Abstract
The severity of skin and soft tissue infections caused by group A Streptococcus (GAS) is variable, and there are only a limited number of studies evaluating the characteristics of these infections in the literature. From May 2005 to November 2007, 73 patients with skin and soft tissue infections caused by group A Streptococcus were included in this study. Among these patients, 34 (46.6%) had invasive diseases. Diabetes mellitus, alcoholism, and hypertension were the most common underlying disorders. The overall mortality rate was 6.8%, and the elderly were predisposed to invasive infections (P < 0.001). Neutrophil percentages of ≥80, serum creatinine levels of ≥2 mg/dl, and high serum C-reactive protein levels were noted more frequently in patients with invasive infections than in patients with noninvasive infections, as were bacteremia and a high mortality rate. Of the 73 isolates, 93.2%, 97.3%, and 37% exhibited susceptibility to erythromycin, clindamycin, and tetracycline, respectively. The five most prevalent emm types were emm106 (24.7%), emm11 (12.3%), emm102 (9.6%), emm4 (8.2%), and emm12 (8.2%). Compared to other types, the emm106 type was significantly more likely to be associated with invasive diseases (P = 0.012). Dendrogram analysis showed a unique SmaI-digested pulsed-field gel electrophoresis pattern of the emm106 type that was particularly prone to cause invasive skin and soft tissue infections (P < 0.001). The results of this study suggest that isolates with the emm106 gene may be an emerging group A Streptococcus strain that causes invasive skin and soft tissue infections. Further surveillance study to understand the significance of this invasive strain is critical.
INTRODUCTION
Group A Streptococcus (GAS), also known as Streptococcus pyogenes, is a Gram-positive coccus that is arranged in pairs and chains. These organisms are ubiquitous in the environment and are responsible for a broad spectrum of human infections that result in significant morbidity and mortality, including pharyngitis, scarlet fever, skin and soft tissue infection (SSTI), streptococcal toxic shock syndrome, septicemia, pneumonia, and meningitis (10). It is estimated that severe GAS diseases lead to more than 500,000 deaths each year via infections such as acute rheumatic fever, rheumatic heart disease, poststreptococcal glomerulonephritis, and invasive diseases (6).
SSTIs are common and account for approximately 7 to 10% of hospitalizations in North America (12, 13). The manifestations of SSTIs caused by GAS range from mild superficial impetigo, erysipelas, pyoderma, and cellulitis to life-threatening necrotizing fasciitis or myonecrosis. The early discrimination between noninvasive SSTIs that can be resolved with antimicrobial treatment and invasive infections that may additionally require surgical intervention is critical. Unfortunately, the clinical symptoms and signs of invasive SSTIs, including disproportionate pain, bullae, ecchymosis, sloughing, skin anesthesia, rapid progression, gas in the tissue, and systemic toxicity, often appear late in the course of infection (29). The delayed diagnosis and treatment of invasive SSTIs can result in catastrophic outcomes.
Several virulence factors involved in the pathogenesis of GAS have been discussed, including M protein, streptolysin, hyaluronic acid capsular polysaccharide, and the other exotoxins (5). The M proteins of GAS, which are encoded by emm genes and form elongated structures on the bacterial surface, play an important role in the pathogenesis of this microorganism. M proteins are known to be a critical antiphagocytic constituent of GAS due to their role in resistance to opsonization (4, 11). These proteins could trigger the activation and degranulation of neutrophils through the formation of complexes with fibrinogen (16, 19). Nucleotide sequence analysis of the emm gene (2) provides important epidemiological information about GAS infections.
We conducted a retrospective study examining the demographic information, clinical characteristics, and outcomes of patients with SSTIs caused by GAS in order to compare the risk factors for groups of patients with invasive and noninvasive infections. To understand the epidemiological information and explore the molecular characteristics of the infecting strains, we performed emm typing and pulsed-field gel electrophoresis (PFGE) of all GAS isolates involved in SSTIs. In this work, we found that GAS with the emm106 gene, which has been rarely reported, and GAS with a unique SmaI PFGE pattern of the emm106 gene were particularly prone to cause invasive SSTIs.
