Abstract
Saksenaea erythrospora is a newly described species of the order Mucorales which has not previously been reported as a cause of human infection. We report a fatal case of S. erythrospora invasive burn wound infection in a 26-year-old male injured during combat operations in Iraq.
CASE REPORT
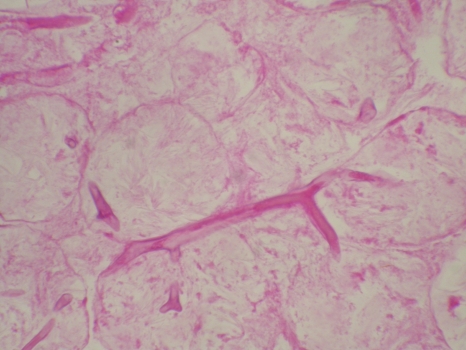
A 26-year-old male sustained 56% total body surface area burns following an improvised explosive device blast in Iraq in November 2005. Injuries were significant for deep, full-thickness facial burns and partial-thickness and full-thickness burns affecting his head, arms, and legs. No evidence of significant smoke inhalation injury was noted on fiber-optic bronchoscopy. At a combat support hospital in Iraq, he was resuscitated and taken to the operating room for bilateral canthotomies, four extremity escharotomies, and four extremity fasciotomies. After initial stabilization, he was evacuated to Germany, where he was found to be in persistent circulatory shock and taken back to the operating room. He was found to have nonviable muscle in the right anterior tibialis compartment, and muscle debridement was performed. He was subsequently stabilized and transferred to the U.S. Army Institute of Surgical Research burn center approximately 4 days postburn. On postburn day 5, the patient underwent tangential excision of his extremity burn wounds and split-thickness skin graft placement. On postburn day 14, the patient was found to have proptosis of his left eye on a routine physical evaluation and underwent a head computed tomography (CT) scan. The CT scan revealed displacement of his left orbit in the inferior lateral direction due to swelling in the orbital fossa. He was rushed to the operating room, where he underwent enucleation of the left eye and debridement of nonviable-appearing tissue in the orbital fossa. Histopathology of the surgical specimen revealed mucormycosis (Fig. 1). Broad-width, aseptate hyphae consistent with Mucorales infection (mucormycosis) were noted on histopathologic examination of tissue from his head and neck multiple times during subsequent serial wound debridements over the course of his hospitalization (days 19, 20, 21, 52, and 85). Multiple cultures were obtained over the course of this management, including mycologic cultures. Most of the fungal cultures were overgrown with bacteria, but they occasionally revealed broad, ribbon-like hyphae on Calcofluor staining. A single culture recovered Saksenaea erythrospora during the course of his hospitalization. The mold was recovered from scalp wound fluid placed into blood culture (aerobic) medium on day 19 postinjury.
Fig. 1.
Histopathology of a section of facial tissue showing broad, aseptate hyphae with wide-angle branching (periodic acid-Schiff staining; magnification, ×40). (Photo courtesy of Seung Kim, reproduced with permission.)
In addition to radical debridement, flap coverage of the left side of his face was attempted on two separate occasions during the course of his hospitalization. Both of these attempts were unsuccessful. His course was also complicated by recurrent bacterial infections and sepsis, in addition to this inability to control his facial mucormycosis, and 100 days following his initial injury, he expired. At autopsy, invasive fungal infection of the left side of the face (including the palate) consistent with mucormycosis on histopathology was noted. The causes of death included burns, fungal and bacterial wound infections, acute myocardial infarction, diffuse interstitial pneumonia, lung hemorrhage, and hepatic necrosis.
Fungal identification.
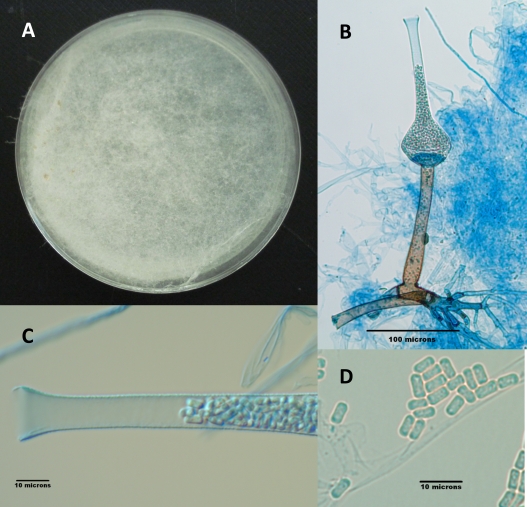
The mold recovered was referred to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio for further identification. Cultures incubated at 25°C on potato flake agar (PFA) displayed rapid growth, with white, cobweb-like colonies, and remained sterile after 4 weeks. Although the isolate had been submitted as a Mucor species, the white colonial morphology was suggestive of an Apophysomyces or Saksenaea species, prompting additional subculturing on Czapek Dox agar (CZA) at 25°C and in a yeast extract-water culture at 35°C. The water culture remained sterile after 4 weeks; however, a few fruiting structures were seen on the CZA plate and the isolate was reported as Saksenaea vasiformis. Subsequent to this identification, a multilocus molecular study of Saksenaea species (ITS, D1/D2, and EF-1α; GenBank accession numbers FR687329, HM776681, and HM776692, respectively) (1) identified the isolate as a new species within the genus, i.e., S. erythrospora. A reexamination of the isolate in a yeast extract-water culture (6) confirmed the phenotypic features described for this species. Colonies were white to off-white and woolly (Fig. 2A), remaining sterile on routine media. Temperature studies on PFA indicated growth at 37°C and 40°C but no growth at 45°C and 50°C. Sporangiophores were long (from 96.8 μm to 232 μm), straight, and brown, with small encrustations. Sporangia were 41 μm to 51 μm at the widest part and were 113 μm to 157 μm in length (Fig. 2B and C). Sporangiospores were 2 to 2.5 by 5.5 μm, resembling biconcave erythrocytes (Fig. 2C and D).
