Abstract
Parvovirus B19 infection during pregnancy is a potential hazard to the fetus because of the virus' ability to infect fetal erythroid precursor cells and fetal tissues. Fetal complications range from transitory fetal anemia and nonimmune fetal hydrops to miscarriage and intrauterine fetal death. In the present study, 72 pregnancies complicated by parvovirus B19 infection were followed up: fetal and neonatal specimens were investigated by serological and/or virological assays to detect fetal/congenital infection, and fetuses and neonates were clinically evaluated to monitor pregnancy outcomes following maternal infection. Analysis of serological and virological maternal B19 markers of infection demonstrated that neither B19 IgM nor B19 DNA detected all maternal infections. IgM serology correctly diagnosed 94.1% of the B19 infections, while DNA testing correctly diagnosed 96.3%. The maximum sensitivity was achieved with the combined detection of both parameters. B19 vertical transmission was observed in 39% of the pregnancies, with an overall 10.2% rate of fetal deaths. The highest rates of congenital infections and B19-related fatal outcomes were observed when maternal infections occurred by the gestational week 20. B19 fetal hydrops occurred in 11.9% of the fetuses, and 28.6% resolved the hydrops with a normal neurodevelopment outcome at 1- to 5-year follow-up. In conclusion, maternal screening based on the concurrent analysis of B19 IgM and DNA should be encouraged to reliably diagnose maternal B19 infection and correctly manage pregnancies at risk.
INTRODUCTION
Parvovirus B19 is a common human pathogen. The infection can occur asymptomatically, or it can be associated with a broad range of clinical features such as erythema infectiosum, postinfectious arthropathy, or transient aplastic crises in patients with hemolytic anemia and can complicate a pregnancy course. B19 is mainly transmitted via respiratory secretions and vertically by the transplacental route. B19 iatrogenic transmission has also been described by blood products (3).
The prevalence of specific B19 IgG ranges from 15 to 60% in children to 30 to 60% in adults and is >85% in the geriatric population (13, 14, 18). In pregnant women, susceptibility to B19 infection has been estimated at 26 to 43.5%, with a seroconversion incidence of 0.61 to 2.4%. In an epidemic period, the incidence may increase up to 13.5% (12, 15, 22).
The infection during pregnancy can have very different outcomes, ranging from the absence of maternal and fetal symptoms to transitory fetal anemia and nonimmune hydrops fetalis (NIHF) to miscarriage and intrauterine fetal death (IUFD). Fetal injuries such as fetal myocarditis, endothelial lesions, and fetal cerebral damages have been reported (9, 19, 21). Thrombocytopenia secondary to B19 infection can also occur (19), as well as congenital anomalies, including chronic anemia (4), meconium peritonitis (24), congenital heart disease (23), fetal hepatic calcifications (20), and bilateral opacification of the cornea (17). The pathogenesis of fetal damages due to B19 infection has been intensively investigated (5, 15, 16); however, little is known of the virological and/or clinical parameters that can predict the risk of fetal complications.
In the present study, 72 pregnancies complicated with maternal B19 infection were followed up. The serological and virological data obtained from the 72 pregnant women were analyzed to define the most accurate diagnostic procedure to detect and manage B19 infection during pregnancy. Fetal and neonatal specimens were investigated by serological and/or virological assays to detect B19-specific antibodies and B19 DNA. Moreover, fetuses and neonates were clinically evaluated till delivery to monitor gestational outcomes and at 1- to 5-year follow-up to assess the neurodevelopment of congenitally infected children.
MATERIALS AND METHODS
Clinical specimens.
Specimens obtained from 72 pregnancies complicated by maternal B19 infection were collected from June 2005 to December 2010. The inclusion criterion was the presence of specific B19 IgM and/or B19 DNA in maternal serum sample. Serological and virological investigations were performed at the Division of Microbiology, S. Orsola-Malpighi Hospital (University of Bologna). Fetal ultrasonographic (US) scans, fetal samplings, and intrauterine transfusions (IUTs) were carried out at the Division of Prenatal Medicine, S. Orsola-Malpighi Hospital (University of Bologna).