(This study was presented, in part, at the 2011 Annual Conference of the Infectious Diseases Society of Taiwan, Taipei, Taiwan, 16 January 2011.)
MATERIALS AND METHODS
Study design.
This study was carried out at E-Da Hospital, a 1,000-bed university-affiliated hospital, which serves more than 1 million people in Kaohsiung in southern Taiwan. The study was approved by the Institutional Review Board of E-Da Hospital.
The clinical laboratory database of E-Da Hospital, Kaohsiung, Taiwan, was searched to identify patients who had positive group A Streptococcus (GAS) cultures between May 2005 and November 2007. These medical records were reviewed, and patients with skin and soft tissue infections (SSTIs) caused by GAS were enrolled in the study. Demographic information, underlying illnesses, clinical conditions, and outcomes were collected from the medical records. The purified bacterial isolates used in this study had been collected from patients and stored as glycerol stocks at −80°C. Streptococcus pyogenes ATCC 12344 was used as a control strain.
Case definition.
An SSTI was defined as an infection that was associated with inflammation of the skin or soft tissue but that excluded cervical adenitis, pharyngeal infection, and cutaneous inflammation overlying septic arthritis (27). An invasive SSTI was defined as an infection meeting the criteria of SSTI, with the addition of isolation of GAS from a normally sterile body site, such as blood samples, surgical specimens, intraoperative swabs, or aspirates of soft tissue or abscesses (14, 27). A noninvasive SSTI was defined as an infection meeting the criteria of SSTIs, with the addition of the isolation of GAS from a nonsterile site, such as a superficial wound, impetigo, or pyoderma (30). Necrotizing fasciitis was defined as described previously (20). A hospital-acquired infection was defined as an SSTI that occurred >48 h after hospitalization. Shock was defined as systolic pressure of <90 mm Hg, a reduction of 40 mm Hg in the systolic blood pressure from the baseline, or a condition requiring inotropic agents to maintain blood pressure during the hospital stay.
Identification of isolates and testing of samples for antimicrobial susceptibility.
All GAS isolates were identified by standard methods (26). Briefly, isolates were identified as GAS based on beta-hemolysis on sheep blood agar, Gram staining, a negative catalase test, a positive pyrrolidonyl arylamidase test, and agglutination with Lancefield group A antiserum. The MICs of antimicrobial agents were defined using the Phoenix automated microbiology system (BD Diagnostics, Sparks, MD). D-zone tests were performed if erythromycin-resistant, clindamycin-susceptible isolates were identified (9). Susceptibility testing was determined according to the Clinical and Laboratory Standards Institute criteria (9).
emm typing.
GAS emm DNA fragment preparation was conducted according to the protocol of the Centers for Disease Control and Prevention (CDC) (7a). As specified by the CDC protocol, primers 1 and 2 were used for amplifying the N-terminal region of the emm gene [primer 1 was 5′-TATT(C/G)GCTTAGAAAATTAA-3′, and primer 2 was 5′-GCAAGTTCTTCAGCTTGTTT-3′]. The DNA amplicons and primer 1 were sent to a biotechnology company (Genomics BioSci & Tech Corp., Taipei, Taiwan) for DNA sequencing. The 5′ emm sequences were submitted to the emm database at the CDC web site to determine the emm type (7b).
PFGE analysis.
All isolates were digested by SmaI and then sent for pulsed-field gel electrophoresis (PFGE), according to the previously described protocol (8). The digested fragments of DNA were separated by contour-clamped homogeneous electric field (CHEF) Mapper XA Chiller system (Bio-Rad). The genetic relationship between the bacterial strains was evaluated based on the levels of similarity among the SmaI PFGE patterns. A dendrogram was constructed using the unweighted-pair group method with arithmetic mean (UPGMA) algorithm by GelCompar II software (version 6; Applied Maths NV).
