Abstract
Human respiratory syncytial virus (HRSV) is the leading cause of hospitalization of children aged <5 years due to respiratory illness in industrialized countries, and pneumonia is the leading cause of mortality among children aged <5 years worldwide. Although HRSV was first identified in 1956, a preventative vaccine has yet to be developed. Here we report the results of the first study to investigate the circulation and genetic diversity of HRSV in Cambodia among an all-ages population over 5 consecutive years. The incidences of HRSV infection among all-ages outpatient and hospitalized populations were equivalent, at 9.5% and 8.2%, respectively. Infection was most prevalent among children aged <5 years, with bronchiolitis being the most frequently observed clinical syndrome in the same age group. Circulation of HRSV was seasonal, typically coinciding with the rainy season between July and November annually. Strains belonging to HRSV groups A and B were detected with equivalent frequencies; however, we observed a potentially biennial shift in the predominant circulating HRSV genotype. The majority of HRSV group B strains belonged to the recently described BA genotype, with the exception of 10 strains classified as belonging to a novel HRSV group B genotype, SAB4, first reported here.
INTRODUCTION
First identified in 1956, human respiratory syncytial virus (HRSV) is a member of the Pneumovirus subfamily of the family Paramyxoviridae (25). Infection with HRSV can result in mild to severe illness, including lower respiratory syndromes such as bronchiolitis and pneumonia. HRSV is the leading cause of hospitalization of children aged <5 years due to respiratory illness in industrialized countries, and pneumonia is the leading cause of mortality among children aged <5 years worldwide (13, 17, 30). HRSV has a negative-sense, nonsegmented single-stranded RNA genome that carries 10 distinct mRNAs, each of which codes for a single protein, as follows: nonstructural protein 1 (NS1); nonstructural protein 2 (NS2); three proteins associated with nucleocapsid, N, P, and L; two matrix proteins, M and M2; small hydrophobic protein (SH); attachment glycoprotein (G); and fusion protein (F) (42).
Expressed on the virion surface, the F and G proteins are the most immunogenic of the HRSV proteins, capable of eliciting production of neutralizing antibodies (26, 27, 29). The G protein, a type II glycoprotein, is the most variable region both within and between the major HRSV genotypes. The ectodomain is the least conserved region within the G protein, containing 2 hypervariable regions (HVR). The second HVR (HVR2) carries the C terminus of the protein and is commonly sequenced to investigate genetic diversity of HRSV strains within a given population (14, 34, 37, 49).
Although functioning as an attachment glycoprotein, studies have shown that strains lacking the G protein retain the ability to replicate in vitro (20, 45). Hence, antigenic variation within the HRSV G protein is thought to occur partially because structural or functional constraints do not restrict amino acid substitutions within this region (19). This high level of genetic variation has resulted in sufficient antigenic heterogeneity to enable the virus to establish repeat infections within the same host (16, 19).
On the basis of antigenic and genetic variation within the G protein, HRSV isolates are classified into 2 distinct groups, A and B (10, 28, 35, 36). Ten HRSV A genotypes, GA1 to GA7, SAA1, NA1, and NA2, have been identified (35, 36, 41, 49). Up to 19 genotypes have been proposed for the classification of HRSV B strains, as follows: GB1 to GB4, BA1 to BA10, URU1, URU2, and SAB1 to SAB3 (4, 11, 35, 36, 48, 49). As there is currently no HRSV vaccine available, ongoing global surveillance and characterization of circulating HRSV strains are required to aid vaccine development. Here we report the findings of the first study to investigate the genetic diversity of HRSV circulating within Cambodia. Diversity within the HRSV G protein was investigated in a study conducted using strains collected over 5 consecutive years.
MATERIALS AND METHODS
Sample collection and screening.
Samples tested in this study were collected under the guidelines of the Surveillance and Investigation of Epidemic Situations in Southeast Asia (SISEA) project, the H5N1 national surveillance program, and the influenza-like illness (ILI) national surveillance program. Under the guidelines of the ILI and H5N1 surveillance programs, between June 2005 and December 2009, a total of 5,056 nasopharyngeal specimens were collected nationwide from patients hospitalized with suspicion of H5N1 virus infection and from outpatients presenting with ILI symptoms at 5 sentinel sites (Takeo, Kampong Cham, and Battambang provincial hospitals, Angkor Hospital for Children in Siem Reap, and the National Pediatric Hospital in Phnom Penh) and included in this study (http://www.canbypublications.com/maps/simpleprov.htm) (22). The case definition used for inclusion of patients with ILI symptoms was according to WHO guidelines: sudden onset of fever (≥38°C, armpit), cough, sore throat, and the absence of any other diagnosis (6). Each ILI surveillance sentinel site was required to randomly collect nasopharyngeal swabs from between 5 and 10 patients per week. Swabs were collected in 1 ml of viral transport medium and stored at −80°C prior to testing.
Under the guidelines of the SISEA project, between April 2007 and December 2009, a total of 2,773 samples were collected from patients admitted with symptoms of acute lower respiratory tract illness (ALRI) to Takeo (southern Cambodia) and Kampong Cham (north central Cambodia) provincial hospitals (see map URL given above). For patients aged <5 years, ALRI was defined as an illness <10 days in duration that consisted of cough or difficult breathing, plus tachypnea (respiratory rate of ≥50 breaths/min in infants aged <1 year and ≥40 breaths/min in children aged 1 to 4 years). For patients aged between 5 and 14 years, the case definition included the above-described symptoms as well as a fever of >38°C, and the tachypnea was defined by a respiratory rate of ≥30 breaths/min. For patients 15 years or older ALRI was defined as a fever of >38°C plus chest pain, tachypnea (respiratory rate, ≥30 breaths/min), or auscultatory crackles. The age-specific criteria for diagnosis of a severe case were defined previously (2). Nasopharyngeal swabs were collected from each patient. Samples were kept in liquid nitrogen following collection and during transport to the Institut Pasteur in Cambodia (IPC), where samples were stored at −80°C. Additional clinical specimens and data, including sputum samples and chest X-rays, were collected from patients when possible.
Samples collected from patients with ALRI symptoms (SISEA project) were screened for the presence of 18 common and novel viral respiratory pathogens, including HRSV, human metapneumovirus (HMPV), human bocavirus (HBoV), influenza A and B viruses, human coronaviruses OC43, 229E, HKU1, and NL63, severe acute respiratory syndrome-associated coronavirus (SARS-CoV), parainfluenza viruses 1 to 4, adenoviruses, human rhinovirus, and enterovirus, by multiplex reverse transcriptase PCR (RT-PCR)/PCR, as previously reported (2, 6). Specimens obtained from patients included in the ILI national surveillance program or patients who were suspected of infection with H5N1 virus were tested using a multiplex RT-PCR for the simultaneous detection of influenza A, influenza B, HMPV, and HRSV. All samples that tested positive for HRSV were included in this study.