At study entry, the mean age of pregnant women was 34 years (standard deviation [SD], 5.5 years; median age, 34 years [range, 19 to 46 years]); 33% of the women had no prior deliveries, 41.7% had one, and 20.9% had two or more. The mean gestational age (GA) was 21 weeks and 3 days (21 full gestational weeks plus 3 days; SD, 1 week and 2 days; median GA, 22 weeks and 6 days [range, 6 weeks and 6 days to 36 weeks and 4 days]). No multiple pregnancies were observed.
Maternal serum samples were analyzed with serological and/or virological assays at study entry and, when possible, one more time during the pregnancy (n = 133). Fetal samples for B19 DNA detection were investigated at the time of amniocentesis (n = 7). Neonatal serum samples were collected by 3 days from delivery and at 30 days (n = 58) and tested for B19-specific antibodies and/or DNA. Placental biopsy samples were analyzed for B19 DNA when available (n = 4). Blood tests for standard TORCH infections were performed on maternal samples and proved negative. Fetal karyotypes, when assessed, were normal.
Serological investigations.
B19 IgG and IgM antibodies in maternal and neonatal serum samples were detected by using enzyme-linked immunosorbent assays (EIA Biotrin, Dublin, Ireland) according to the manufacturer's instructions. Quantitative results were obtained by using positive reference samples and were expressed as index values (IVs). Samples with IVs of >1 were considered positive, those with values from 0.8 through 1 were borderline, and those with IVs of <0.8 were considered negative.
Virological investigations.
B19 DNA was detected by means of a real-time PCR assay on maternal, fetal, and neonatal specimens and by an in situ hybridization assay on placental biopsy specimens. For the real-time PCR assay, the total nucleic acids from serum and blood specimens (100 μl) were extracted by using a bioMerieux NucliSENS easyMAG instrument. An exogenous analytical control (11) was added to the magnetic silica supplied by bioMerieux and copurified with nucleic acids to check both purification and amplification procedures.
The real-time PCR assay was performed on a RotorGene 3000 real-time PCR system (Corbett Research). The amplification mixture of 20 μl consisted of 10 μl of extracted DNA, 0.1 μM concentrations of primer pairs, and a 1× QuantiTect SybrGreen master mix (Qiagen). Amplification of B19 DNA was performed with primers (forward primer, 5′-GCGGGAACACTACAACAACT-3′; reverse primer, 5′-GTCCCAGCTTTGTGCATTAC-3′) able to detect B19 DNA genotype 1 with a sensitivity of 102 IU/ml. The analytical sensitivity was assessed on the International Standard for B19 DNA (code 99/800, National Institute of Biological Standards and Control, United Kingdom). Amplification of the analytical control was performed in the same run with the specific primers (forward primer, 5′-TATTTCTCCTGCTACTTGTC-3′; reverse primer, 5′-CCTCGTCATCTTGCTGGTGA-3′). For both targets, the cycling conditions were 40 cycles of 15 s at 95°C, 30 s at 60°C, and 15 s at 72°C, following a preheating step of 10 min at 95°C. Fluorescence emissions were recorded in the FAM/Sybr channel of the instrument and analyzed by using the functions available in the RotorGene 6.0 software. B19 DNA quantitation was performed by using the algorithm described previously (11) and was expressed as B19 DNA IU/ml of sample. For the in situ hybridization assay, paraffin-embedded sections of placental biopsy specimens were dewaxed sequentially in two changes of fresh xylene, washed in absolute ethanol, and then analyzed as described previously (1).
Statistical analysis.
Statistical tests included the chi-square test and, when appropriate, the Fisher exact test. A P value of <0.05 was considered statistically significant.
RESULTS
Maternal B19 infection.
A total of 72 pregnant women with B19 infections were enrolled in the present study over a period of 6 years (June 2005 to December 2010). The inclusion criteria were the presence of specific B19 IgM (with or without IgG) and/or B19 DNA in maternal serum sample (Table 1). In particular, 68/72 (94.4%) pregnant women underwent B19 serological investigation, and the presence of IgM correctly diagnosed B19 infection in 64/68 (94.1%). Of the 27/72 (37.5%) pregnant women who underwent virological testing, DNA tested positive in 26/27 (96.3%).
Table 1.
