Abstract
Pythium aphanidermatum is a fungus-like plant pathogen which has never been reported as a cause of human infection. We report a case of P. aphanidermatum invasive wound infection in a 21-year-old male injured during combat operations in Afghanistan.
CASE REPORT
A 21-year-old Hispanic male sustained extensive soft tissue injuries to his bilateral lower extremities, a right midshaft fibula fracture, and soft tissue injuries of his buttocks and right upper extremity in an improvised explosive device (IED) blast in Afghanistan in August 2009. On the day of the injury, he underwent debridement and irrigation of his wounds and placement of vacuum-assisted closure devices. He was aeromedically evacuated to a tertiary care medical center in Germany and then to the Brooke Army Medical Center (BAMC), arriving 4 days after the initial trauma.
On initial evaluation at the BAMC, the patient was intubated and sedated, with a Glasgow coma scale score of 3T. He was tachycardic (heart rate, 121 beats per minute) and normotensive (blood pressure, 134/65 mm Hg), with a respiratory rate of 14 breaths per minute and a temperature of 100°F. Physical examination revealed minimally reactive pupils and bibasilar crackles upon lung auscultation. Wound examination revealed complete exposure of the posterior thighs to the deep muscle layers and, in some areas, to the bone. He was noted to have recent muscle debridement, focal muscle excision, and a left lower extremity fasciotomy. An examination of his right elbow revealed a deep wound with palpable bone. Initial labs revealed a white blood cell count of 7,700 cells/μl (86.1% neutrophils, 33% bands) and a creatine kinase level of 2,057 units/liter. The patient's renal function panel and other laboratory values were unremarkable.
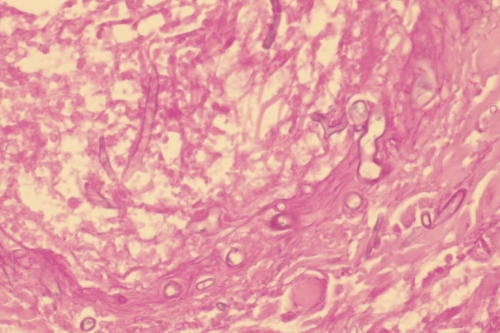
During his hospital course, the patient underwent numerous debridements and irrigations of his bilateral lower extremity wounds, ultimately requiring disarticulation of both lower extremities. Approximately 7 days after initial trauma, significant necrosis of subcutaneous fat and muscle was noted in both lower extremities. Histopathology from the left calf showed many broad, aseptate hyphae with ribbon forms, consistent with mucormycosis. Nine days after his initial trauma, the patient underwent below-knee amputation of the left lower extremity, with healthy, viable tissue noted at the proximal margins postamputation. Despite these initially clear margins, operative reexploration 24 h later revealed severely devitalized musculature, complete avascularity, and loss of contractility. These findings of rapid advancement of necrosis led to the decision to perform the potentially lifesaving procedure of bilateral lower extremity disarticulations. Pre- and postdisarticulation histopathology of the bilateral lower extremities revealed hyphae consistent with mucormycosis in the skin, subcutaneous fat, muscles, and viable blood vessels. Histopathology of the left sartorius muscle showed multiple scattered hyphae, characterized as large, broad, and rarely septate (Fig. 1). Postdisarticulation histopathology revealed an invasive fungal infection with clear proximal margins. Intravenous (i.v.) liposomal amphotericin B (10 mg/kg of body weight i.v. daily) and voriconazole (4 mg/kg i.v. every 12 h) were initiated at the time of disarticulation.
Fig. 1.
Histopathology from a section of the left sartorius muscle showing broad, sparsely septate hyphae (periodic acid Schiff stain; magnification, ×40).
Following disarticulation, the patient required the placement of a wound vacuum device on his bilateral stumps and serial debridements of the lower extremity wounds, and several attempts for final wound closure were made. He had a complicated hospital course, which included a cerebral vascular accident, numerous surgeries for gastric necrosis and perforation, intra-abdominal abscess formation, ventilator-associated pneumonias, and recurrent bacteremias. The patient died of multiorgan system failure 16 weeks after his disarticulation surgery (18 weeks after his initial combat trauma). Autopsy did not reveal any evidence of residual fungal infection.