The SISEA project was approved by the National Ethics Committee of Cambodia. All patients/parents of sick children who participated provided written informed consent. Samples collected from patients suspected of H5N1 infection, or with ILI symptoms, were tested using a multiplex RT-PCR for influenza in addition to HRSV within the framework of the national influenza surveillance program of Cambodia. Influenza surveillance was conducted by the Cambodian National Influenza Centre, which was established by the Cambodian Ministry of Health and is a joint collaboration between the Communicable Disease Control Department of Cambodia (Ministry of Health) and the Virology Unit at IPC.
RT-PCR and sequencing.
Viral RNA was extracted from nasopharyngeal specimens that tested positive for HRSV following the initial screening, using the Qiagen viral RNA minikit (Qiagen, CA), as per the manufacturer's instructions. RNA was eluted in 60 μl of Qiagen AVE buffer and stored at −80°C until required.
Seminested RT-PCR/PCR was performed to amplify a region within the C-terminal second hypervariable region (HVR2) of the HRSV G protein for both genotype A and B strains. RT-PCR was performed using the Qiagen One-Step RT-PCR kit (Qiagen, CA), as per the manufacturer's instructions, and the following published primers: ABG490, 5′-ATGATTWYCAYTTTGAAGTGTTC-3′, and F164, 5′-GTTATGACACTGGTATACCAACC-3′ (34). The forward primer ABG490 corresponds to bases 497 to 519 of the G gene of the HRSV A prototype strain A2 (GenBank accession number M11486) and bases 491 to 513 of the HRSV B prototype strain CH18537 (GenBank accession number M17213). The reverse primer F164 corresponds to bases 164 to 186 of the F gene of strain CH18537 and to the F gene of strain A2, with one mismatch (the mismatch is underlined) (43). Both primers have been used previously to successfully amplify HRSV A and B G genes (34, 43). Thermocycling was performed under the following conditions: reverse transcription at 50°C for 30 min, PCR activation at 95°C for 15 min, 40 cycles of denaturation at 94°C for 40 s, annealing at 50°C for 45 s, and extension at 72°C for 45 s, followed by a final extension at 72°C for 10 min.
A seminested, HRSV genotype-specific PCR was then performed using primers AG655, 5′-GATCYCAAACCTCAAACCAC-3′, and F164, 5′-GTTATGACACTGGTATACCAACC-3′ (HRSV A), and primers BG517, 5′-TTYGTTCCCTGTAGTATATGTG-3′, and F164, 5′-GTTATGACACTGGTATACCAACC-3′ (HRSV B) (34). The AG655 forward primer corresponds to bases 655 to 674 of the HRSV A prototype strain A2, and the BG517 forward primer corresponds to bases 517 to 538 of the HRSV B prototype CH18537 strain (34). Thermocycling was performed under the following conditions: PCR activation at 94°C for 2 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. All PCR products were visualized using ethidium bromide under UV light on a 1.5% agarose gel. When required, PCR products were purified using the QIAquick gel extraction protocol of the QIAquick PCR purification kit (Qiagen, CA), as per the manufacturer's instructions, prior to sequence analysis. Single-pass sequencing reactions were performed at a contract sequencing facility using the ABI BigDye Terminator cycle sequencing kit on an ABI 3730XL automatic DNA analyzer (Macrogen, Seoul, South Korea).
Phylogenetic analysis.
Consensus sequences were generated using CLC Main Workbench software, version 5.6.1 (CLC bio). Nucleotide sequences of reference strains from established HRSV A and B genotypes were obtained from GenBank and used to construct alignments and phylogeny (see Tables S1 and S2 in the supplemental material). Cambodian and reference HRSV nucleotide sequences were aligned using the ClustalW alignment program of MEGA software, version 4.0 (44). The average pairwise Jukes-Cantor distance was found to be 0.06 and 0.07 for the HRSV A and B alignments, respectively, indicating that the data were suitable to generate neighbor-joining trees (32). Neighbor-joining trees were constructed using the p-distance nucleotide substitution model with 1,000 bootstrap replicates, using MEGA 4.0 software. An HRSV group A strain (GenBank accession number AF233911) was used to root the HRSV B phylogenetic tree, and a HRSV group B strain (GenBank accession number M555633) was used to root the HRSV A phylogenetic tree. Pairwise nucleotide distances (p-distances), the proportion of sites at which nucleotide sequences differ divided by the total number of nucleotides compared, were calculated using the pairwise distance function of MEGA software. Pairwise amino acid distances (p-distances), the proportion of sites at which amino acid sequences differ divided by the total number of residues compared, were calculated using the Poisson model in the pairwise distance function of MEGA software.
Deduced amino acid sequences and N- and O-glycosylation site analysis.
Deduced amino acid sequences of the C-terminal second HVR of the HRSV G gene were generated by translating nucleotide sequences with the standard genetic code using MEGA software. Potential N-glycosylation sites were predicted by the NXT motif, where X is not a proline (8, 34, 37, 38, 41). Potential O-glycosylation sites were predicted by KPXn and TTKXn motifs (in which X is any amino acid) (8, 34, 37, 38, 41).
Nucleotide sequence accession numbers.
The nucleotide sequences of HRSV strains obtained in this study were deposited in GenBank under accession numbers JN119875 to JN120019.
RESULTS
HRSV distribution and seasonality.
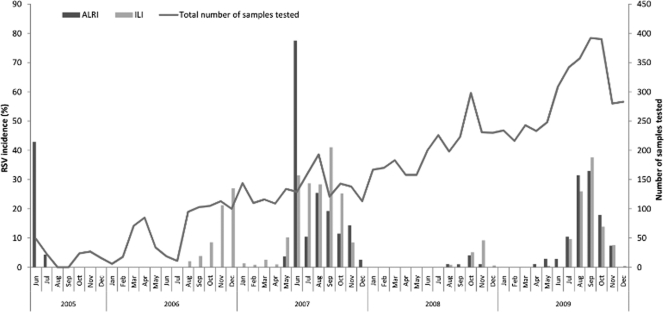
Of the combined 7,829 samples collected under the guidelines of the SISEA, ILI, and H5N1 programs between June 2005 and December 2009, 739 (9.4%) tested positive for HRSV. To investigate the incidence of HRSV infection, data relating to the H5N1 surveillance samples were combined with the results from the SISEA surveillance study samples, as patients included in both studies required hospitalization for respiratory illness; however, only cases included in the SISEA project were fully clinically documented. The combined H5N1 surveillance and SISEA study data are represented in Fig. 1 as ALRI. The yearly incidences of HRSV among the ILI and the ALRI study populations were compared and found to vary annually within both groups (Fig. 1). Among patients with ALRI symptoms, the annual incidence of HRSV infection was 15.8% in 2005 (June to December only), 0% (out of the 315 samples tested) in 2006, 20% in 2007, 0.7% in 2008, and 12% in 2009. Similar variation with respect to HRSV incidence was observed within the ILI study, as follows: 13% in 2006 (August to December only), 14.5% in 2007, 1.7% in 2008, and 9% in 2009 (Fig. 1). The annual incidences of HRSV infection were comparable between the ALRI and ILI groups, with the exception of 2005 and 2006, as ILI surveillance started only in August 2006.