Detection of B19 markers in 72 maternal serum samples at study entry
| B19 DNA marker status | B19 IgM (no. of samples)a |
|||
|---|---|---|---|---|
| Positive | Negative | NA | Total | |
| Positive | 18b | 4 | 4 | 26 |
| Negative | 1 | – | – | 1 |
| NA | 45c | – | – | 45 |
| Total | 64 | 4 | 4 | 72 |
NA, data not available; –, not suitable at enrollment.
Sixteen samples were IgG positive, and two samples were IgG negative.
A total of 43 samples were IgG positive, and 2 samples were IgG negative.
Of the 72 women, 29 (40.3%) were tested because of the onset of B19-related symptoms, 17 (23.6%) were tested because fetal anomalies were observed during ultrasound examination, 14 (19.4%) were tested in the course of a routine screening, and 12 (16.7%) were tested following contact with cases of erythema infectiosum. Of the 29 symptomatic pregnant women, 23 (79.3%) experienced rash with or without fever and polyarthralgia, 3 (10.3%) experienced fever, 2 (6.9%) experienced general malaise, and 1 (3.5%) experienced polyarthralgia.
Serological and virological data of the 72 maternal serum samples at the time of enrolment were grouped according to the clinical or epidemiological suspicions for B19 testing (Table 2). Differences in the median IgM index values and B19 viremias were observed among groups.
Table 2.
Quantitative serological and virological data of 72 maternal serum samples at study entry categorized based on clinical or epidemiological suspicionsa
| Reason for B19 testing | No. of women (n = 72) | Parameter | IVb |
B19 DNA (IU/ml) | |
|---|---|---|---|---|---|
| IgG | IgM | ||||
| Symptoms | 29 | Median | 4.10 | 5.65 | 2.14 × 104 |
| Mean | 4.45 | 6.98 | 4.94 × 104 | ||
| SD | 1.79 | 4.08 | 4.98 × 104 | ||
| Suspected contacts | 12 | Median | 4.00 | 2.80 | 1.00 × 104 |
| Mean | 4.62 | 3.01 | 1.79 × 104 | ||
| SD | 1.19 | 1.93 | 2.24 × 104 | ||
| Routine screening | 14 | Median | 3.90 | 1.70 | 5.19 × 102c |
| Mean | 3.52 | 2.38 | 4.50 × 102 | ||
| SD | 2.35 | 1.26 | 2.46 × 102 | ||
| Abnormal US image | 17 | Median | 4.20 | 1.15 | 1.00 × 104 |
| Mean | 4.20 | 1.32 | 1.99 × 109 | ||
| SD | 1.90 | 0.71 | 5.25 × 109 | ||
The table reports median and mean values and standard deviations for B19 specific IgG and IgM IVs (index values) and DNA detected in serum samples from pregnant women that tested for B19 infection following the onset of B19-related symptoms or suspected contact with cases of erythema infectiosum during the course of a routine screening, or because fetal anomalies were observed during US examination, as indicated in column 1.
The median IgM IV for women with B19-related symptoms is statistically higher than in the other groups.
The median B19 viremia for women tested in the course of a routine screening is lower than in the other groups.
Fetal clinical manifestations and outcomes.
Of the 72 fetuses clinically evaluated at ultrasound examination, 22 (30.6%) showed anomalies. In four cases, the development of fetal symptoms were observed during the clinical follow-up after maternal diagnosis of B19 infection, and the time that elapsed between the onset of maternal and fetal manifestations was calculated and ranged from 3 to 15 weeks (Table 3).
Table 3.
Time elapsed from the onset of maternal and fetal symptomsa
| Maternal symptoms | Fetal symptoms | B19 DNA detection |
|---|---|---|
| Polyarthralgia at 5 wks | Moderate hyperechogenic bowel at 20 wks + 6 days, spontaneously resolved | Positive on amniotic fluid sample and on serum sample at birth |
| Rash at 6 wks | Moderate hyperechogenic bowel at 17 wks, spontaneously resolved | Negative at birth |
| Rash at 17 wks | Normal US image at 21 wks, ascites at 23 wks, and IUFD at 26 wks | Positive on placental specimen |
| Rash at 17 wks | Moderate cerebral ventriculomegaly at 20 wks + 4 days | Lost to virological follow-up |
US, ultrasound; IUFD, intrauterine fetal death. The table details the time that elapsed between the onset of maternal and fetal symptoms. The time is expressed in gestational weeks (+ additional days [where applicable]), starting from the date of conception.