Data analysis.
The SPSS software package (Statistical Package for Social Sciences, version 14.0) was used to analyze the results. Categorical variables were analyzed using the chi-square test or Fisher exact tests, as appropriate. Continuous variables were analyzed for differences in mean values by the Student t test. The chi-square test for trend was employed to define the invasive ratio of SSTIs in different age groups. To identify the risk factors for invasive SSTIs compared with noninvasive SSTIs, underlying illnesses that could contribute to invasive SSTIs and were associated with a level of significance of <0.20 in univariate analyses were included in a logistic regression model for multivariate analysis. The Hosmer-Lemeshow goodness-of-fit test was used to assess the fitness of the model. The odds ratio (OR), 95% confidence interval (95% CI), and P value were calculated for each factor. All P values were 2-tailed, and a P value of <0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics.
During the study period, 75 isolates from 75 consecutive patients with skin and soft tissue infections (SSTIs) caused by group A Streptococcus (GAS) met the inclusion criteria of the study. After the GAS stocks were thawed, 2 isolates died, resulting in a total of 73 isolates/patients that were included in our study. There were 57 (78.1%) males and 16 (21.9%) females (Table 1). Diabetes mellitus (28.8%) was the most common underlying disease. Hospital-acquired infections were noted in 2 patients. Five patients (6.8%) died of GAS infection with a median age of 66 years (range, 34 to 75 years). Of these five patients, four were diagnosed as having necrotizing fasciitis, and three had diabetes mellitus.
Table 1.
Demographic and clinical characteristics of the patients with invasive and noninvasive skin and soft tissue infections caused by group A Streptococcus
| Characteristic | No. of patients (%) witha: |
Odds ratio (95% CI)b | P valueb | ||
|---|---|---|---|---|---|
| SSTI (n = 73) | Invasive SSTI (n = 34) | Noninvasive SSTI (n = 39) | |||
| Demographic characteristics | |||||
| Age (yr) (mean ± SD) | 45.6 ± 24 | 58.5 ± 14.6 | 34.3 ± 25 | <0.001 | |
| Male | 57 (78.1) | 26 (76.5) | 31 (79.5) | 0.84 (0.28–2.55) | 0.756 |
| Underlying illnesses | |||||
| Diabetes mellitus | 21 (28.8) | 15 (44.1) | 6 (15.4) | 4.34 (1.44–13.07)c | 0.007c |
| Hypertension | 16 (21.9) | 12 (35.3) | 4 (10.3) | 4.77 (1.37–16.67)d | 0.01d |
| Congestive heart failure | 4 (5.5) | 3 (8.8) | 1 (2.6) | 3.68 (0.36–37.14) | 0.333 |
| Malignancy | 4 (5.5) | 3 (8.8) | 1 (2.6) | 3.68 (0.36–37.14) | 0.333 |
| Liver cirrhosis | 2 (2.7) | 1 (2.9) | 1 (2.6) | 1.15 (0.07–19.14) | 0.999 |
| Alcoholism | 18 (24.7) | 8 (23.5) | 10 (25.6) | 0.89 (0.31–2.6) | 0.835 |
| Clinical diagnoses | |||||
| Cellulitis/wound infection | 47 (64.4) | 12 (35.3) | 35 (89.7) | 0.06 (0.02–0.22) | <0.001 |
| Necrotizing fasciitis | 19 (26) | 19 (55.9) | 0 | <0.001 | |
| Deep abscess | 2 (2.7) | 2 (5.9) | 0 | 0.213 | |
| Furuncle/carbuncle/pustule | 4 (5.5) | 0 | 4 (10.3) | 0.118 | |
| Deep neck infection | 1 (1.4) | 1 (2.9) | 0 | 0.466 | |
| Clinical manifestations | |||||
| Shock | 10 (13.7) | 10 (29.4) | 0 | <0.001 | |
| Bacteremia | 12 (16.4) | 12 (35.3) | 0 | <0.001 | |
| Hospital-acquired infection | 2 (2.7) | 2 (5.9) | 0 | 0.213 | |
| Intensive care unit admission | 12 (16.4) | 11 (32.4) | 1 (2.6) | 18.17 (2.2–150.14) | 0.001 |
| Hospitalization | 55 (75.3) | 34 (100) | 21 (53.8) | 2.62 (1.87–3.67) | <0.001 |
| Surgical intervention | 38 (52.1) | 29 (85.3) | 9 (23.1) | 19.33 (5.79–64.61) | <0.001 |
| Mortality | 5 (6.8) | 5 (14.7) | 0 | 0.019 | |
The number of patients with skin and soft tissue infections (SSTIs) is given unless specified otherwise (i.e., age).