Fig. 1.
Seasonal distribution of HRSV-positive samples. Data represent the incidence of HRSV infection among the ALRI and ILI study populations between 2005 and 2009. The total number of samples tested during the coincident time period is also shown.
The annual peak incidence of HRSV cases occurred during the rainy season, typically between May/June and October/November. However, timing of the onset of the HRSV season and timing of the annual peak incidence of infection varied annually. In 2006 and 2008, HRSV infections were detected from August to December, with the peak incidence occurring in December and November, respectively (Fig. 1). In 2007 and 2009, HRSV cases were detected primarily from May to December, with the peak incidence occurring during June and September, respectively; however, HRSV cases occurred sporadically year-round in 2007 (Fig. 1). As HRSV detection among respiratory specimens began only in June of 2005, it is not possible to comment on HRSV seasonality during 2005. Annual fluctuations in the number of HRSV-positive cases detected did not occur as a result of similarly high or low numbers of ALRI or ILI patient samples collected during coincident periods (Fig. 1). Instead, these data suggest a trend of alternating seasons with “low” and “high” levels of HRSV circulation in Cambodia for the duration of the study period.
Patient characteristics.
Of the 739 HRSV-positive patients identified, detailed information regarding coinfections and clinical symptoms was available from 245 patients, all of whom were enrolled in the SISEA project. Considering the small number of cases and the absence of clinical descriptions for patients recruited by the H5N1 study, these data were not included in the analysis presented in Table 1. Demographic data only were available from the 472 HRSV-positive ILI patients.
Table 1.
Incidence of HRSV infections according to age and clinical diagnosisa
| Patient age | Incidence (no. [%]) |
|||||
|---|---|---|---|---|---|---|
| HRSV cases |
Clinical diagnosis of ALRI patients |
|||||
| ILI | ALRI | Bronchiolitis | Bronchitis | Pneumonia/ bronchopneumonia | Other respiratory symptoms | |
| 0–6 mo | 48 (10.8) | 71 (24.1) | 54 (76.0) | 1 (1.4) | 16 (22.6) | 0 |
| 7–12 mo | 79 (10.4) | 51 (20.1) | 35 (68.6) | 1 (2.0) | 14 (27.4) | 1 (2.0) |
| 13–23 mo | 68 (11.8) | 39 (20.3) | 23 (59.0) | 0 | 16 (41.0) | 0 |
| 2–5 yr | 151 (14.5) | 34 (14.5) | 9 (26.4) | 4 (11.8) | 18 (53.0) | 3 (8.8) |
| 6–10 yr | 40 (5.3) | 5 (6.0) | 0 | 1 (20.0) | 4 (80.0) | 0 |
| 11–20 yr | 22 (5.2) | 4 (4.4) | 0 | 2 (50.0) | 1 (25.0) | 1 (25.0) |
| 21–40 yr | 46 (8.0) | 8 (2.5) | 0 | 3 (37.5) | 5 (62.5) | 0 |
| 41–60 yr | 12 (3.9) | 18 (2.6) | 0 | 3 (16.6) | 3 (16.6) | 12 (67.0) |
| >60 yr | 6 (7.5) | 15 (2.4) | 0 | 3 (20.0) | 2 (13.0) | 10 (67.0) |
| Total no. of patients | 472 | 245 | 121 | 18 | 79 | 27 |
Shown is the incidence of HRSV infection among the ALRI and ILI cohorts according to age. Incidence was calculated relative to the total number of patients within each age group. The clinical diagnosis is shown only among patients experiencing ALRI symptoms.
The ALRI HRSV-positive individuals were between 8 weeks and 79 years of age (median age, 1 year). The HRSV-positive ILI patients were between 3 weeks and 67 years of age (median age, 2.1 years). The incidence of HRSV infection was highest among ALRI patients aged 0 to 2 years and among ILI patients aged 0 to 5 years; overall, HRSV incidence decreased with increasing age among both the ALRI and ILI groups (Table 1). The most common diagnosis among ALRI patients younger than 2 years of age was bronchiolitis; bronchiolitis was not observed in hospitalized patients older than 6 years of age. Bronchitis was associated mainly with HRSV infection in patients older than 2 years, especially in adolescents and young adults. Pneumonia or bronchopneumonia was observed among patients of all age groups (Table 1).
Overall, of the 245 HRSV-positive patients experiencing ALRI symptoms, 23 (9.4%) were coinfected with an additional virus (Table 2). Twelve (4.8%) of the patients were coinfected with human rhinovirus, 58% of which were classified as having severe illness. Coronavirus 229E, parainfluenza viruses 1 and 3, human bocavirus, adenovirus, and influenza virus B were also detected in coinfections (Table 2).
Table 2.
Viruses detected in addition to HRSV among specimens collected from Cambodian patients hospitalized with symptoms of ALRIa
| Virus | No. of cases | Overall (%) | No. of severe cases (%) |
|---|---|---|---|
| Human rhinovirus | 12 | 4.8 | 7 (58) |
| Parainfluenza virus type 3 | 3 | 1.2 | 2 (66) |
| Coronavirus 229E | 2 | 0.8 | 2 (100) |
| Adenovirus | 2 | 0.8 | 1 (50) |
| Influenza B virus | 2 | 0.8 | 1 (50) |
| Human bocavirus | 1 | 0.4 | 1 (100) |
| Parainfluenza virus type 1 and adenovirus | 1 | 0.4 | 0 |
| Total | 23 | 9.3 |
Shown are the number of cases detected, the incidence of each coinfection among the HRSV-positive ALRI cohort, and the percentage of coinfected patients classified as having severe illness.
Molecular epidemiology of HRSV. (i) Circulation and distribution of HRSV genotypes.