Of the 22 cases of fetal anomalies, 8 (36.4%) were nonimmune hydrops fetalis (NIHF), 8 (36.4%) were moderate hyperechogenic bowel, 2 (9.1%) were moderate cerebral ventriculomegaly, 2 (9.1%) were meconium peritonitis, 1 (4.5%) was subcutaneous edema, and 1 (4.5%) was pleural effusion.
NIHF was ascribed to fetal B19 infection in 7/8 hydropic fetuses by detection of B19 DNA on fetal or placental specimens. In 2/8 (25%) NIHF, TOPs (terminations of pregnancy) were decided. NIHF led to fetal loss in four of the remaining six cases (66.7%): two miscarriages (17 and 14 weeks, respectively) and two IUFDs shortly after IUTs of red packed cells (26 and 25 weeks, respectively). Of the two surviving hydropic fetuses, one resolved spontaneously and the other one after IUT. Both neonates were asymptomatic at birth and proved negative for B19 DNA on serum samples. All B19-derived NIHFs were observed in maternal infections documented in the first trimester.
Of the eight fetuses with hyperechogenic bowel (gestational age at clinical evaluation ranging from 17 weeks to 29 weeks+6 days), two (25%) were positive for B19 DNA. They all recovered spontaneously.
Both fetuses with meconium peritonitis were positive for B19 DNA at birth. One fetus resolved spontaneously and the other one was surgically treated at delivery (the gestational ages at clinical evaluation were 23 weeks+5 days and 27 weeks, respectively).
The fetus with pleural effusion (gestational age at clinical evaluation 35 weeks) underwent thoracentesis soon after delivery (36 weeks). The newborn required ventilation support; he was transfused several times and was discharged at 6 weeks of life. A neonatal serum sample was available for serological and virological analysis and proved positive both for B19 DNA and specific IgM.
The fetuses with moderate cerebral ventriculomegaly and subcutaneous edema were born at term and were lost at virological follow-up.
An overall fetal adverse outcome was observed in 8/72 (11.1%) pregnancies and, apart from six deaths definitively attributed to B19 infection, one TOP was decided, one miscarriage occurred, and no specimens were available for diagnosis. In the TOP, the natural outcome of the pregnancy cannot be predicted.
Congenital B19 infections.
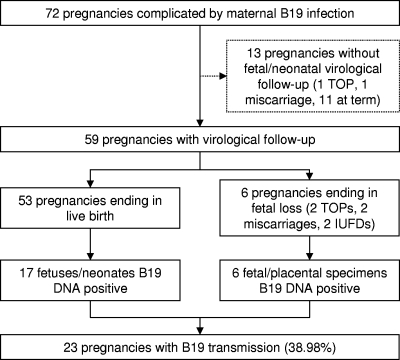
Of the 72 pregnancies complicated by maternal B19 infection, 13/72 (18%) were lost to fetal or neonatal virological follow-up (Fig. 1), while the remaining 59 were analyzed to detect B19 congenital infection. In particular, 69 specimens from the 59 pregnancies were tested: 7 amniotic fluid samples, 4 placental biopsy specimens, and 58 neonatal serum samples.
Fig. 1.
Follow-up of 72 pregnancies complicated by maternal B19 infection. TOP, termination of pregnancy; IUFD, intrauterine fetal death.
Of the 53 pregnancies ending in live birth, a B19 congenital infection was detected in 17 neonates by a real-time PCR assay (3 neonates tested B19 DNA positive in both amniotic fluid and neonatal serum samples, 2 tested positive in amniotic fluid but negative in a neonatal serum sample, and 12 tested positive on neonatal serum sample). The mean viral load for neonatal serum samples was 9.8 × 103 IU/ml (SD, 2.2 × 104 IU/ml), with a median viral load of 1.5 × 103 IU/ml. Ten of the seventeen congenitally infected fetuses (58.8%) never showed anomalies upon US analysis. Moreover, only 2/15 (13.3%) neonates tested IgM positive, and both mothers were infected at the third trimester. Five of the fifteen (33.3%) positive neonates could be retested at 30 days and proved negative for B19 DNA.
