LETTER
Culture-independent approaches to studying the microbiota of the airways of cystic fibrosis (CF) patients have employed analysis of the bacterial 16S rRNA gene through Sanger sequencing of clone libraries (5, 6, 12), terminal restriction fragment length polymorphism analysis (8, 11), microarray hybridization (3, 7), or pyrosequencing (2, 5) and have revealed the presence of far more complex bacterial communities than has been appreciated with culture-based methods. Expectorated sputum has been used in many of these studies and, when collected properly, is estimated to contain a relatively low abundance of bacteria from the oropharynx (9). However, sputum samples typically require transportation and/or storage before culture-independent analysis. We sought to assess the effects of sputum sample storage conditions on bacterial community measures obtained by using pyrosequencing analysis.
A sputum specimen, obtained with the approval of the University of Michigan Institutional Review Board during the course of routine care of a 23-year-old man with CF, was stored at 4°C for 2 h and then divided into multiple 0.5-ml aliquots. Aliquots were either immediately frozen at −80°C or stored at room temperature (RT) (∼25°C), 4°C, or −20°C for 1, 2, or 4 weeks before being transferred to −80°C. To isolate DNA, sputum aliquots were thawed on ice, treated with Sputolysin (EMD Chemicals, San Diego, CA), mechanically disrupted, and processed on an automated nucleic acid purification platform (MagNA Pure compact system; Roche Diagnostics, Indianapolis, IN). Bar-coded pyrosequencing of the 16S rRNA V3-V5 region was performed using protocols developed for the Human Microbiome Project (http://www.hmpdacc.org/tools_protocols/tools_protocols.php). The software package mothur V1.19 was used to process sequences and calculate alpha and beta diversities (http://www.mothur.org/wiki/Costello_stool_analysis) (10).
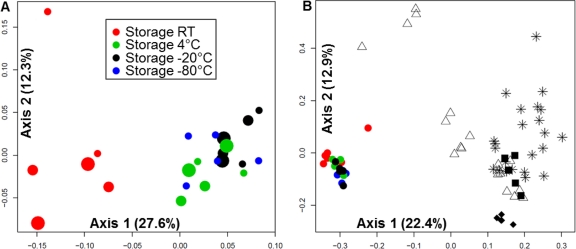
Pyrosequencing with the Roche 454 Titanium platform produced an average of 4,988 sequences (range, 2,625 to 7,241) and 15 operational taxonomic units (OTUs) (range, 10 to 20) per specimen. To compare bacterial community structures, Bray-Curtis distances were calculated using normalized and log2-transformed abundance data for each OTU from individual samples and assessed with principal coordinate analysis (PCoA) (4). Based on nonparametric analysis of molecular variance (NP-AMOVA), the communities in the six samples stored at RT were significantly different (P < 0.001) (1) from the 17 samples stored at other temperatures, which showed insignificant variation (Fig. 1A). For each of the temperatures studied, community structure was not influenced by storage time (P = 0.65, NP-AMOVA). By two-way analysis of variance (ANOVA), community diversity and richness were not significantly different between samples from different storage temperatures or durations (P > 0.05). However, sequences representing Pseudomonas aeruginosa increased significantly (P < 0.05), while sequences representing Gemella haemolysans, Rothia dentocariosa, and Lactococcus lactis decreased significantly (P < 0.05), in samples stored at RT compared to those stored at all other temperatures, irrespective of storage duration. Despite the variation in community structure observed in samples stored at RT, the overall variation within the test samples was comparable to that found within five interrun and five intrarun control samples (Fig. 1B).
Fig. 1.