The odds ratio (95% confidence interval [95% CI]) and P value for patients with invasive and noninvasive SSTIs are shown.
OR = 3.32 (95% CI, 1.05 to 10.51) and P = 0.042 by multivariate logistic regression analysis.
OR = 3.47 (95% CI, 0.93 to 12.87) and P = 0.063 by multivariate logistic regression analysis.
Characteristics distinguishing invasive infections from noninvasive infections.
Patients with invasive SSTIs were older than patients with noninvasive SSTIs (P < 0.001) (Table 1). The elderly were predisposed to invasive SSTIs by the trend analysis (P < 0.001) (Fig. 1). Patients with diabetes mellitus and hypertension were significantly more likely to have invasive SSTIs. Multivariate logistic regression analysis showed that diabetes mellitus was an independent risk factor for invasive SSTIs (odds ratio [OR] = 3.32 [95% confidence interval {95% CI}, 1.05 to 10.51]; P = 0.042). Bacteremia and mortality occurred more frequently in patients with invasive SSTIs (P < 0.001 and P = 0.019, respectively). The incidence of invasive infections was significantly associated with neutrophil percentages of ≥80, creatinine levels of ≥2 mg/dl, and high serum C-reactive protein levels (Table 2).
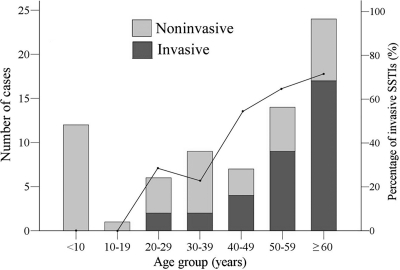
Fig. 1.
Patients with group A Streptococcus skin and soft tissue infections (SSTIs) (bars) and the percentage of invasive SSTIs in different age groups (line). There were significant differences in the percentage of invasive SSTIs with the age of the patients, as measured by the chi-square test for trend (chi-square, 20.355; P < 0.001).
Table 2.
