Abstract
Systemic infections caused by Clostridium sordellii are rare. They are usually reported in cases of skin and soft tissue infections and sometimes in cases of toxic shock syndrome involving exotoxins. We report here the first case of Clostridium sordellii infection associated with acute constrictive pericarditis and with pyopericardium and tamponade.
CASE REPORT
The patient, an 8-month-old infant, was admitted to a local hospital with a history of about 2 months of fever and an abscess in the right thigh that had developed following hepatitis B vaccination. The abscess was drained, and the pus culture revealed Staphylococcus aureus, for which the patient was given vancomycin intravenously per the antimicrobial susceptibility report. The abscess subsequently healed; however, the patient remained sick, with development of a dry cough and swelling of legs. Echocardiography and chest X-ray results showed a pericardial effusion with an increased cardiothoracic ratio, and the patient was referred to our institute. On admission, the patient was afebrile, with mild pedal edema, tender hepatomegaly, and liver palpable to 4 cm below the costal margin. Routine investigations showed hemoglobin at 12.8 g/dl; total leukocyte count at 14,100/μl; differential leukocyte counts of neutrophils at 50, lymphocytes at 46, eosinophils at 3, and monocytes at 1; an erythrocyte sedimentation rate at 5 mm in the first hour; blood urea at 35 mg/dl; serum creatinine at 0.4 mg/dl; serum sodium at 128 mEq/liter; serum potassium at 4.3 mEq/liter; serum aspartate transaminase (SGOT) at 115 U/liter; serum glutamic pyruvic transaminase (SGPT) at 235 U/liter; total bilirubin at 1.8 mg/dl; total protein at 5.1 g/dl; and serum albumin at 2.8 g/dl. The urine output was within normal limits. Central nervous system and respiratory system examinations showed no abnormalities. The patient's heart rate was 128 beats per min. Electrocardiogram showed low-voltage complexes, and echocardiography revealed organized pericardial effusions with features of tamponade. A chest X-ray showed a cardiothoracic ratio of 0.65. A computerized tomography (CT) scan of the chest showed features suggestive of effusive constrictive pericarditis, and a diagnosis of constrictive pericarditis with pyopericardium and tamponade was made. Pigtail drainage of pericardial pus was performed, and the patient was empirically administered intravenous piperacillin-tazobactam and linezolid. On examination, the pericardial fluid was turbid, with protein at 40 mg/dl; sugar at 45 mg/dl; a total leukocyte count of 2,600/μl; and differential leukocyte counts of neutrophils at 90 and lymphocytes at 10. Gram staining on a direct smear showed no organisms, and Ziehl-Neelsen staining showed no acid-fast bacilli. Aerobic cultures of pericardial fluid and blood were sterile; however, pericardial fluid was not sent for anaerobic culture. Meanwhile, the patient had stabilized. A follow-up computerized tomography angiogram showed a localized constriction at the atrioventricular groove, and pericardiectomy was performed. The postoperative period was uneventful, and the patient was discharged with an oral amoxicillin-clavulanic acid treatment regimen as prescribed.
The excised pericardial tissue was sent for both aerobic and anaerobic culture. Aerobic culture showed no growth. On anaerobic culture, odorless, flat, grayish, nonhemolytic colonies with irregular margins were seen. They were metronidazole sensitive. Gram staining showed Gram-positive bacilli with subterminal nonbulging spores. The isolate was further identified biochemically and tested positive for lecithinase, urease, gelatin liquefaction, indole production, glucose fermentation, and esculin hydrolysis. The isolate was identified as Clostridium sordellii. The isolate was also tested using API 20A (bioMérieux, France) and was again identified as C. sordellii.
A PCR using published primers for the 16S rRNA gene was also done. Briefly, DNA was extracted from the culture by the use of a QIAmp kit (Qiagen, Germany) and was amplified using broad-range PCR primers, i.e., forward primer (F8) 5′-AGTTTGATCCTGGCTCAG-3′ and reverse primer (357R) 5′-CTGCTGCCTCCCGTA-3′ (6, 14) and C. sordellii-specific forward primer (C1SOR-F) 5′-TCGAGCGACCTTCGG-3′ and reverse primer (C1SOR-R) 5′-CACCACCTGTCACCAT-3′, respectively, targeting the 16S rRNA gene (10). Amplified products, i.e., 330 bp (Fig. 1) for broad-range PCR and 944 bp (Fig. 2) for Clostridium sordellii-specific PCR, were purified using a Qiagen gel extraction kit. The purified products were commercially sequenced and compared with sequences available in the GenBank database by the use of the Basic Local Alignment Search Tool (BLAST). The 16S rRNA gene sequences amplified from the isolate showed 99% identity with the C. sordellii sequence (GenBank accession no. AB448946.1) available in the database. Thus, C. sordellii was identified as the cause of constrictive pericarditis with pyopericardium and tamponade. The patient responded favorably to the therapy.
Fig. 1.