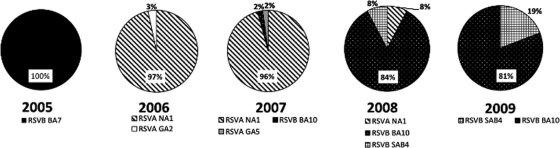
Monthly, dependent on the number available, up to 10 samples found to be positive for HRSV following multiplex RT-PCR testing were randomly selected for sequencing, regardless of clinical presentation. Partial sequences of the HRSV G gene C-terminal HVR2 were obtained from 140 HRSV-positive patient samples. Overall, 73 (52%) of the strains were classified as HRSV group A, and 67 (48%) of the strains belonged to HRSV group B (Fig. 2 and 3). Group B strains accounted for 100% of the cases in 2005, whereas group A strains accounted for 100% and 98% of the HRSV infections in 2006 and 2007, respectively. In 2008 and 2009, group B strains predominated, accounting for 92% and 100% of cases, respectively (Fig. 4).
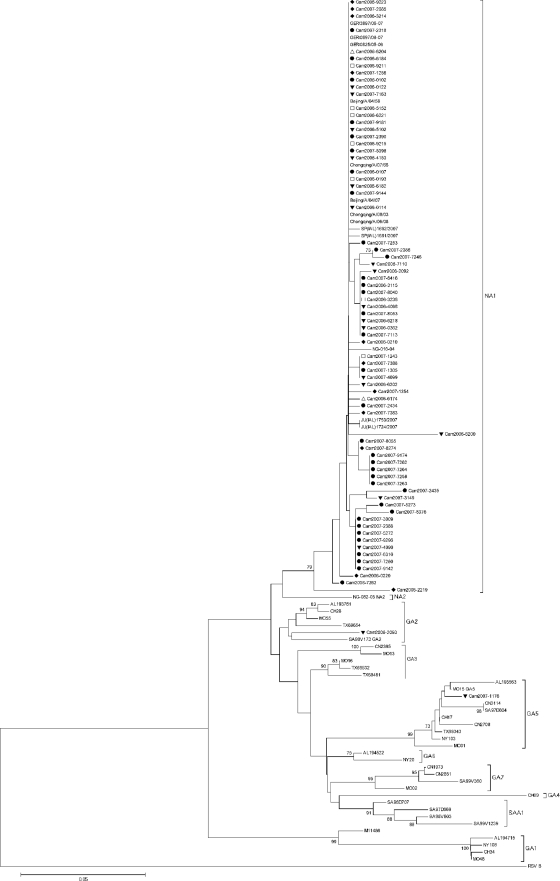
Fig. 2.
Phylogenetic analysis of the C-terminal second hypervariable region of the G gene of the Cambodian and reference HRSV group A isolates. The prototype HRSV group A strain A2 (GenBank accession number M11486) was included. An HRSV group B strain (GenBank accession number M55633) was used as an outgroup. Phylogeny was constructed using the neighbor-joining method with 1,000 bootstrap replicates. Only bootstrap values of >70% are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and were measured in the units of the number of base differences per site. Codon positions included were first, second, third, and noncoding. Cambodian strains are indicated by “Cam,” followed by the year of collection. Provinces in which samples were collected are indicated as follows: Kampong Cham, □; Kandal, ▵; Phnom Penh, ▾; Takeo, ●; and Battambang, ♦.
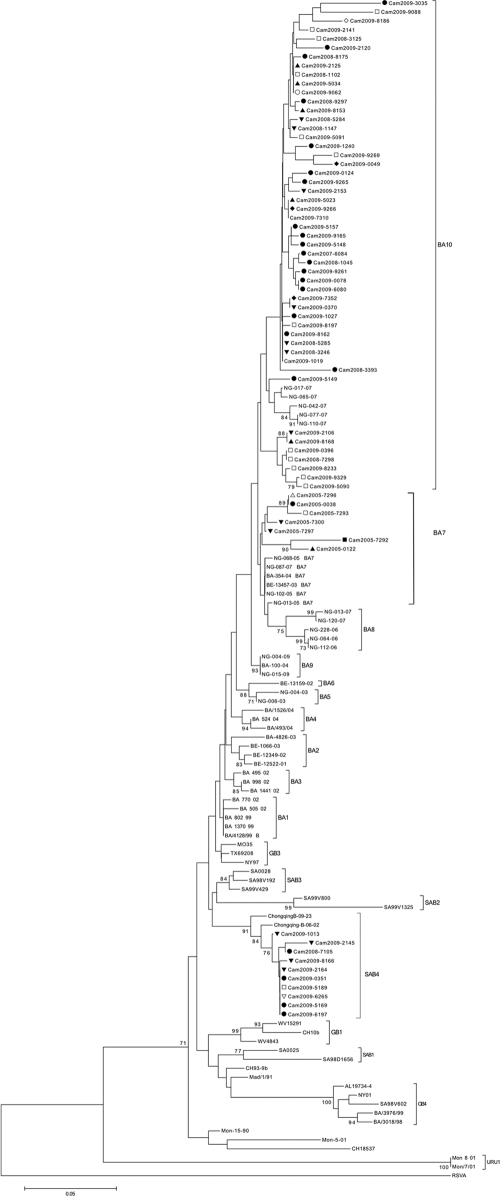
Fig. 3.
Phylogenetic analysis of the C-terminal second hypervariable region of the G gene of the Cambodian and reference HRSV group B isolates. An HRSV group A strain (GenBank accession number AF233900) was used as an outgroup. The prototype HRSV group B strain CH18537 (GenBank accession number M17213) and the prototype BA strain BA4128/99B (GenBank accession number AY333364) were also included. Phylogeny was constructed using the neighbor-joining method with 1,000 bootstrap replicates. Only bootstrap values of >70% are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and were measured in the units of the number of base differences per site. Codon positions included were first, second, third, and noncoding. Cambodian strains are indicated by “Cam,” followed by the year of collection. Provinces in which samples were collected are indicated as follows: Koh Kong, ○; Prey Veng, *; Kampong Speu, ■; Kampong Cham, □; Kandal, ▵; Siem Reap, ▴; Phnom Penh, ▾; Takeo, ●; Sihanoukville, ▿; Battambang, ♦; Banteay Meanchey, ★; and Kampot, ♢. Following our phylogenetic analysis, we reclassified strains ChongqingB-09-23 and ChongqingB-06-02 as belonging to genotype SAB4.
Fig. 4.
Circulation of HRSV A and B genotypes in Cambodia between 2005 and 2009. Relative annual percentages of each HRSV genotype detected are shown.