Laboratory findings for patients with invasive and noninvasive skin and soft tissue infections caused by group A Streptococcus
| Laboratory findings | No. of patients (%) witha: |
Odds ratio (95% CI)b | P valueb | |
|---|---|---|---|---|
| Invasive SSTI | Noninvasive SSTI | |||
| White blood cell count ≥ 11,000 cells/mm3 | 27/34 (79.4) | 16/25 (64) | 2.17 (0.68–6.96) | 0.188 |
| Neutrophil percentage ≥ 80 | 25/31 (80.6) | 12/25 (48) | 4.51 (1.38–14.8) | 0.01 |
| Band percentage ≥ 5 | 5/31 (16.1) | 0/25 (0) | 0.058 | |
| Hemoglobin level ≤ 10 g/dl | 7/34 (20.6) | 1/25 (4) | 6.22 (0.71–54.29) | 0.122 |
| Platelet count ≤ 100,000 cells/mm3 | 5/34 (14.7) | 2/25 (8) | 1.98 (0.35–11.17) | 0.687 |
| Blood urea nitrogen level ≥ 40 mg/dl | 8/25 (32) | 1/13 (7.7) | 5.65 (0.62–51.29) | 0.126 |
| Creatinine level ≥ 2 mg/dl | 12/32 (37.5) | 2/19 (10.5) | 5.1 (1–26.05) | 0.037 |
| Aspartate aminotransferase level ≥ 80 U/liter | 5/27 (18.5) | 1/16 (6.3) | 3.41 (0.36–32.19) | 0.386 |
| Alanine aminotransferase level ≥ 80 U/liter | 4/26 (15.4) | 2/12 (16.7) | 0.91 (0.14–5.81) | 0.999 |
| Glucose level ≥ 200 mg/dl | 9/27 (33.3) | 3/13 (23.1) | 1.67 (0.37–7.61) | 0.716 |
| Creatinine phosphokinase level ≥ 600 U/liter | 2/9 (22.2) | 1/2 (50) | 0.29 (0.01–6.91) | 0.491 |
| C-reactive protein level (mg/liter) (mean ± SD)c | 205.4 ± 161.6 | 77.5 ± 79.3 | 0.002 | |
The number of patients with a positive result for the laboratory finding/total number of patients in each group (percentage) is shown unless specified otherwise (i.e., C-reactive protein).
The odds ratio (95% confidence interval [95% CI]) and P value for patients with invasive and noninvasive SSTIs are shown.
The mean C-reactive protein level ± standard deviation (SD) for 39 patients is shown.
Testing for antimicrobial susceptibility.
None of the isolates exhibited resistance to penicillin, cefepime, cefotaxime, chloramphenicol, levofloxacin, meropenem, linezolid, or vancomycin. Of 73 isolates, 5 (6.8%) and 2 (2.7%) demonstrated resistance to erythromycin and clindamycin, respectively. Three isolates were resistant to erythromycin but susceptible to clindamycin. No inducible clindamycin resistance was detected by the D-zone test in these 3 isolates. Forty-six (63%) isolates were resistant to tetracycline. Four of 5 erythromycin-resistant isolates were identified from patients with invasive SSTIs (P = 0.177), and 23 (50%) of 46 tetracycline-resistant isolates were found from patients with invasive SSTIs (P = 0.444).
emm types.
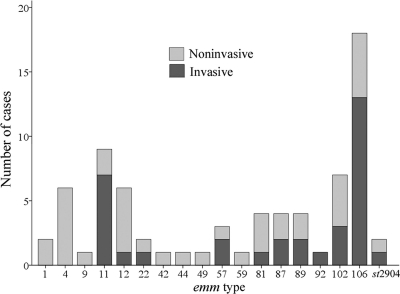
All the isolates were typeable for emm genes, and 18 different emm types were identified (Fig. 2). The five most prevalent emm types, accounting for 63.0% of the collection, were emm106 (24.7%), emm11 (12.3%), emm102 (9.6%), emm4 (8.2%), and emm12 (8.2%). Among the 34 isolates of invasive GAS, the emm106 type accounted for 38.2% and the emm11 type accounted for 20.6%. The emm106 type was more significantly associated with invasive SSTIs than the other emm types (OR = 4.21 [95% CI, 1.31 to 13.51]; P = 0.012). Older patients (P = 0.037) and those who had malignancy (OR = 10.8 [95% CI, 1.05 to 111.49]; P = 0.044) were predisposed to suffer from infections caused by bacteria with the emm106 gene. The antimicrobial susceptibility profiles did not differ between patients infected with GAS with the emm106 gene and GAS with genes other than emm106. The theoretical coverage of invasive isolates by the experimental 26-valent M-protein-based GAS vaccine was 41.2% (24). The 5 patients who died were infected with bacteria with the following genes: emm106 (2 patients), emm11 (1 patient), emm57 (1 patient), and emm102 (1 patient).
Fig. 2.
Distribution of group A Streptococcus emm types in invasive and noninvasive skin and soft tissue infections.
PFGE pattern.