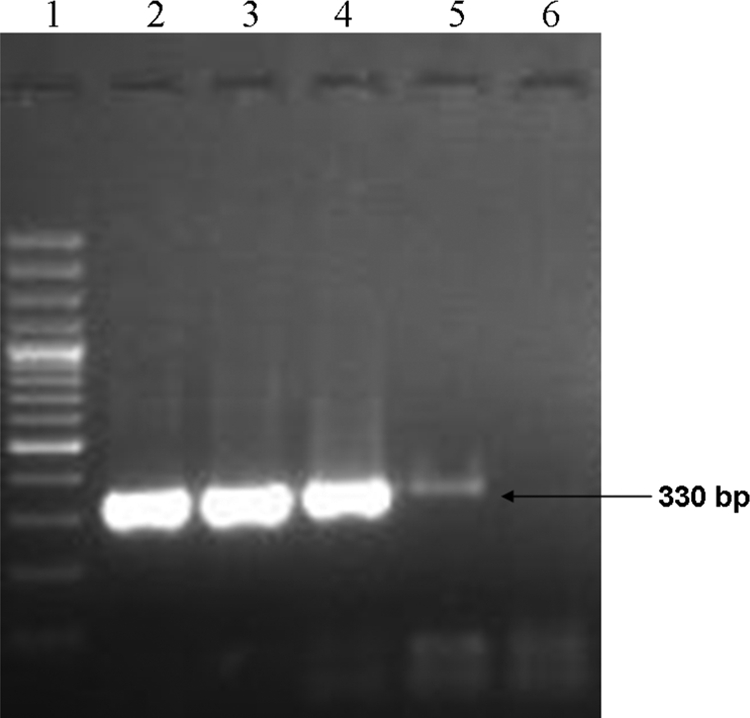
Broad-range PCR assay. The band corresponding to the 330-bp sequence of 16S rRNA gene of the genus Clostridium is indicated (arrow). Lane 1, 100-bp ladder; lane 2, pericardial isolate; lane 3, C. sordellii laboratory isolate; lane 4, C. difficile ATCC 9689; lane 5, C. perfringens laboratory isolate; lane 6, negative control.
Fig. 2.
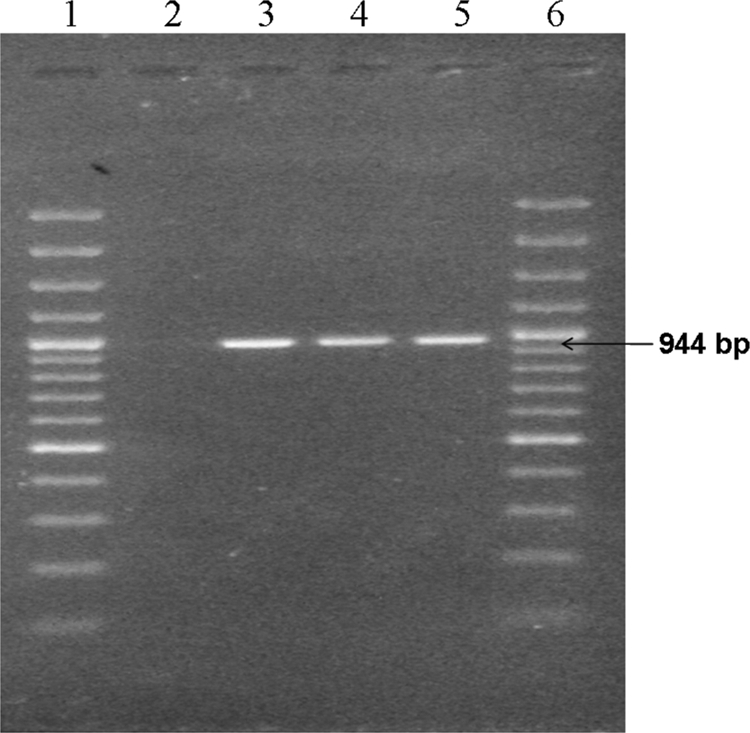
Clostridium sordellii-specific PCR. The band corresponding to the 944-bp sequence of the 16S rRNA gene is indicated (arrow). Lane 1, 100-bp ladder; lane 2, negative control; lane 3, C. sordellii pericardial isolate; lanes 4 and 5, C. sordellii laboratory isolate; lane 6, 100-bp ladder.