Principal coordinate analysis (PCoA) of sputum bacterial community structures using log2-transformed Bray-Curtis distances. Each symbol represents a sputum sample. The distance between symbols on the ordination plot indicates the relative similarity in their community structures. (A) PCoA of the 23 sputum storage aliquots. Two aliquots each were stored at RT (red), 4°C (green), or −20°C (black) for each of the storage times indicated by small (1 week), medium (2 weeks), or large (4 weeks) circles. Blue circles represent samples stored immediately at −80°C (n = 5). (B) PCoA of 77 sputum samples, including the 23 storage test aliquots (colored circles in lower left), the 5 interrun control samples (solid black diamonds), the 5 replicate control samples (solid black squares), and the 44 serial sputum samples from two other CF patients (stars and open triangles). For the interrun controls, a single specimen was analyzed in five separate pyrosequencing runs using the same barcoded primer. For the intrarun controls, five aliquots from a single sample were analyzed on the same pyrosequencing run.
To put these results in the context of CF lung bacterial community dynamics, communities from the storage aliquots were compared to two sets of sputum samples serially collected from two adult CF patients during the course of 9 and 8 years, respectively. PCoA using log2-transformed Bray-Curtis distances indicated that the bacterial communities from the CF patient samples were widely distributed throughout the ordination plot relative to the storage test samples and control samples (Fig. 1B).
In summary, while sputum samples stored at RT showed greater variation in community structure than samples stored at the other temperatures tested, the overall variation within the storage test samples was comparable to that found within the intra- and interrun control samples. Importantly, these variations were insignificant relative to those found among samples serially obtained from CF patients during prolonged periods of observation, suggesting that storage at any of these temperatures, at least for the durations of time included in this study, does not significantly affect pyrosequencing-based measures of community structure. The variation observed in intra- and interrun controls illustrates the limits of reproducibility of pyrosequencing-based bacterial community profiling. This limitation warrants further study and must be taken into account in studies designed to assess the fine-scale dynamics of the CF lung microbiota.
Acknowledgments
We thank Dr. Richard Gibbs, Donna Muzny, and the Baylor College of Medicine Human Genome Sequencing Center production team for their assistance with this effort.
This work was supported by the National Institutes of Health (NHLBI grant 1RC1HL100809-01 and CTSA grant UL1RR024986) and the Cystic Fibrosis Foundation.
Footnotes
Published ahead of print on 24 August 2011.
Contributor Information
Jiangchao Zhao, Department of Pediatrics and Communicable Disease University of Michigan Medical School Ann Arbor, Michigan.
Jun Li, Department of Human Genetics University of Michigan Medical School Ann Arbor, Michigan.
Patrick D. Schloss, Department of Microbiology and Immunology University of Michigan Medical School Ann Arbor, Michigan
Tracy A. Raymond, Department of Pediatrics and Communicable Disease University of Michigan Medical School Ann Arbor, Michigan
Joseph F. Petrosino, Human Genome Sequencing Center Department of Molecular Virology and Microbiology Baylor College of Medicine Houston, Texas
Vincent B. Young, Department of Internal Medicine University of Michigan Medical School Ann Arbor, Michigan
John J. LiPuma, Department of Pediatrics and Communicable Disease University of Michigan Medical School 8323 MSRB III 1150 W. Medical Center Dr. Ann Arbor, Michigan 48109-5646.
REFERENCES
- 1. Anderson M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46 [Google Scholar]
- 2. Armougom F., et al. 2009. Microbial diversity in the sputum of a cystic fibrosis patient studied with 16S rDNA pyrosequencing. Eur. J. Clin. Microbiol. Infect. Dis. 28:1151–1154 [DOI] [PubMed] [Google Scholar]
- 3. Cox M. J., et al. 2010. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One 5:e11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dethlefsen L., Relman D. A. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 1):4554–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guss A. M., et al. 2011. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J. 5:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris J. K., et al. 2007. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A. 104:20529–20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klepac-Ceraj V., et al. 2010. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ. Microbiol. 12:1293–1303 [DOI] [PubMed] [Google Scholar]
- 8. Rogers G. B., et al. 2004. Characterization of bacterial community diversity in cystic fibrosis lung infections by use of 16s ribosomal DNA terminal restriction fragment length polymorphism profiling. J. Clin. Microbiol. 42:5176–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers G. B., et al. 2006. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J. Clin. Microbiol. 44:2601–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schloss P. D., et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sibley C. D., et al. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 105:15070–15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Gast C. J., et al. 2011. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 5:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]