Fungal identification.
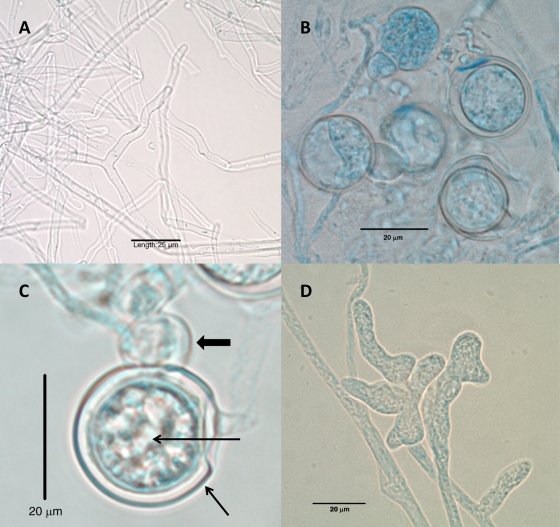
Fungal cultures of necrotic tissue from both lower extremities recovered Mucor circinelloides (left calf muscle, 9 days after injury), Aspergillus flavus (right thigh muscle, 9 days after injury), and Pythium aphanidermatum (left sartorius muscle, 9 days after injury) isolates. The Pythium aphanidermatum isolate recovered from the left sartorius muscle was submitted for phenotypic identification and accessioned into the University of Texas Health Science Center at San Antonio Fungus Testing Laboratory culture collection under accession number UTHSCSA 09-2282. The white, woolly isolate grew rapidly, filling a 60-mm potato flake agar plate within 3 days, but produced only sterile hyphae (Fig. 2A). It was initially thought to be an Apophysomyces or Saksenaea species based upon its morphology and lack of fruiting; however, a water agar culture at 35°C, used to induce fruiting in these genera, remained sterile after 1 month (18). The isolate also demonstrated very poor growth and remained sterile on hay infusion agar used for sporulation in Saksenaea species (15). The isolate was subsequently reevaluated for the production of sexual and asexual features on cornmeal agar with irradiated-carnation-leaf agar (CMACLA) (12), V8 agar, and CLA (12), all prepared in-house. Plates were overlaid with a few drops of sterile water, incubated at 25°C, and examined at 12, 24, 36, and 48 h. Growth on the CMACLA was the most informative. After 24 h, colonies were white, woolly, and 40 mm in diameter in a 60-mm petri dish, and hyphae were approximately 8.0 μm in diameter. Oogonia were abundant, globose, smooth, 22 μm to 27 μm in diameter, and mostly formed at the ends of hyphae. Oospores were loosely arranged within the oogonia. Antheridia, approximately 8 to 10 μm by 10 to 12 μm, formed adjacent to the oogonia (Fig. 2B and C). Toruloid sporangia, consisting of terminal complexes of swollen hyphal branches of various lengths and up to 20 μm wide (Fig. 2D), were also present. Zoospores were not observed. Temperature studies conducted on potato flake agar slants at 25°C, 30°C, 35°C, and 40°C and read after 4 days were recorded as 4+, 3+, 3+, and 1+, respectively. The isolate has been deposited into the Centraalbureau voor Schimmelcultures Fungal Biodiversity Centre under accession number CBS 128995. Antifungal susceptibility testing, using the Clinical and Laboratory Standards Institute's M38-A2 document for testing of filamentous fungi, revealed resistance to all agents examined, with results as follows: an amphotericin B MIC of 16 μg/ml, an itraconazole MIC of >16 μg/ml, a posaconazole MIC of >16 μg/ml, and a terbinafine MIC of >2 μg/ml (3). Genotypic identification of Pythium aphanidermatum (accession number UTHSCSA R-4449) was performed by sequencing internal transcribed spacer (ITS) and D1/D2 ribosomal DNA regions as described previously (14). A BLASTn search was performed (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using cutoffs of ≥97% identity and ≥90% query coverage for significance. The results of the BLASTn search with the ITS sequence showed the top three hits as Pythium aphanidermatum accession number EU245039 (821/822 nucleotides match; 99% identity), Pythium aphanidermatum accession number AF452146 (795/796 nucleotides match; 99% identity), and Pythium aphanidermatum accession number AY151180 (785/786 nucleotides match; 99% identity). The top-rated hit was also Pythium aphanidermatum, which matched at 100% identity, but it had only 80% query coverage and was not considered. The BLASTn results of the D1/D2 search showed the top three hits as Pythium aphanidermatum accession number AB468700 (704/705 nucleotides match; 99% identity), Pythium aphanidermatum accession number AY598622 (704/705 nucleotides match; 99% identity), and Pythium monospermum accession number AY598621 (680/705 nucleotides match; 97% identity). The results of the UTHSCSA R-4449 molecular identification were consistent with an identification of Pythium aphanidermatum.
