Abstract
Sputum smear microscopy is an easy, inexpensive, and rapid method for detecting tubercle bacilli when there are more than 10,000 bacilli/ml in the original sputum. Furthermore, because the microscopic method provides not only quantitative, but also qualitative information, such as the shape of bacilli, it has remained significant. We have previously developed and reported panel test slides made from polyacrylamide-based artificial sputum (PBAS) mixed with both cultured THP-1 cells and nonpathogenic mycobacteria. In this paper, we report an improved preparation method for PBAS for panel test slides that provides a simplified method and enhanced availability with high consistency in each grade and in which only negative PBAS is prepared from polyacrylamide and cultured THP-1 cells and mixed with graded formalin-fixed Mycobacterium tuberculosis solution (FFTBS) containing oral flora and Pseudomonas aeruginosa on the slides. In the smears prepared using this improved method, the numbers (average ± standard deviation [SD]) of acid-fast bacilli (AFB) in 300 fields (2- by 3-cm smear) in eight smears of each grade ranged from 5 to 9 (6.4 ± 1.4), from 59 to 88 (74.6 ± 10.0), from 503 to 912 (705.0 ± 145.7), and from 1,819 to 3,256 (2133.3 ± 478.0) in ±, +, ++, and +++ smears, respectively. In addition, this preparation method provided high similarity to the microscopic appearance of bacilli and background seen in the actual patient sputum, with high feasibility. These results revealed that our new PBAS had high authenticity in the appearance and consistency in each grade, which could make it valuable as a reliable artificial sputum for the training of microscopists.
INTRODUCTION
The World Health Organization (WHO) global tuberculosis (TB) control 2010 report estimated that there were 9.4 million new cases, 14 million existing cases, and 1.3 million deaths among HIV-negative cases of tuberculosis in 2009. The estimated global incidence rate is 137 cases per 100,000 population, and while the rate continues to fall from the high in 2004, this is occurring too slowly. Furthermore, there were 440,000 new multiple-drug-resistant TB (MDR-TB) cases in 2008, and 150,000 deaths from MDR-TB. Extensively drug-resistant TB (XDR-TB) cases have been confirmed in 58 countries (13).
The detection of tuberculosis cases can be performed by several methods, including microscopic examination, liquid culture, and nucleic acid amplification tests (2, 4, 8, 9, 11). Among the methods, sputum smear examination can detect mycobacteria rapidly, within 1 hour after sputum collection, and inexpensively if there are >10 kCFU/ml of the bacilli in the sputum (12).
It is therefore crucial to foster superior microscopists who can detect mycobacteria in sputum smears rapidly and correctly. However, there are few tools available for training (1, 3, 5, 6, 10). We have developed and introduced a first-generation polyacrylamide-based artificial sputum (PBAS1) for preparation of panel test slide sets, which consists of cultured cells, Mycobacterium bovis BCG bacilli, and polyacrylamide (14, 15). PBAS1 has high similarity to actual sputum in its macro- and microscopic appearance and viscosity properties. Furthermore, PBAS1 can be prepared easily, with four positive grades (±, +, ++, and +++) and a negative grade, in any laboratory with cell culture facilities. Because negative PBAS is prepared from cultured cells and polyacrylamide, it is absolutely negative for mycobacteria, which cannot be the case if actual sputa are used. Precise positivity grades in PBAS1 can be controlled by changing the volume of bacillary solution with known numbers of CFU that is mixed with cultured cells and polyacrylamide.
However, because PBAS1 already contains bacilli in a viscous entity, it is difficult to strictly adjust the bacillary concentration in the smear while maintaining constant thickness. In addition, PBAS1 uses the BCG-Pasteur strain, which is slightly shorter than actual tubercle bacilli, which may give a less natural impression to microscopists. Because the positive grades of PBAS1 are separately prepared with 10-fold serially diluted bacillary solutions, it is necessary to divide cell components into five grades, including a negative grade, and this requires a large amount of cell culture.