Multiple genotypes cocirculated within the same season, and the predominant circulating genotypes varied annually (Fig. 4). NA1 was the predominant circulating HRSV group A genotype, detected for 3 consecutive years, from 2006 to 2008. Overall, 97% of all HRSV A strains belonged to the NA1 genotype. HRSV A genotypes GA2 and GA5 were also detected, accounting for 3% and 2% of HRSV infections in 2006 and 2007, respectively. The HRSV B genotype BA10, detected in 3 consecutive seasons from 2007 to 2009, was the predominant circulating HRSV B genotype, accounting for 74.6% of all HRSV B strains. Strains belonging to the HRSV B BA7 genotype were identified only in 2005. Detected in 2008 and 2009, 10 strains were classified as belonging to a novel genotype first described here that we propose to name SAB4. With the exceptions of HRSV A genotype NA1 and HRSV B genotypes BA10 and SAB4, circulation of all additional HRSV genotypes was confined to a single season (Fig. 4).
(ii) Intra- and intergenotype diversity among HRSV A strains.
Seventy-three partial sequences of the HRSV A G gene C-terminal HVR2 were obtained. Of these, 71 (97.2%) belonged to genotype NA1, 1 (1.4%) to genotype GA2, and 1 (1.4%) to genotype GA5 (Fig. 2). When directly comparing all HRSV A strains, homology at the nucleotide level varied between 86.5% and 100%. Diversity at the amino acid level was much higher, with up to 23.6% divergence between strains. When comparing only genotype NA1 strains, divergence at the nucleotide level was 5.8%, and diversity at the amino acid level was up to 14.9%.
Analysis of HRSV group A amino acid sequences.
The predicted length of all Cambodian HRSV group A G proteins was between 297 to 298 amino acids (see Fig. S2 in the supplemental material). Several genotype-specific amino acid substitutions relative to the HRSV A reference strain A2 were observed (Fig. S2). The majority of HRSV A strains isolated in this study belonged to the recently described NA1 genotype (41). Four previously reported NA1 genotype-specific amino acid substitutions, Asp237 (100%), Leu274 (97.2%), Ser292 (97.2%), and a premature stop codon at position 298 (97.2%), were also observed here (41). A stop codon was present at position 299 in all of the Cambodian HRSV A isolates included in this study (Fig. S2).
The amino acid substitutions Thr269 and Ser289 were observed in this study and have been reported previously to be genotype GA2 specific (37). However, sequence analysis revealed that the Thr269 and Ser289 substitutions also occurred in Cambodian strains belonging to the NA1 subgenotype (see Fig. S2 in the supplemental material) (41). Potential GA2 genotype specific amino acid substitutions may also include Gln234 and Tyr297 (Fig. S2). In addition to the previously described GA5 genotype-specific amino acid substitutions Asn250, Ser251, Thr274, Ile279, Ile295, and Asp297, the Cambodian GA5 strain also differentiated from Cambodian NA1 and GA2 strains by three further amino acid substitutions, Ile238, Pro241, and Leu256 (Fig. S2) (37).
Potential N- and O-glycosylation sites among HRSV group A strains.
We observed 3 potential N-glycosylation sites among the Cambodian HRSV A strains. The first site, located at amino acid position 237, was present only in the GA2 and GA5 strains; the motif was not present in the NA1 strains due to amino acid substitution Asp237 (see Fig. S2 in the supplemental material). The second potential site, located at amino acid position 251, was conserved among all strains, with the exception of the GA5 strain due to the Ser251 substitution (Fig. S2). For the GA5 strain, the second potential N-glycosylation site was located at position 250 instead (Fig. S2). The third potential N-glycosylation site was located at amino acid position 294 and was conserved among all of the Cambodian HRSV A strains (Fig. S2). All potential N-glycosylation sites have been reported previously (34, 37, 41).
We observed 3 potential O-glycosylation sites that were highly conserved among the Cambodian HRSV A strains (see Fig. S2 in the supplemental material). Located at amino acid position 227, the TTKP motif was conserved among all strains. The second potential O-glycosylation site, a KPT motif located at amino acid position 233, was present in all isolates except the GA2 strain. The third potential site, a TTKT motif located at amino acid position 238, was conserved among all isolates, with the exception of the GA5 strain (Fig. S2). All potential O-glycosylation sites have been reported previously (34, 37).
Intra- and intersubtype diversity among HRSV B strains.
Sixty-seven partial sequences of the HRSV B G gene C-terminal HVR2 were obtained. Fifty (74.6%) and seven (10.4%) of the strains belonged to the recently described HRSV B genotypes BA10 and BA7, respectively (Fig. 3) (11). In addition, 10 (15%) of the strains clustered separately and were classified as belonging to a novel genotype, SAB4 (Fig. 3). To be classified as a separate HRSV genotype, the criteria used previously were that sequences must cluster together with a minimum bootstrap of 70%, with a maximum pairwise distance (p-distance) of 0.07 between cluster members (4, 41, 49). Strains belonging to the genotype SAB4 clustered together with a bootstrap value of 91% (Fig. 3). Furthermore, the maximum p-distance values, calculated using the nucleotide and amino acid sequences of Cambodian SAB4 strains, were 0.02 and 0.05, respectively (data not shown). According to our phylogenetic analysis, strains ChongqingB/09/23 and ChongqingB/06/02, previously classified as genotype GB3 strains, should be reclassified as members of the new SAB4 genotype (Fig. 3) (51). The new genotype was named SAB4 on account of strains clustering closely with strains of the existing SAB2 and SAB3 genotypes following phylogenetic analysis (Fig. 3).
With the exception of the 10 SAB4 strains, all Cambodian HRSV B isolates were classified as belonging to 2 of the novel BA genotypes recently reported by Dapat and colleagues (11). When directly comparing all of the Cambodian HRSV B strains, homology at the nucleotide level was higher than that at the amino acid level, with 12% and 24.9% divergence, respectively. A higher degree of divergence at the amino acid level relative to the nucleotide level was also observed when strains belonging to the same genotype were compared. Nucleotide divergence among genotype BA7 strains was 10%, compared with 18% divergence at the amino acid level. Diversity at the amino acid level was more than double that at the nucleotide level for BA10 strains, at 18% and 7.5%, respectively. The SAB4 genotype strains displayed the highest intragenotype similarity, with only 2% divergence at the nucleotide level and 5% divergence at the amino acid level.
Analysis of HRSV group B amino acid sequences.