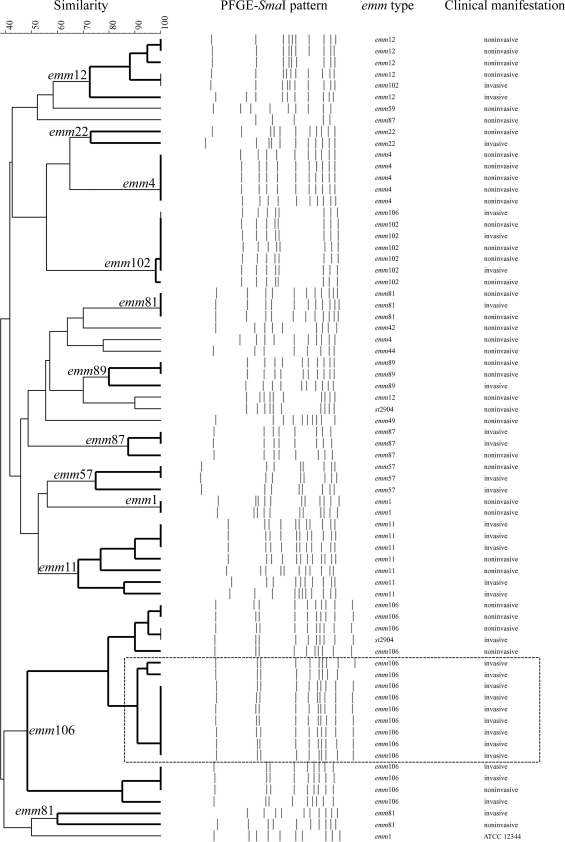
There were 4 isolates with DNA resistance to SmaI digestion, including 1 isolate with the emm9 gene, 2 isolates with the emm11 gene, and 1 isolate with emm92. Figure 3 shows the dendrogram of SmaI PFGE patterns. Strikingly, a cluster of invasive isolates with the emm106 gene with a similarity of 85% formed a unique SmaI PFGE pattern (P < 0.001). However, epidemiologic links, such as the type of transmission, the location where patients lived, or the time of presentation, did not exist between these cases with emm106 infections.
Fig. 3.
Dendrogram showing pulsed-field gel electrophoresis (PFGE) of group A Streptococcus isolates digested by the SmaI restriction enzyme and the corresponding emm types and clinical manifestations. The clustering analysis was constructed using the unweighted pair group method with arithmetic mean (UPGMA) algorithm. A cluster of invasive GAS isolates with the emm106 gene that exhibited a similarity of 85% are shown boxed (in the rectangle outlined by a broken line). Streptococcus pyogenes ATCC 12344 was used as a control strain.
DISCUSSION
In this work, we found that age is an important factor for the presence of invasive skin and soft tissue infections (SSTIs) caused by group A Streptococcus (GAS). This finding was similar to a finding of a previous report from Canada (27), which showed that the incidence of invasive soft tissue infection was highest among elderly patients. Several nationwide studies have demonstrated that the rates of invasive GAS infections were higher in males than in females (18, 22, 25). Although there was no statistical difference in gender in the groups of patients with invasive and noninvasive infections, our study showed a predominance of males with SSTIs caused by GAS.
Patients with noninvasive SSTIs usually appear stable, but invasive SSTIs can be life-threatening. It is important, therefore, to recognize patients with invasive SSTIs early to provide timely treatment. Unfortunately, determining the severity of SSTIs may be difficult. Some laboratory data can be helpful in the early recognition of invasive SSTIs caused by GAS, and the practice guideline of SSTIs suggests that surgical intervention is necessary if patients present with an elevated creatinine level, low serum bicarbonate level, elevated creatinine phosphokinase level, marked left shift, or a C-reactive protein level of >13 mg/liter (29). Our study also supports the relevance of these biomarkers.