To resolve these drawbacks to the PBAS method, we have developed a novel preparation procedure for improved PBAS (PBAS2) with formalin-fixed Mycobacterium tuberculosis solution (FFTBS) with a >50-MCFU/ml tubercle bacillus solution. Although this new procedure is similar to the PBAS1 method, the appearance of PBAS2 is more similar to actual sputum than PBAS1 because the H37Rv strain is used for positive grades and mixed with negative PBAS after fixation with formalin containing oral flora and Pseudomonas aeruginosa as a non-acid-fast bacillus contaminant. Improvements in the preparation of PBAS2 include greater simplicity in the preparation of PBAS, where only negative PBAS is prepared, enhanced reality of the bacillary appearance, and greater ease in adjusting the positivity in each grade by controlling the volume of bacillary solution that is applied and mixed with negative PBAS without changing the thickness of the smear. The PBAS2 method will therefore likely play a significant role in training for tuberculosis microscopy because of its superior panel test slide sets, which consist of both defined positive grades and absolutely negative smears, with more authentic bacilli.
MATERIALS AND METHODS
Bacterium and preparation of the bacillary solutions.
M. tuberculosis H37Rv (ATCC 25618) was cultured in 50 ml of Middlebrook 7H9 (Becton Dickinson, MD) broth supplemented with albumin, dextrose, and catalase enrichment (Becton Dickinson, MD) and Tween 80 (Sigma-Aldrich, MD) for 2 weeks. Next, the fully grown bacillary solution was passed through an Acrodisk filter with a pore size of 5.0 μm (catalog no. 4650; Pall Corporation, Cornwall, United Kingdom) to obtain a single bacillus solution, which was placed on 1% Ogawa medium. After 4 weeks of culture, the number of CFU was determined, and it was confirmed that the bacillary concentration was higher than the 50 MCFU/ml that was needed to obtain the +++ smear. At the same time, aliquots (1 ml) of the filtrate were transferred to sterile Nalgene cryogenic vials (catalog no. 5001-1020; Agene; Thermo Fisher Scientific Inc.) and stored frozen at −80°C until use. Clinical isolates of oral flora, including Corynebacterium spp., Streptococcus viridans, Neisseria spp., and P. aeruginosa, were plated on Trypticase soy agar with blood. Single-colony isolations were performed for oral flora, and the bacteria were cultured together in a brain heart infusion (BHI) broth. P. aeruginosa was cultured in LB broth, and fully grown bacillary solutions (optical density [OD] > 0.5) were stored frozen at −80°C as aliquots (1 ml) in cryogenic vials as described above. A total of 2 ml (one aliquot each) of the thawed bacillary solutions of both the mixed oral flora and P. aeruginosa were added to 10 ml of formalin solution (Mildform 20MN; Wako Pure Chemical Industries, Ltd., Osaka, Japan), and this solution was used both as the dilution solution for preparation of positive smears and as the acid-fast bacillus (AFB)-negative solution for preparing negative smears. This solution can be stored at 4°C. Then, the FFTBS used for each positive grade was prepared by fixation and dilution of the stored H37Rv solution with the dilution solution described above. Briefly, the stored H37Rv solution with more than 50 MCFU/ml was fixed with formalin solution at a 3× to 5× dilution, depending on the number of CFU, and was used for preparation of the +++ smear. Next, three serial 10-fold dilutions were made from the dilution solution to obtain bacillary solutions for ++, +, and ± smears (Fig. 1).
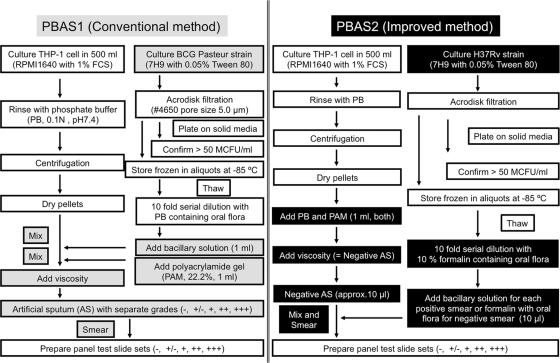
Fig. 1.
Flowchart displaying the procedures for preparing PBAS1 (Conventional method) and PBAS2 (Improved method). The gray and black boxes indicate steps specific to the PBAS1 and PBAS2 methods, respectively.
Cell culture.
THP-1 cells (TIB-20; American Type Culture Collection, Manassas, VA) were cultured from the frozen stock containing nearly 107 cells for about 2 weeks in 175-cm2 flasks (Nunc; catalog no. 156449) containing 250 ml RPMI 1640 (Gibco; catalog no. 11875-093) supplemented with 1% fetal bovine serum (Gibco; catalog no. 10437085) and 5 ml of penicillin-streptomycin (Gibco; catalog no. 15140-122) at 37°C in 5% CO2. The subculture started with adding 5 to 10 ml of the cell suspension containing more than 1 million cells per ml to the new culture medium described above. Cultures of five flasks were prepared to obtain a total of 4 ml of negative sputum.