Fig. 2.
(A) Sterile hyphae of Pythium aphanidermatum grown on potato flake agar (lactophenol cotton blue mount); (B) oogonia, oospores, and antheridia (lactophenol cotton blue mount; magnification, ×20); (C) higher magnification of the oogonium (short thin arrow), the oospore (long thin arrow), and the antheridium (fat arrow) (lactophenol cotton blue mount); (D) toruloid sporangium. All were produced on CMACLA after 24 h of incubation at 25°C.
Discussion.
Pythium species are known plant pathogens that infect seeds, plant roots, and mature plants (4, 5, 17). Pythium insidiosum is the only member of the genus implicated previously in human and animal infections (2, 9, 10, 11, 17, 19). Pythium species originally belonged to the oomycetous kingdom of fungi but were reclassified to the kingdom Stramenopila, phylum Oomycota, class Oomycetes, and family Pythiaceae. These organisms are typically found in tropical and subtropical regions and are described as a group of aquatic, parafungal microbes with hyaline elements that form motile spores (2, 8, 9, 10, 11, 13, 17, 19). Pythium is often confused with mucoraceous fungi, as the sparsely septate, hyaline hypha-like elements of Pythium species resemble the sparsely septate, hyaline hyphae seen in the Mucorales (11, 13). The cell wall of Pythium aphanidermatum contains cellulose and 1-3,1-6 β-d-glucans rather than mannan and chitin (1, 13).
Human infections with P. insidiosum are exceptionally rare and occur by attachment of motile biflagellate zoospores that are attracted chemotactically to traumatized human tissues and that are believed to encyst in the damaged tissues (2, 7, 10, 13, 19). These infections are life-threatening and often result in limb amputation or death (8, 11, 13). Extensive surgical debridement is indicated, due to their aggressive and deeply invasive nature (6, 8, 17). Pythium species do not possess ergosterol in their cytoplasmic membranes and thus do not respond to most antifungal agents that are active against ergosterol. The mainstay of treatment is aggressive debridement and, if necessary, amputation of infected tissues (6, 7, 9, 11). Pythium infection in humans was first recognized in Thailand in 1985. Additional human cases have been reported from tropical and subtropical regions of the world, mostly in aquatic or wet environments (8, 9, 11, 13, 19). Cutaneous, subcutaneous, vascular, ocular, and disseminated cases of Pythium insidiosum infection have been reported, with significant morbidity and high case fatality rates (8, 9, 13, 19). Histopathology of human pythiosis indicates necrotizing arteritis and thrombosis, with evidence of sparsely septate, irregularly branched hyphae in the vessel walls, later replaced by granulomas with the presence of hyphae (13). As was seen in this case, vascular pythiosis affects the arteries of lower extremities, resulting in dissections of involved vessels, aneurysm formation, and thrombosis. These events may lead to fatal hemorrhage despite aggressive surgical intervention (6, 8, 13).
Pythium aphanidermatum is pathogenic to multiple plants, often attacking the roots of the plants, preventing further growth or causing plant death (5). Pythium aphanidermatum has not previously been reported to cause infections in animals or humans, nor has it been reported to be recovered in clinical laboratories as a contaminant. We identified this organism from wound cultures with histopathology consistent with invasive, aggressive infection and similar to that seen in previously described P. insidiosum infections. Although M. circinelloides was also recovered in this wounded soldier, it is not clear whether this was a coinfection or whether it was restricted to the left lower leg.