Although penicillin is the drug of choice for treating GAS infections, macrolides, lincosamides, and tetracycline are the first-line substitutes for patients with an allergy to β-lactams. However, increasing rates of GAS resistance to these alternatives have been reported (1, 15, 18). Compared to previous studies (1, 15, 18), our study found similar rates of resistance to erythromycin and clindamycin but a much higher rate of resistance to tetracycline. The extensive use of tetracycline in the treatment of a variety of human and veterinary infections may have contributed to the dissemination of tetracycline-resistant GAS (23). Based on the data in our report, the high incidence of macrolide- and tetracycline-resistant GAS makes the choice of these agents as empirical therapy for SSTIs caused by GAS a matter of concern, especially in invasive cases. Susceptibility testing may be warranted preceding the administration of such agents for suspicious GAS infections.
Bacterial traits could influence the clinical syndromes and geographic distribution of GAS infection. The distribution of emm types of GAS differs among different global regions (10, 27). In a meta-analysis of GAS isolates worldwide (28), the most common type was emm1 (18.3%), followed by emm12 (11.1%), emm28 (8.5%), emm3 (6.9%), and emm4 (6.9%). Our study found that GAS with the emm106 gene played an important role in the invasive SSTIs. This emm type has only rarely been mentioned in the global epidemiologic surveys (28). As shown by the data from the Streptococcus Laboratory of the CDC (7), the emm106 type was noted only for pharyngeal diseases in Africa, where this type ranked 21 of the 25 most common emm types. The emm genes can be categorized into 5 subfamilies (A to E) according to chromosomal analysis, and bacteria with different emm genes infect different tissues (3, 17). The opsonization and fibrinogen-binding capabilities of M proteins vary among different emm types of GAS (11, 21). As GAS emm106 type is infrequently recognized, the exact mechanism by which this serotype leads to invasive SSTIs has not been delineated. Our study showed a unique cluster of SmaI PFGE patterns in the isolates with the emm106 gene. This SmaI PFGE pattern appeared to be associated with special epidemic virulence for invasive SSTIs. However, the exact role of this unique invasive cluster is not yet clear.
In conclusion, SSTIs caused by GAS are commonly encountered in daily practice. It is important to understand the epidemiology, clinical manifestations, microbiologic characteristics, and prognosis of this disease. Our study revealed the risk factors, clinical manifestations, and molecular characteristics of invasive SSTIs caused by GAS. Specifically, for the first time, GAS with the emm106 gene and the distinctive SmaI PFGE pattern of emm106 were shown to play an important role in invasive SSTIs. It is essential that further studies should be conducted to understand the significance and exact pathogenesis of GAS with the emm106 gene in invasive SSTIs.
ACKNOWLEDGMENTS
This study was supported by E-Da Hospital (EDAH-99014 and EDAHI-98001).
We declare that we have no conflicts of interest in relation to this work.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Ayer V., et al. 2007. Tetracycline resistance in group A streptococci: emergence on a global scale and influence on multiple-drug resistance. Antimicrob. Agents Chemother. 51:1865–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beall B., Facklam R., Thompson T. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bessen D. E., Sotir C. M., Readdy T. L., Hollingshead S. K. 1996. Genetic correlates of throat and skin isolates of group A streptococci. J. Infect. Dis. 173:896–900 [DOI] [PubMed] [Google Scholar]
- 4. Bisno A. L. 1979. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect. Immun. 26:1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bisno A. L., Brito M. O., Collins C. M. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3:191–200 [DOI] [PubMed] [Google Scholar]
- 6. Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694 [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2009. Streptococcus library: emm types as proportions of total disease isolates in six global regions. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ncidod/biotech/strep/emmtype_proportions.htm [Google Scholar]