The predicted length of the HRSV group B G protein was 315 amino acids for Cambodian genotype SAB4 strains, between 260 and 319 amino acids for genotype BA7 strains, and between 312 and 319 amino acids for genotype BA10 strains (see Fig. S3 in the supplemental material). Fifty-seven (85%) of the Cambodian HRSV group B strains were classified as genotype BA viruses and, as a result, contained the 20-amino-acid duplication characteristic of all HRSV BA genotype strains (48). As reported previously, amino acid substitutions within the duplicated 20 amino acid region varied between strains (51). As the majority of HRSV B strains belonged to the BA10 or BA7 genotype, predicted amino acid sequences were compared relative to the BA prototype strain BA4128/99B (GenBank accession number AY333364) (48) (Fig. S3). The prototype HRSV B strain CH18537 was also included in the analysis to enable comparison with non-BA strains.
Several genotype-specific amino acid substitutions were identified. Strains belonging to the SAB4 genotype were characterized by amino acid substitutions Thr229, Glu278, Ile300, Phe307, Glu314, and Pro315 (see Fig. S3 in the supplemental material). The combination of the Glu278, Phe307, Glu314, and Pro315 amino acid substitutions was present in all of the Cambodian SAB4 strains and were not observed in either HRSV group B reference strains or closely related HRSV group B strain sequences obtained following a BLAST search (data not shown). Genotype BA10 strains could be characterized by Ser231 and Gly292 substitutions, conserved among 86% of BA10 sequences. Dapat et al. also reported conservation of the Gly292 substitution among Japanese genotype BA10 strains (11). A premature stop codon at position 313, resulting in production of a truncated protein missing 6 amino acids at the C terminus reported previously to occur in the BA2, BA4, BA9 and BA10 strains, was present in all Cambodian BA10 strains with the exception of Cam2008-3393 (Fig. S3) (11). Analysis of the genotype BA7 amino acid sequences revealed premature stop codons in 5 of the 7 strains (Fig. S3). Stop codons were located at positions 261, 262, and 278, resulting in production of truncated G proteins lacking between 46 and 59 C-terminal amino acids (Fig. S3). The nucleotide and amino acid mutations resulting in premature stop codons for the 5 HRSV genotype BA7 strains are summarized in the Table S3 in the supplemental material.
Potential N- and O-glycosylation sites among HRSV group B strains.
Two potential N-glycosylation sites, located at amino acid positions 296 and 310, were conserved among all of the Cambodian HRSV B strains analyzed, with the exception of Cam2009-9088 (see Fig. S3 in the supplemental material). Both of the potential N-glycosylation sites have been reported previously to be conserved among HRSV group B strains (11). An additional novel potential N-glycosylation site at position 230, resulting from a Ser231 substitution, was present among 80% of the BA10 strains (Fig. S3). An Asn253 substitution, present in eight BA10 isolates and in one BA7 isolate, generated an additional potential novel N-glycosylation site (Fig. S3). Only one potential O-glycosylation site was identified at amino acid position 234, which was conserved among all of the Cambodian HRSV B isolates analyzed.
DISCUSSION
HRSV is a leading cause of ALRI among children and immunocompromised adults worldwide (19, 30). In the present study, the circulation and genetic diversity of HRSV strains within Cambodia were investigated over 5 consecutive years, from June 2005 to December 2009. Among an all-ages population of patients hospitalized with ALRI symptoms, the incidence of HRSV infection was 8.2%, with infants and children aged less than 5 years being most frequently infected. Similarly, among an all-ages outpatient population with influenza-like illness, the incidence of HRSV infection was 9.5%, with the highest age-specific incidence of infection also observed among children aged less than 5 years. Overall, HRSV group A and B strains were detected at an equivalent frequency. The majority of HRSV group B strains detected were of the recently described BA genotype, with the exception of 10 strains, which we have classified as belonging to a novel HRSV group B genotype, SAB4, first reported here.
Little is known regarding the seasonality, genetic diversity, and circulation of HRSV in tropical and subtropical developing countries, where the disease burden is the greatest (31, 33). During the study period in Cambodia, annual HRSV epidemics typically occurred from July to November, coinciding with the rainy season, similar to reports of HRSV circulation in neighboring Thailand and Vietnam (5, 13, 33, 50). In addition, a potentially biennial pattern of alternating low and high HRSV transmission seasons, as previously reported both globally and regionally, was observed (13, 46).
Globally, the incidence of HRSV infection has been reported to decrease with increasing age (37, 52), which was indeed observed among both hospitalized and outpatient Cambodian populations (Table 1). The decreasing incidence of infection over time is due to the development of anti-HRSV immune responses upon initial infection that are boosted following subsequent reinfections, which are common due to the high antigenic variability between and within HRSV group A and B strains (3, 18, 19, 28, 37, 40). Indeed, the results of two recent studies of hospitalized children and infants reported the incidence of HRSV infection to be 23 to 24% among pediatric populations in Vietnam (12, 50), compared with an overall incidence of 9.4% among the all-ages Cambodian patients tested in this study. Similarly, Fry et al. reported the incidence of RSV infection to be 8.9% among an all-ages hospitalized Thai population (13).
Cambodian HRSV group A and B strains were classified according to all known genotypes, including genotypes BA1 to BA10 recently described by Dapat et al. (11, 35, 36, 41, 48, 49). Despite genotype BA7 not meeting the strict criteria defined by Venter et al. for the classification of novel HRSV genotypes, all Cambodian HRSV group B isolates aligned and clustered closely with strains belonging to the novel BA7 and BA10 genotypes, with the exception of the novel genotype SAB4 strains (11, 49). Hence, the findings of this study are analogous to those of Dapat et al., showing that BA7 to BA10 represent distinct HRSV group B genotypes.
Ten Cambodian HRSV group B strains were identified as belonging to a novel genotype, SAB4. The first genotype SAB4 strain, Cam2008-7105, was identified in 2008, followed by nine additional strains identified in 2009 in distinct provinces of Cambodia (Fig. 3; see also Fig. S1 and S3 in the supplemental material). Strains sharing the highest homology, at the nucleotide level, with the genotype SAB4 strains were strains originally isolated in Chongqing, China, in 2006 and 2009, suggesting concurrent circulation of SAB4 strains within and outside Cambodia during the study period (51).
It has been observed that HRSV strains do not necessarily cluster with those circulating regionally within a given year (4, 15). Boonyasuppayakorn et al. reported that in Thailand in 2007, all HRSV B strains were exclusively BA strains, and that the HRSV A strains belonged only to genotypes GA2 and GA5, with GA2 strains predominating (5). While HRSV B genotype BA strains predominated in both Thailand and Cambodia in 2007, the predominant circulating HRSV group A genotype was NA1 in Cambodia (5). That distinct HRSV genotypes circulated within the same season in two neighboring countries sharing a land border emphasizes both the localized nature of HRSV epidemics and the challenges facing development of a globally effective HRSV vaccine as a result of high viral diversity.