Considering the six pregnancies ending in fetal loss, all specimens (two amniotic fluid samples and four placental biopsy specimens) tested positive for B19 DNA by means of real-time PCR and/or in situ hybridization assays.
In summary, B19 vertical transmission was observed in a total of 23/59 pregnancies (39%) and was analyzed in relation to the GA at the time of documented maternal infection (Table 4). The highest rate of vertical transmission occurred when B19 infection was acquired during the second trimester, by gestational week 20 (57.1%, P < 0.05). No fetal deaths were observed when maternal B19 infection was documented after gestational week 20.
Table 4.
Analysis of B19 vertical transmission in relation to the trimester of documented maternal infectiona
| Maternal B19 infection | Total no. (%) of pregnancies | No. (%) of pregnancies with vertical transmission | No. (%) of various pregnancy outcomes |
|---|---|---|---|
| First trimester | 23/59 (38.98) | 8/23 (34.78) | 6/8 (75) at term |
| 2/8 (25) miscarriages | |||
| Second trimester (<20 wks) | 14/59 (23.73) | 8/14 (57.14) | 6/8 (75) at term |
| 2/8 (25) IUFDs | |||
| Second trimester (>20 wks) | 13/59 (22.03) | 3/13 (23.07) | 3/3 (100) at term |
| Third trimester | 9/59 (15.25) | 4/9 (44.44) | 4/4 (100) at term |
| Total | 59/59 (100) | 23/59 (38.98) | 19/23 (82.61) at term |
| 4/23 (17.39) fetal loss |
IUFD, intrauterine fetal death.
Finally, of 23 congenital infections, 9 (39.1%) occurred when mothers were symptomatic and 14 (69.9%) were asymptomatic. Ten of the fourteen (71.4%, P < 0.05) asymptomatic pregnant women were tested because of an abnormal US scan, and the remaining four women (28.7%) were tested because of contacts with cases of erythema infectiosum.
DISCUSSION
Our study presents laboratory and clinical data for 72 pregnancies complicated by maternal B19 infection. Mother-fetus pairs were included in the study when a positive B19 IgM and/or DNA result was ascertained from maternal serum samples; specimens from fetuses and neonates were analyzed to detect B19-specific antibodies and/or DNA. Pregnancies were clinically evaluated to monitor the gestational outcomes, following the documented viral infection.
Serological and virological data of the women at enrollment were evaluated to identify the most accurate diagnostic procedure to detect B19 maternal infection. The analysis demonstrated that neither B19 IgM nor B19 DNA detected all maternal infections. IgM serology correctly diagnosed 94.1% of B19 infections, while DNA testing correctly diagnosed 96.3%. Regarding IgM detection, it is important to underline that the absence of IgM in maternal serum samples cannot exclude B19 fetal infection since fetal manifestations can occur 8 to 15 weeks after maternal infection, when the signs of acute infection could have already disappeared (1, 9). Therefore, reliance on a negative IgM serologic result alone can be misleading in pregnant women; concurrent detection of B19 DNA should be considered to avoid misdiagnosis. At enrollment, 17.4% of the pregnant women proved to be B19 IgG and DNA positive (in the absence of specific IgM). In the course of the natural infection, detection of these B19-specific biomarkers may represent a late phase of viral clearance or a reactivation of past infection (2). In the present study, four asymptomatic pregnant women were enrolled with B19 IgG (without IgM) and DNA positive results (three women tested at first and one at the second trimester). Pregnancies ended with miscarriages in 2 of 4 cases at 2 and 4 weeks, respectively, after the laboratory diagnosis of B19 infection.
Maternal B19-specific antibodies and DNA were quantitatively evaluated among the different groups of women classified considering the clinical or epidemiological suspicions of B19 infection (maternal and fetal symptoms, maternal screening, or suspected contacts). The median IgG index values did not vary significantly, while the median IgM value was higher in the group of women that was tested for B19-related symptoms than in the others. Indeed, the clinical symptoms experienced by these pregnant women (rash and/or polyarthralgia) are related to the specific immune response. Regarding B19 DNA, the median viremia of women that tested in the course of a routine screening was lower than in the other groups of women.