Fig. 2.
(A) S. erythrospora on CZA after 3 weeks of incubation at 25°C. (B) Fruiting structure of S. erythrospora depicting lateral rhizoids; a brown, straight, encrusted sporangiophore; the columella at the base of the sporangium; and the sporangium with its long neck filled with sporangiospores. (C) Higher magnification of the neck of the sporangium filled with sporangiospores. (D) Ellipsoidal, biconcave sporangiospores.
Antifungal susceptibility data for the isolate, obtained by a broth macrodilution modification of the CLSI M38-A2 method (2), are as follows: amphotericin B, 8 μg/ml; itraconazole, 0.5 μg/ml; posaconazole, 0.5 μg/ml; voriconazole, 16 μg/ml; terbinafine, 0.03 μg/ml; anidulafungin, >16 μg/ml; caspofungin, >16 μg/ml; micafungin, >16 μg/ml.
Discussion.
S. vasiformis has been recovered from soil and diseased humans and animals. Human infection with this mucoraceous mold is typically seen after trauma, including burns, and is associated with contamination with soil (4, 5, 7, 9, 10, 11). Severe infection following penetrating trauma with contamination with soil is commonly associated with members of the family Saksenaeaceae (which includes the genera Apophysomyces and Saksenaea) (5, 11). Soft-tissue infection is the most common manifestation of mucormycosis due to Saksenaea, and the majority of patients have been previously healthy and without preexisting immune compromise (5, 8). Infection associated with combat trauma in Afghanistan has been reported (3).
Until recently, S. vasiformis was considered the only species in the family Saksenaeaceae. Alvarez et al. (1) have determined not only that S. vasiformis is a complex of species but also that two new species reside within this family, Saksenaea oblongispora and S. erythrospora (1).
As seen with our isolate, testing of the susceptibility of Saksenaea species to the antifungals posaconazole, itraconazole, and terbinafine has produced low MICs. The activity of amphotericin B, the echinocandins, and voriconazole against these species has been low (higher MICs) (1, 5). The decreased susceptibility of Saksenaea to amphotericin B should be considered when selecting empirical antifungal therapy of infections with this fungus.
As it has only recently been discovered that there are additional species of Saksenaea, it is highly likely that the newly described species S. erythrospora has been the cause of human infection (mucormycosis). The extent to which this species is the cause of human disease, compared with other Saksenaea species in the complex, has yet to be determined. Our case is the first description of infection with S. erythrospora.
Culture collection accession numbers.
The isolate described here has been deposited in the University of Alberta Microfungus Collection under accession number UAMH 11526 and in the University of Texas Health Science Center culture collection as UTHSC 06-576.
Acknowledgments
The views expressed herein are ours and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government. D.R.H., K.K.C., K.L., and E.M.R. are employees of the U.S. Government.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Alvarez E., et al. 2010. Molecular phylogeny and proposal of two new species of the emerging pathogenic fungus Saksenaea. J. Clin. Microbiol. 48:4410–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Evriviades D., et al. 2011. Shaping the military wound: issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldschmied-Reouven A., Shvoron A., Topaz M., Block C. 1989. Saksenaea vasiformis infection in a burn wound. J. Med. Vet. Mycol. 27:427–429 [PubMed] [Google Scholar]
- 5. Gomes M. Z. R., Lewis R. E., Kontoyiannis D. P. 2011. Mucormycosis caused by unusual Mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 24:411–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padhye A. A., Ajello L. 1988. Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis. J. Clin. Microbiol. 26:1861–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribes J. A., Vanover-Sams C. L., Baker D. J. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roden M. M., et al. 2005. Epidemiology and outcome of zygomycosis: a report of 929 reported cases. Clin. Infect. Dis. 41:634–653 [DOI] [PubMed] [Google Scholar]
- 9. Skiada A., Petrikkos G. 2009. Cutaneous zygomycosis. Clin. Microbiol. Infect. 15(Suppl. 5):41–45 [DOI] [PubMed] [Google Scholar]
- 10. Vega W., Orellana M., Zaror L., Gene J., Guarro J. 2006. Saksenaea vasiformis infections: case report and literature review. Mycopathologica 162:289–294 [DOI] [PubMed] [Google Scholar]
- 11. Vitrat-Hincky V., et al. 2009. Severe filamentous fungal infections after widespread tissue damage due to traumatic injury: six cases and review of the literature. Scand. J. Infect. Dis. 41:491–500 [DOI] [PubMed] [Google Scholar]