Analogous to published reports, diversity among Cambodian HRSV group A and B strains was higher at the amino acid level than at the nucleotide level (9, 48, 49). It has been reported that HRSV group A strains are globally more frequent, potentially due to higher genetic variability in comparison to HRSV group B strains (34, 38). When performing intragenotype pairwise distance analysis, we observed that diversity at both the nucleotide and amino acid levels for HRSV group A and B strains was equivalent; therefore, the level of nucleotide and amino acid diversity observed among Cambodian HRSV group B strains was higher than previously reported (34, 38). This observation did not occur due to inclusion of novel SAB4 genotype strains in pairwise distance calculations; nucleotide and amino acid diversity among BA genotype strains only remained high. Similarly, Zhang et al. reported nucleotide and amino diversity as high as 6.8% and 14.4%, respectively, between Chinese HRSV genotype BA strains (51). Together, these results suggest that diversity among HRSV group B strains may be higher than previously thought. Indeed, genotype B strains have been shown to be evolving at a higher rate than HRSV A strains, evidenced by greater variability in terms of stop codon usage as well as insertions and deletions within the variable regions of the G protein (23, 24). Gaunt et al. identified amino acid residues under positive selection as a result of targeted immune pressure, all of which are located within the HRSV group B HVR2, the genomic region investigated in this study (15). Analysis of such mutations is important to investigate whether the changes observed confer a fitness loss or advantage to HRSV strains (15). An example of this is the emergence of strains belonging to the HRSV BA genotypes. BA strains, first detected in Argentina in 1999, are characterized by a 60-nucleotide duplication within the C terminus of the G gene and have since become the predominant global HRSV group B genotype (47). Hence, it is thought that the duplication confers a fitness advantage. All of the HRSV B strains investigated in this study contained the 60-nucleotide duplication, with the exception of the novel genotype SAB4 strains. Potentially, it is widespread infection with strains belonging to the BA genotypes that will drive the emergence of strains such as those of the novel SAB4 genotype, which do not contain the duplication, to escape developing immunity to BA strains globally (1). Indeed, two of five residues identified by Gaunt et al. as under positive selection within the HRSV G protein are located within or close to the 20-amino-acid duplicated region within HRSV BA strains, suggestive of the development of targeted immune responses to the duplicated BA genotype region (15).
In this study, premature stop codons were observed in the predicted amino acid sequences of five of the seven HRSV BA7 strains detected, at positions 261, 262, and 278, potentially resulting in the production of severely truncated G proteins. Similarly, Lazar et al. reported that premature stop codons were present at positions 257 and 278 of the G protein of an HRSV A strain isolated from an immunocompromised child regularly receiving intravenous immunoglobulin and speculated that the mutations developed as a result of prolonged immunological pressure (21). Premature stop codons located at five positions, including positions 261 and 278, within the G protein C termini of laboratory-generated HRSV group A strains have been associated with escape from neutralization by monoclonal antibodies in vitro (39). Identification of residues under positive selection by immune responses is crucial to identify potential vaccine candidates (15). Whether mutations occurred as a result of targeted immune responses or not, truncation of the HRSV G protein may have resulted in reduced viral fitness and therefore pathogenicity of the mutated Cambodian BA7 strains. Indeed, in vivo studies have demonstrated that although HRSV strains lacking the G protein retain the ability to replicate, replication occurs at a reduced rate relative to wild-type strains (20, 45). The Cambodian strains with a heavily truncated G protein were detected only during a single transmission season, suggestive of potentially reduced transmissibility.
In addition, two novel potential N-glycosylation sites were identified among the Cambodian HRSV BA strains. Differences in N- and O-glycosylation patterns between HRSV group A and B strains have been speculated to contribute to differences in virus antigenicity between the genotypes (38). Although not within the scope of this study, whether the observed truncations and novel potential glycosylation sites confer a fitness advantage, or affect the antigenicity of the HRSV B G protein, should be investigated subsequently to determine the capacity of these HRSV strains to replicate and thus spread.
In conclusion, we report the detection of strains belonging to a novel HRSV group B genotype, SAB4. The findings of this study also suggest that genetic variability among HRSV group B viruses may be higher than previously thought. The incidence and seasonality of HRSV infection were similar to those reported for other tropical countries. The results from this and similar studies highlight that HRSV circulation within distinct communities is highly variable, emphasizing the need for ongoing, annual surveillance of HRSV infections.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Surveillance and Investigation of Epidemic Situations in Southeast Asia (SISEA) project, by a grant from the French Agency for Development, and by the Office of the Assistant Secretary for Preparedness and Response within the U.S. Department of Health and Human Services. The ILI surveillance was supported by the U.S. CDC influenza program and by WHO Cambodia.