- 7a. Centers for Disease Control and Prevention 2010. Streptococcus laboratory protocols. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ncidod/biotech/strep/protocols.htm [Google Scholar]
- 7b. Centers for Disease Control and Prevention 2011. CDC Streptococcus laboratory. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm [Google Scholar]
- 8. Chiou C.-S., et al. 2004. Epidemiology and molecular characterization of Streptococcus pyogenes recovered from scarlet fever patients in central Taiwan from 1996 to 1999. J. Clin. Microbiol. 42:3998–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing, 17th informational supplement, document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Cunningham M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dale J. B., Washburn R. G., Marques M. B., Wessels M. R. 1996. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect. Immun. 64:1495–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiNubile M. J., Lipsky B. A. 2004. Complicated infections of skin and skin structures: when the infection is more than skin deep. J. Antimicrob. Chemother. 53(Suppl. 2):ii37–ii50 [DOI] [PubMed] [Google Scholar]
- 13. Dong S. L., Kelly K. D., Oland R. C., Holroyd B. R., Rowe B. H. 2001. ED management of cellulitis: a review of five urban centers. Am. J. Emerg. Med. 19:535–540 [DOI] [PubMed] [Google Scholar]
- 14. Factor S. H., et al. 2003. Invasive group A streptococcal disease: risk factors for adults. Emerg. Infect. Dis. 9:970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green M. D., et al. 2006. Multicentre surveillance of the prevalence and molecular epidemiology of macrolide resistance among pharyngeal isolates of group A streptococci in the USA. J. Antimicrob. Chemother. 57:1240–1243 [DOI] [PubMed] [Google Scholar]
- 16. Herwald H., et al. 2004. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 116:367–379 [DOI] [PubMed] [Google Scholar]
- 17. Hollingshead S. K., Arnold J., Readdy T. L., Bessen D. E. 1994. Molecular evolution of a multigene family in group A streptococci. Mol. Biol. Evol. 11:208–219 [DOI] [PubMed] [Google Scholar]
- 18. Imöhl M., Reinert R. R., Ocklenburg C., van der Linden M. 2010. Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003-2007. FEMS Immunol. Med. Microbiol. 58:389–396 [DOI] [PubMed] [Google Scholar]
- 19. Kahn F., et al. 2008. Antibodies against a surface protein of Streptococcus pyogenes promote a pathological inflammatory response. PLoS Pathog. 4:e1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaul R., McGeer A., Low D. E., Green K., Schwartz B. 1997. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am. J. Med. 103:18–24 [DOI] [PubMed] [Google Scholar]
- 21. Kotb M., et al. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 8:1398–1404 [DOI] [PubMed] [Google Scholar]
- 22. Lamagni T. L., et al. 2008. Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 46:2359–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma Y. P., Chang S. K., Chou C. C. 2006. Characterization of bacterial susceptibility isolates in sixteen dairy farms in Taiwan. J. Dairy Sci. 89:4573–4582 [DOI] [PubMed] [Google Scholar]
- 24. McNeil S. A., et al. 2005. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114–1122 [DOI] [PubMed] [Google Scholar]
- 25. O'Loughlin R. E., et al. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853–862 [DOI] [PubMed] [Google Scholar]
- 26. Ruoff K. L., Whiley R. A., Beighton D. 2003. Streptococcus, p. 405–421 In Murray P. R., Baron E. J., Pfaller M. A., Tenover F. C., Yolken R. H. (ed.), Manual of clinical microbiology, 8th ed ASM Press, Washington, DC [Google Scholar]
- 27. Sharkawy A., et al. 2002. Severe group A streptococcal soft-tissue infections in Ontario: 1992-1996. Clin. Infect. Dis. 34:454–460 [DOI] [PubMed] [Google Scholar]
- 28. Steer A. C., Law I., Matatolu L., Beall B. W., Carapetis J. R. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect. Dis. 9:611–616 [DOI] [PubMed] [Google Scholar]
- 29. Stevens D. L., et al. 2005. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 41:1373–1406 [DOI] [PubMed] [Google Scholar]
- 30. Su Y.-F., et al. 2009. Changing epidemiology of Streptococcus pyogenes emm types and associated invasive and noninvasive infections in southern Taiwan. J. Clin. Microbiol. 47:2658–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]