher B. J., Woods G. L. 2005. Clinical evaluation of the DiversiLab microbial typing system using repetitive-sequence-based PCR for characterization of Staphylococcus aureus strains. J. Clin. Microbiol. 43:1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Small P. M., Chambers H. F. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soriano A., et al. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200 [DOI] [PubMed] [Google Scholar]
- 32. Walsh T. R., et al. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welsh K. J., et al. 2010. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J. Clin. Microbiol. 48:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang K., McClure J. A., Elsayed S., Louie T., Conly J. M. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5026–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]