We gratefully thank Alain Viari and Eric Coissac for invaluable advice and help with bioinformatic and phylogenetic analyses. We thank all of the technical staff in the Virology and Epidemiology units at the Institut Pasteur in Cambodia for their assistance. We are also grateful to all the staff from the hospitals, from the Ministry of Health, and from the WHO office in Cambodia for their collaboration on the ILI sentinel surveillance and on the SISEA project.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Agoti C. N., et al. 2010. Intrapatient variation of the respiratory syncytial virus attachment protein gene. J. Virol. 84:10425–10428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnott A., et al. 2011. Genetic variability of human metapneumovirus amongst an all ages population in Cambodia between 2007 and 2009. Infect. Genet. Evol. [Epub ahead of print.] doi:10.1016/j.meegid.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhattarakosol P., Pancharoen C., Mungmee V., Thammaborvorn R., Semboonlor L. 2003. Seroprevalence of anti-RSV IgG in Thai children aged 6 months to 5 years. Asian Pac. J. Allergy Immunol. 21:269–271 [PubMed] [Google Scholar]
- 4. Blanc A., Delfraro A., Frabasile S., Arbiza J. 2005. Genotypes of respiratory syncytial virus group B identified in Uruguay. Arch. Virol. 150:603–609 [DOI] [PubMed] [Google Scholar]
- 5. Boonyasuppayakorn S., Kowitdamrong E., Bhattarakosol P. 2010. Molecular and demographic analysis of respiratory syncytial virus infection in patients admitted to King Chulalongkorn Memorial Hospital, Thailand, 2007. Influenza Other Respi. Viruses 4:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buecher C., et al. 2010. Use of a multiplex PCR/RT-PCR approach to assess the viral causes of influenza-like illnesses in Cambodia during three consecutive dry seasons. J. Med. Virol. 82:1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Cane P. A., Matthews D. A., Pringle C. R. 1991. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J. Gen. Virol. 72(Pt. 9):2091–2096 [DOI] [PubMed] [Google Scholar]
- 9. Cane P. A., Pringle C. R. 1995. Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J. Virol. 69:2918–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cane P. A., Pringle C. R. 1991. Respiratory syncytial virus heterogeneity during an epidemic: analysis by limited nucleotide sequencing (SH gene) and restriction mapping (N gene). J. Gen. Virol. 72(Pt. 2):349–357 [DOI] [PubMed] [Google Scholar]
- 11. Dapat I. C., et al. 2010. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. J. Clin. Microbiol. 48:3423–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Do A. H., et al. 2011. Viral etiologies of acute respiratory infections among hospitalized Vietnamese Children in Ho Chi Minh City, 2004–2008. PLoS One 6:e18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fry A. M., et al. 2010. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One 5:e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galiano M. C., et al. 2005. Intragroup antigenic diversity of human respiratory syncytial virus (group A) isolated in Argentina and Chile. J. Med. Virol. 77:311–316 [DOI] [PubMed] [Google Scholar]
- 15. Gaunt E. R., et al. 2011. Molecular epidemiology and evolution of human respiratory syncytial virus and human metapneumovirus. PLoS One 6:e17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall C. B., Walsh E. E., Long C. E., Schnabel K. C. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693–698 [DOI] [PubMed] [Google Scholar]
- 17. Hall C. B., et al. 2009. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffman S. J., Laham F. R., Polack F. P. 2004. Mechanisms of illness during respiratory syncytial virus infection: the lungs, the virus and the immune response. Microbes Infect. 6:767–772 [DOI] [PubMed] [Google Scholar]
- 19. Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. U. S. A. 84:5625–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karron R. A., et al. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. U. S. A. 94:13961–13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazar I., Canaan A., Weibel C., Kahn J. S. 2006. Novel mutations in the respiratory syncytial virus G gene identified in viral isolates from a girl with severe combined immune deficiency treated with intravenous immune globulin. J. Clin. Virol. 37:168–173 [DOI] [PubMed] [Google Scholar]
- 22. Mardy S., et al. 2009. Influenza activity in Cambodia during 2006–2008. BMC Infect. Dis. 9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez I., et al. 1999. Evolutionary pattern of the G glycoprotein of human respiratory syncytial viruses from antigenic group B: the use of alternative termination codons and lineage diversification. J. Gen. Virol. 80(Pt. 1):125–130 [DOI] [PubMed] [Google Scholar]
- 24. Matheson J. W., et al. 2006. Distinct patterns of evolution between respiratory syncytial virus subgroups A and B from New Zealand isolates collected over thirty-seven years. J. Med. Virol. 78:1354–1364 [DOI] [PubMed] [Google Scholar]
- 25. McIntosh K., Chanock R. M. 1990. Respiratory syncytial virus, p. 1045–1072 In Fields B. N. (ed.), Virology, 2nd ed., vol. 1 Raven Press Ltd., New York, NY [Google Scholar]
- 26. McLellan J. S., et al. 2010. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J. Virol. 84:12236–12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McLellan J. S., et al. 2010. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat. Struct. Mol. Biol. 17:248–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mufson M. A., Orvell C., Rafnar B., Norrby E. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66(Pt. 10):2111–2124 [DOI] [PubMed] [Google Scholar]
- 29. Murata Y., Lightfoote P. M., Falsey A. R., Walsh E. E. 2010. Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin. Vaccine Immunol. 17:695–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nair H., et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nair H., et al. 2011. An evaluation of the emerging interventions against respiratory syncytial virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health 11(Suppl. 3):S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nei M., Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 33. Olsen S. J., et al. 2010. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol. Infect. 138:1811–1822 [DOI] [PubMed] [Google Scholar]
- 34. Parveen S., et al. 2006. Genetic variability in the G protein gene of group A and B respiratory syncytial viruses from India. J. Clin. Microbiol. 44:3055–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peret T. C., et al. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 181:1891–1896 [DOI] [PubMed] [Google Scholar]
- 36. Peret T. C., Hall C. B., Schnabel K. C., Golub J. A., Anderson L. J. 1998. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79(Pt. 9):2221–2229 [DOI] [PubMed] [Google Scholar]
- 37. Reiche J., Schweiger B. 2009. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J. Clin. Microbiol. 47:1800–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roca A., et al. 2001. Genetic variability among group A and B respiratory syncytial viruses in Mozambique: identification of a new cluster of group B isolates. J. Gen. Virol. 82:103–111 [DOI] [PubMed] [Google Scholar]
- 39. Rueda P., Delgado T., Portela A., Melero J. A., Garcia-Barreno B. 1991. Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J. Virol. 65:3374–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruuskanen O., Ogra P. L. 1993. Respiratory syncytial virus. Curr. Probl. Pediatr. 23:50–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shobugawa Y., et al. 2009. Emerging genotypes of human respiratory syncytial virus subgroup A among patients in Japan. J. Clin. Microbiol. 47:2475–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sullender W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1–15, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sullender W. M., Sun L., Anderson L. J. 1993. Analysis of respiratory syncytial virus genetic variability with amplified cDNAs. J. Clin. Microbiol. 31:1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 45. Teng M. N., Whitehead S. S., Collins P. L. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283–296 [DOI] [PubMed] [Google Scholar]
- 46. Terletskaia-Ladwig E., Enders G., Schalasta G., Enders M. 2005. Defining the timing of respiratory syncytial virus (RSV) outbreaks: an epidemiological study. BMC Infect. Dis. 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trento A., et al. 2003. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 84:3115–3120 [DOI] [PubMed] [Google Scholar]
- 48. Trento A., et al. 2006. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J. Virol. 80:975–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venter M., Madhi S. A., Tiemessen C. T., Schoub B. D. 2001. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J. Gen. Virol. 82:2117–2124 [DOI] [PubMed] [Google Scholar]
- 50. Yoshida L. M., et al. 2010. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr. Infect. Dis. J. 29:75–77 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Z. Y., et al. 2010. Genetic variability of respiratory syncytial viruses (RSV) prevalent in Southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J. Clin. Microbiol. 48:1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zlateva K. T., Vijgen L., Dekeersmaeker N., Naranjo C., Van Ranst M. 2007. Subgroup prevalence and genotype circulation patterns of human respiratory syncytial virus in Belgium during ten successive epidemic seasons. J. Clin. Microbiol. 45:3022–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.