Anaerobes are now being recognized as etiological agents for infections of almost any body site, including the pericardium (4). Pericarditis refers to inflammation of the pericardium and the proximal part of great blood vessels. It may be asymptomatic or may present as a fulminating, life-threatening condition. Brahan et al. (3), François et al. (8), and Pigrau et al. (12) have reported the association of C. septicum infection with pericarditis in patients with underlying carcinoma. Skiest et al. (13) have given a brief review of anaerobic pericarditis occurring in children in which a Bacteroides species was highlighted as the predominant cause (13). Pericarditis was also associated with C. perfringens (9). C. difficile pericarditis complicating pseudomembranous colitis in a patient hospitalized after multiple traumas was first reported by Koehler et al. (11). It is to be noted that C. difficile resembles C. sordellii phenotypically, and, since C. difficile strains of greater virulence such as NAP1 are being reported worldwide, there is a need to carefully differentiate them genotypically (7). Although a few other cases of pericarditis caused by species of the genus Clostridium have been reported, to our knowledge, this is the first case in which C. sordellii infection was found to be associated with pericarditis. C. sordellii is a Gram-positive anaerobe and an infrequent human pathogen associated with skin and soft tissue infections. The exotoxins produced by the bacteria are associated with toxic shock syndrome and with a high mortality rate following childbirth, medically induced abortion, and routine gynecological procedures (1, 2). Recently, C. sordellii has been reported as a cause of brain abscess (15).
In the case reported here, there was no initial clinical suspicion of anaerobic infection. Since all of the other possible causes were systematically ruled out, the clinicians finally decided to send the excised pericardial tissue for anaerobic culture. The sample was transported in anaerobic transport medium with sterility precautions. A pure culture of C. sordellii was confirmed phenotypically and genotypically.
Different prevailing local conditions and host factors and rapid administration of appropriate antimicrobial treatment could have decreased the clinical severity of the infection; however, the patient whose case is reported here was given a good prognosis. The possibility of a low level of virulence of the pathogen as a factor mitigating the severity of the symptoms could not be ruled out (5).
The present case demonstrates that C. sordellii can cause acute constrictive pericarditis. Moreover, the expanding clinical spectrum of C. sordellii infections necessitates application of rapid identification methods such as PCR and gene sequencing and increased clinical awareness of this unrecognized human pathogen.
Acknowledgments
We thank Mohanlal Sharma, laboratory technician, and S. K. Choudhary and his teammates at the Cardiothoracic and Vascular Surgery Centre at AIIMS for sending the sample.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Aldape M. J., Bryant A. E., Stevens D. L. 2006. Clostridium sordellii infections: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin. Infect. Dis. 43:1436–1446 [DOI] [PubMed] [Google Scholar]
- 2. Aronoff D. M., Ballard J. D. 2009. Clostridium sordellii toxic shock syndrome. Lancet Infect. Dis. 9:725–726 [DOI] [PubMed] [Google Scholar]
- 3. Brahan R. B., Kahler R. C. 1990. Clostridium septicum as a cause of pericarditis and mycotic aneurysm. J. Clin. Microbiol. 28:2377–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brook I. 2009. Pericarditis caused by anaerobic bacteria. Int. J. Antimicrob. Agents 33:297–300 [DOI] [PubMed] [Google Scholar]
- 5. Brüggemann H. 2005. Genomics of clostridial pathogens: implication of extrachromosomal elements in pathogenicity. Curr. Opin. Microbiol. 8:601–605 [DOI] [PubMed] [Google Scholar]
- 6. Daly J. S., et al. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elsayed S., Zhang K. 2006. Positive Clostridium difficile stool assay in a patient with fatal C. sordellii infection. N. Engl. J. Med. 355:1284–1285 [DOI] [PubMed] [Google Scholar]
- 8. François B. L., et al. 1994. Gas gangrene and purulent pericarditis during Clostridium septicemia revealing a cecal carcinoma. Intensive Care Med. 20:309. [DOI] [PubMed] [Google Scholar]
- 9. Ivey M. J., Gross B. H. 1993. Back pain and fever in an elderly patient. Chest 103:1851–1853 [DOI] [PubMed] [Google Scholar]
- 10. Kikuchi E., Miyamoto Y., Narushima S., Itoh K. 2002. Design of species-specific primers to identify 13 species of Clostridium harboured in human intestinal tracts. Microbiol. Immunol. 46:353–358 [DOI] [PubMed] [Google Scholar]
- 11. Koehler R., et al. 2003. Clostridium difficile pericarditis complicating pseudomembranous colitis in a trauma patient. J. Trauma 55:771–773 [DOI] [PubMed] [Google Scholar]
- 12. Pigrau C. M., Ruiz P., Sagrista J. 1995. Purulent pericarditis due to Clostridium septicum associated with carcinoma of the colon. Clin. Infect. Dis. 20:202–203 [DOI] [PubMed] [Google Scholar]
- 13. Skiest D. J., Steiner D., Werner M., Garner J. G. 1994. Anaerobic pericarditis: case report and review. Clin. Infect. Dis. 19:435–440 [DOI] [PubMed] [Google Scholar]
- 14. Stackebrandt E., Charfreitag O. 1990. Partial 16S rRNA primary structure of five Actinomyces species: phylogenetic implications and development of an Actinomyces israelii-specific oligonucleotide probe. J. Gen. Microbiol. 136:37–43 [DOI] [PubMed] [Google Scholar]
- 15. Valour F. S., et al. 2010. Clostridium sordellii brain abscess diagnosed by 16S rRNA gene sequencing. J. Clin. Microbiol. 48:3443–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]