Preparation of polyacrylamide.
A total of 22.2 g of acrylamide and 0.6 g of N,N′-methylenebisacrylamide were dissolved in 80 ml of distilled water (DW). After complete dissolution, DW was added to give a volume of 100 ml, and the solution was stored at 4°C as a stock. For polyacrylamide preparation, 1.75 ml of the stock acrylamide solution described above, 6.25 ml DW, 2.0 ml of 5× Tris-borate-EDTA (TBE) buffer, and 100 μl of freshly prepared 10% ammonium persulfate were mixed in 15 ml polypropylene centrifuge tubes. Just before addition to the cell pellet, 8 μl of tetramethylethylenediamine (TEMED) was added to the mixture described above, mixed well, and placed on ice. DW and 5× TBE solutions were filtered with nylon filters with a pore size of 0.2 μm (catalog no. 17845; Sartorius, Germany).
Preparation of negative PBAS.
THP-1 cells were cultured as described above. After being cultured for about 2 weeks, full-grown cell cultures from five 175-cm2 flasks were collected into 50-ml polypropylene centrifuge tubes, centrifuged at 1,200 rpm for 10 min, and finally collected into two tubes. Then, the cells were rinsed with 0.2-μm-filter-passed 0.1 N phosphate buffer (pH 7.4) (PB) three times. During the final centrifugation, polyacrylamide was prepared as described above. After the final rinse, the supernatants were discarded completely, and 1 ml of PB was added to the pellet and mixed well by pipetting, followed by the addition of 1 ml of polyacrylamide solution, to which TEMED had just been added.
Smear preparation.
As shown in Fig. S1 in the supplemental material, positive smears were prepared by placing 10 μl of the H37Rv bacillary solution diluted to different grades (as described above) and negative PBAS on a glass slide. Negative panel test smears were prepared by replacement of the H37Rv bacillary solution with a formalin solution containing only oral flora and P. aeruginosa. Then, the solution and the negative PBAS were mixed well and spread in a 2-cm by 3-cm ellipse. The smears were then subjected to Ziehl-Neelsen staining and microscopic examination.
Confirmation of consistency by PBAS2 smears.
In order to confirm the consistency of the positive grades, 8 smears (2- by 3-cm ellipses) were prepared using the PBAS2 procedure for each positive grade, and 300 fields per smear were examined, counting the bacilli, with blind evaluations performed by three different individuals. The data were expressed as means and standard deviations (SD).
RESULTS
We previoulsy developed and reported PBAS1, which consisted of the cultured BCG-Pasteur strain, THP-1 cells, and polyacrylamide (14, 15). The PBAS1 procedure can be easily performed, with definite positive grades, and can provide valuable panel test slide sets for training of tuberculosis sputum smear microscopists. Here, we report an improved preparation method we have named the PBAS2 method, in order to enhance the simplicity of preparation and the authenticity of the appearance of the bacilli and to expand availability. The improved protocol consists of preparation of PBAS2 smear panel test slides with five grades, including a negative grade, by mixing and smearing negative PBAS with graded positive H37Rv bacillary solutions, with dilution solutions for positive smears or with dilution solution alone for negative smears.
There are two major differences between these two methods in preparation and in the resulting smears. During the preparation procedure for PBAS1, the five grades, including a negative grade, were prepared individually by mixing cell pellets with bacillary solution or PB, respectively, before adding polyacrylamide, followed by polymerization. In contrast, in the PBAS2 procedure, only the negative PBAS is prepared because bacillary solutions for each positive grade are prepared separately, added, and mixed on the slide glass with the negative PBAS, making it possible to adjust the number of bacilli precisely by changing the volume of FFTBS. A comparison of PBAS1 and PBAS2 with regard to the artificial-smear preparation procedure is shown in Fig. 1. Actual manipulations for smear preparation are shown in Fig. S1 in the supplemental material.