Similar to the rate reported in the literature (7), the rate of vertical transmission observed here was 39%, considering fetuses with clinical and laboratory B19 diagnosis and neonates with markers of B19 infection with or without clinical evidences. However, these diagnostic criteria could lead to an underestimation of the real value of vertical transmission, since not all fetal samples were available for B19 investigations after the diagnosis of maternal B19 infection. Invasive investigations, in fact, cannot be performed on fetuses that do not present with clinical complications. In the present study, indeed, two B19-positive fetuses at the time of amniocentesis, performed at 20 and 23 weeks, respectively, were observed while, at birth, the two neonates tested negative for B19 DNA.
Data regarding the observed vertical transmission were analyzed in relation to the gestational age at the time of maternal viral diagnosis. The highest rates of B19 transmission and fatal outcome were observed when maternal infection occurred by gestational week 20 (43.2 and 25.%, respectively).
Congenital infection remained clinically unrecognized in the 58.8% of fetuses, while in the remaining 41.2% the infected fetuses developed anomalies. When maternal B19 infection was symptomatic, the time between the onset of maternal and fetal manifestations was calculated and ranged from 3 to 15 weeks. Interestingly, fetal anomalies became evident in ultrasound investigations performed at 17 to 23 weeks of gestation irrespective of the gestational age of maternal infection. This observation is strictly related to the pathogenesis of fetal damage. During the period from gestational weeks 17 to 24, the placental trophoblasts express elevated concentrations of P antigen, the specific B19 receptor, and a very intense hematopoiesis is located in the liver; thus, the fetus is vulnerable to damage induced by B19 (3, 10).
B19-derived fetal hydrops occurred only after maternal B19 infection during the first trimester, and the risk was estimated to be 11.9%. Hydrops resolved in 28.6% of the fetuses, and a normal neurodevelopment outcome was reported in survivors at the 1- to 5-year follow-up.
Regarding the rate of fetal hydrops, the data from the literature range from 3.9 to 10% (7, 9); the higher rate of B19-derived fetal hydrops detected in the present study can be attributed to a selection bias due to the referral of pregnant women with B19-related fetal symptoms upon ultrasound examination.
The risk of an adverse outcome definitely attributed to maternal infection was 10.2% and, when the analysis was limited to women infected before gestational week 20, was estimated to be 11.4%. In agreement with the findings of Enders et al. (10), nonhydropic late IUFDs were not observed.
In conclusion, concurrent detection of B19-specific antibodies and DNA should be encouraged in order to reliably diagnose maternal B19 infection and thereby promptly manage pregnancies that are at risk. For the diagnosis of congenital infection, detection of B19 DNA should be performed, since only 13.3% of the infected neonates presented with IgM at birth. Both the immaturity of the fetal immune system and B19 antigenicity can contribute to the absence of IgM (6, 8). It should be also be noted that in the absence of maternal diagnosis of infection, the occurrence of fetal deaths could also be ascribed to B19.