Nucleotide sequence accession numbers.
The ITS and D1/D2 sequences of the P. aphanidermatum isolate were submitted to GenBank under accession numbers JF412451 and JF412452, respectively.
Acknowledgments
B.L.W. is supported by grant PR054228 from the U.S. Army Medical Research and Materiel Command, Office of Congressionally Directed Medical Research Programs.
We acknowledge the assistance of Josep Guarro at the Universitat Rovira i Virgili, Reus, Spain, in comparing our Mucor circinelloides sequence data with his Mucor database to rule out the newly described species Mucor velutinosus and Mucor ellipsoideus.
The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Air Force, Department of the Army, Department of Defense, or the U.S. Government. T.P.C., P.J.B., T.J.V., C.E.W., E.M.R., and D.R.H. are employees of the U.S. Government.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Blaschek W., Kasbauer J., Kraus J., Franz G. 1992. Pythium aphanidermatum: culture, cell-wall composition, and isolation and structure of antitumour storage and solubilised cell-wall (1→3),(1→6) beta-d-glucans. Carbohydr. Res. 231:293–307 [DOI] [PubMed] [Google Scholar]
- 2. Camus A., Grooters A., Aquilar R. 2004. Granulomatous pneumonia caused by Pythium insidiosum in a Central American jaguar, Panthera onca. J. Vet. Diagn. Invest. 16:567–571 [DOI] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed Approved standard M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. El-Tarabily K. A., Nassar A. H., Hardy G. E., Sivasithamparam K. 2009. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 106:13–26 [DOI] [PubMed] [Google Scholar]
- 5. Heine G., Tikum G., Horst W. 2007. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 58:569–577 [DOI] [PubMed] [Google Scholar]
- 6. Imwidthaya P. 1994. Human pythiosis in Thailand. Postgrad. Med. J. 70:558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaufman L. 1998. Penicilliosis marneffei and pythiosis: emerging tropical diseases. Mycopathologia 143:3–7 [DOI] [PubMed] [Google Scholar]
- 8. Krajaejun T., et al. 2006. Identification of a novel 74-kilodalton immunodominant antigen of Pythium insidiosum recognized by sera from human patients with pythiosis. J. Clin. Microbiol. 44:1647–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krajaejun T., et al. 2006. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 43:569–576 [DOI] [PubMed] [Google Scholar]
- 10. Mendoza L., Hernandez F., Ajello L. 1993. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J. Clin. Microbiol. 31:2967–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendoza L., Prasla S. H., Ajello L. 2004. Orbital pythiosis: a non-fungal disease mimicking orbital mycotic infections with a retrospective review of the literature. Mycoses 47:14–23 [DOI] [PubMed] [Google Scholar]
- 12. Nelson P. E., Toussoun T. A., Marasas W. F. O. 1983. Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, University Park, PA [Google Scholar]
- 13. Pfaller M. A., Diekema D. J. 2005. Unusual fungal and pseudofungal infections of humans. J. Clin. Microbiol. 43:1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romanelli A. M., Sutton D. A., Thompson E. H., Rinaldi M. G., Wickes B. L. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schipper M. A. A., Stalpers J. A. 2002. Zygomycetes: the order Mucorales, p. 67–126 In Howard D. H. (ed.), Pathogenic fungi in humans and animals, 2nd ed Marcel Dekker, Inc., New York, NY [Google Scholar]
- 16. Reference deleted.
- 17. Shenep J. L., et al. 1998. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 27:1388–1393 [DOI] [PubMed] [Google Scholar]
- 18. Sutton D. A., Fothergill A. W., Rinaldi M. G. 1998. Guide to clinically significant fungi, p. 420–421 Williams and Wilkins, Baltimore, MD [Google Scholar]
- 19. Vanittanakom N., et al. 2004. Identification of emerging human-pathogenic Pythium insidiosum by serological and molecular assay-based methods. J. Clin. Microbiol. 42:3970–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]