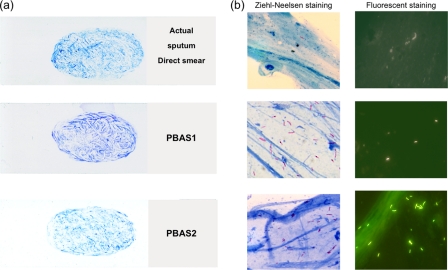
The artificial sputum smears prepared by both methods and an actual sputum smear are shown in Fig. 2. There are no apparent differences in macroscopic appearance among the smears. This result means that it is impossible to distinguish PBAS smears from smears prepared from actual sputum. However, microscopically, the total appearance and stainability of bacteria in the positive smears generated using the PBAS2 method are equivalent to those of the actual sputum smears, because FFTBS containing formalin-fixed tubercle bacilli with oral flora and P. aeruginosa was used. On the other hand, in smears prepared from PBAS1, the unfixed BCG-Pasteur strain demonstrated lower stainability and was shorter than the actual tubercle bacilli, and therefore, they had less authenticity in the absence of supplemented non-acid-fast bacilli compared to actual sputum smears (Fig. 3).
Fig. 2.
Macroscopic and microscopic appearance of PBAS and smears. (a) Macroscopic appearance of smears made from actual sputum (top), PBAS1 (middle), and PBAS2 (bottom). (b) Microscopic appearance of an actual sputum smear (top), PBAS1 (middle), and PBAS2 (bottom) with Ziehl-Neelsen staining (left) and auramine staining (right).
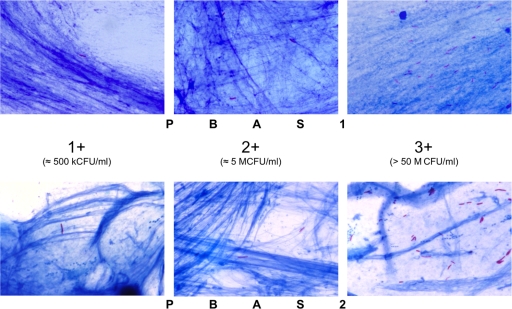
Fig. 3.
Comparative microscopic appearances showing the different positivity grades. An image for the ± grade is not shown, because it cannot be distinguished from the + grade in a pair of single pictures. In PBAS2, bacilli are stained dark and are more clearly distinguished than in the PBAS1 smears.
In our experience while preparing PBAS1 and PBAS2, THP-1 cells appeared in the smears made from freshly prepared PBAS, which had been left for several days after preparation to increase viscosity. However, it was revealed that the cells were lysed when they were mixed with polyacrylamide during storage, and only the DNA fibers derived from the cells could be observed in the smears prepared from PBAS.
PBAS2 provided a much more accurate number of bacilli in the smears than PBAS1. This was because the number of bacilli can be easily controlled by adjusting the volume of FFTBS applied, independent of the volume of viscous artificial sputum to be placed, which provides a number as close as possible to the median value of the grade that is needed through several trial smear preparations made by varying the volume of FFTBS. The results of observations of panel test slides prepared with negative PBAS2 and 10 μl of each positive grade of bacillary solution are shown in Table 1 and support the consistency of the grades in the smears from the improved method. Because it is impossible to pick up the same volume of viscous PBAS1 with premixed AFB for each smear, less consistency in terms of positivity could be obtained than for PBAS2. As shown in Table 1, positive smears prepared with PBAS2 provided high consistency of AFB content in each grade in both 100- and 300-field examinations.
Table 1.
Consistency in PBAS2 smear slides within each grade
| Grade | No. of fields | No. of AFB per smeara |
Avg no. of AFB ± SDb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1e | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| ± | 1–100 | 2 | 2 | 2 | 2 | 3 | 2 | 1 | 3 | 2.1 ± 0.8 |
| 101–200 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | ||
| 201–300 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 4 | ||
| Total | 5 | 6 | 5 | 6 | 8 | 6 | 6 | 9 | 6.4 ± 1.4 | |
| + | 1–100 | 22 | 25 | 25 | 26 | 23 | 27 | 19 | 18 | 24.9 ± 4.1 |
| 101–200 | 22 | 20 | 29 | 29 | 30 | 28 | 19 | 22 | ||
| 201–300 | 24 | 23 | 29 | 27 | 28 | 33 | 21 | 29 | ||
| Total | 68 | 68 | 83 | 82 | 81 | 88 | 59 | 69 | 74.6 ± 10.0 | |
| ++c | 1–100 | 175 (63) | 283 (51) | 197 (65) | 177 (65) | 206 (65) | 232 (66) | 263 (66) | 310 (71) | 235.0 ± 48.9 |
| 101–200 | 156 (57) | 330 (72) | 229 (65) | 183 (65) | 245 (66) | 236 (67) | 296 (66) | 289 (70) | ||
| 201–300 | 172 (64) | 299 (59) | 202 (67) | 191 (65) | 220 (64) | 238 (66) | 257 (66) | 254 (69) | ||