ACKNOWLEDGMENT
We thank Margherita Contoli for her collaboration.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Bonvicini F., et al. 2009. Diagnosis of fetal parvovirus B19 infection: value of virological assays in fetal specimens. BJOG 116:813–817 [DOI] [PubMed] [Google Scholar]
- 2. Bredl S., et al. 2005. Evidence for parvovirus B19 reactivation in seropositive pregnant women, abstr. 34. Abstracts of the XIII International Parvovirus Workshop, Helsinki, Finland [Google Scholar]
- 3. Broliden K., Tolvenstam T., Norbeck O. 2006. Clinical aspects of parvovirus B19 infection. J. Intern. Med. 260:285–304 [DOI] [PubMed] [Google Scholar]
- 4. Brown K. E. 1994. Congenital anaemia after transplacental B19 parvovirus infection. Lancet 343:895–896 [DOI] [PubMed] [Google Scholar]
- 5. Chisaka H., Morita E., Yaegashi N., Sugamura K. 2003. Parvovirus B19 and the pathogenesis of anaemia. Rev. Med. Virol. 13:347–359 [DOI] [PubMed] [Google Scholar]
- 6. de Haan T. R., et al. 2007. Parvovirus B19 infection in pregnancy: maternal and fetal viral load measurements related to clinical parameters. Prenat. Diagn. 27:46–50 [DOI] [PubMed] [Google Scholar]
- 7. de Jong E. P., et al. 2006. Parvovirus B19 infection in pregnancy. J. Clin. Virol. 36:1–7 [DOI] [PubMed] [Google Scholar]
- 8. Dieck D., Shild R. L., Hansmann M., Eis-Hubinger A. M. 1999. Prenatal diagnosis of congenital parvovirus B19 infection: value of serological and PCR techniques in maternal and fetal serum. Prenat. Diagn. 19:1119–1123 [PubMed] [Google Scholar]
- 9. Enders M., Weidner A., Zoellner I., Searle I. K., Enders G. 2004. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat. Diagn. 24:513–518 [DOI] [PubMed] [Google Scholar]
- 10. Enders M., et al. 2010. Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection. J. Clin. Virol. 49:163–169 [DOI] [PubMed] [Google Scholar]
- 11. Gallinella G., et al. 2004. Calibrated real-time PCR for evaluation of parvovirus B19 viral load. Clin. Chem. 4:759–762 [DOI] [PubMed] [Google Scholar]
- 12. Jensen I. P., Thorsen P., Jeune B., Moller B. R., Vestergaard B. F. 2000. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG 107:637–643 [DOI] [PubMed] [Google Scholar]
- 13. Kelly H. A., et al. 2000. The age-specific prevalence of human parvovirus immunity in Victoria, Australia, compared with other parts of the world. Epidemiol. Infect. 124:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manaresi E., et al. 2004. Seroprevalence of IgG against conformational and linear capsid antigens of parvovirus B19 in Italian blood donors. Epidemiol. Infect. 132:857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mossong J., et al. 2008. Parvovirus B19 infection in five European countries: seroepidemiology, force of infection and maternal risk of infection. Epidemiol. Infect. 136:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasquinelli G., Bonvicini F., Foroni L., Salfi N., Gallinella G. 2009. Placental endothelial cells can be productively infected by parvovirus B19. J. Clin. Virol. 44:33–38 [DOI] [PubMed] [Google Scholar]
- 17. Plachouras N., Stefanidis K., Andronikou S., Lolis D. 1999. Severe nonimmune hydrops fetalis and congenital corneal opacification secondary to human parvovirus B19 infection: a case report. J. Reprod. Med. 44:377–380 [PubMed] [Google Scholar]
- 18. Rohrer C., et al. 2008. Seroprevalence of parvovirus B19 in the German population. Epidemiol. Infect. 136:1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segata M., Chaoui R., Khalek N., Bahado-Singh R., Paidas M. J. 2007. Fetal thrombocytopenia secondary to parvovirus B19 infection. Am. J. Obstet. Gynecol. 61:61–64 [DOI] [PubMed] [Google Scholar]
- 20. Simchen M. J., et al. 2002. Fetal hepatic calcifications: prenatal diagnosis and outcome. Am. J. Obstet. Gynecol. 187:1617–1622 [DOI] [PubMed] [Google Scholar]
- 21. Tolfvenstam T., Papadogiannakis N., Norbeck O., Petersson K., Broliden K. 2001. Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet 357:1494–1497 [DOI] [PubMed] [Google Scholar]
- 22. van Gessel P. H., et al. 2006. Incidence of parvovirus B19 infection among an unselected population pf pregnant women in the Netherlands: a prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 128:46–49 [DOI] [PubMed] [Google Scholar]
- 23. Wang X., et al. 2004. Prevalence of human parvovirus B19 DNA in cardiac tissues of patients with congenital heart diseases indicated by nested PCR and in situ hybridization. J. Clin. Virol. 31:20–24 [DOI] [PubMed] [Google Scholar]
- 24. Zerbini M., et al. 1998. Intrauterine parvovirus B19 infection and meconium perotinitis. Prenat. Diagn. 18:599–606 [PubMed] [Google Scholar]