| Total | 503 (184) | 912 (182) | 628 (197) | 551 (195) | 671 (195) | 706 (199) | 816 (198) | 853 (210) | 705.0 ± 145.7 | |
| +++d | 1–100 | 736 [33] | 671 [25] | 615 [27] | 589 [28] | 686 [31] | 1,171 [54] | 581 [28] | 632 [26] | 711.1 ± 158.3 |
| 101–200 | 770 [30] | 621 [23] | 606 [27] | 557 [29] | 760 [33] | 1,051 [48] | 646 [30] | 631 [28] | ||
| 201–300 | 765 [34] | 635 [25] | 611 [28] | 673 [27] | 625 [25] | 1,034 [49] | 637 [33] | 763 [36] | ||
| Total | 2,271 [97] | 1,927 [73] | 1,832 [82] | 1,819 [84] | 2,071 [89] | 3,256 [151] | 1,874 [91] | 2,026 [90] | 2,133.3 ± 478.0 | |
Eight smears (2-by 3-cm ellipses) were examined independently by blind evaluation by three microscopists for 100 fields, and the raw data on bacilli enumerated in 100 (italic figures) and 300 (values in total rows) fields are shown; the latter were derived by adding the values obtained from three 100-field examinations.
The average numbers ± SD were calculated in each grade for 100 (italic figures) and 300 (values in total rows) fields of the eight smears.
The figures in parentheses indicate the number of fields containing at least 1 AFB per 100 (italic figures) or 300 (values in total rows) fields in each smear.
The figures in brackets indicate the number of fields containing 10 or more AFB per 100 (italic figures) or 300 (values in total rows) fields in each smear.
Smear number.
In detail, in ± smears, the numbers of AFB ranged from 1 to 4 and from 5 to 9 per 100 and 300 fields, respectively. The averages were 2.1 ± 0.8 and 6.4 ± 1.4 AFB per 100 and 300 fields, respectively. Likewise, in + smears, the counts ranged from 18 to 33 and from 59 to 88 per 100 and 300 fields, respectively, with an average of 24.9 ± 4.1 and 74.6 ± 10.0 AFB per 100 and 300 fields, respectively. In ++ smears, the counts ranged from 156 to 330 and from 503 to 912 per 100 and 300 fields, respectively. The average was 235.0 ± 48.9 and 705.0 ± 145.7 AFB per 100 and 300 fields. Finally, in +++ smears, the counts ranged from 557 to 1,171 and from 1,819 to 3,256 per 100 and 300 fields, respectively, with averages of 711.1 ± 158.3 and 2,133.3 ± 478.0 AFB per 100 and 300 fields. In addition, it was revealed that more than 50 fields had at least 1 AFB in each ++ smear and that more than 20 fields had 10 or more AFB in each +++ smear (Table 1), conforming to the International Union Against Tuberculosis and Lung Disease (IUATLD) scale.
DISCUSSION
Our original panel test slide sets using PBAS1 can be prepared using a simple procedure with cultured THP-1 cells, BCG-Pasteur bacilli, and polyacrylamide (14, 15). In this report, we provided evidence for the improved PBAS2, which overcame the disadvantages of using PBAS1, including the difficulty in fine adjustment of the AFB content in the positive smears because of its viscosity and an artificial impression derived from unfixed BCG-Pasteur bacilli, which are shorter than tubercle bacilli. Using PBAS2, it is feasible to adjust the final number of bacilli by changing the volume of the bacillary solution that is mixed and smeared with viscous negative PBAS on the slides. Because smears of all grades are prepared with the same negative PBAS, the differences in the thickness of the smears between grades can be minimized, thereby minimizing background differences. Furthermore, PBAS2 uses the H37Rv strain for preparation of the bacillary solution by fixation with formalin containing oral flora and P. aeruginosa, so that the smears made using the new method provide a more realistic impression of the appearance of actual patient slides, as well as the distinct appearance and excellent stainability of the bacteria (Fig. 3).
Another benefit obtained using the improved PBAS2 procedure is that only the negative PBAS has to be prepared. This means that all parts of the cell culture can be used for the preparation of the negative artificial sputum. Through our experiences with the PBAS1 method, it was revealed that better PBAS could be prepared with proper viscosity when more cell components are used. In addition, PBAS2 uses RPMI 1640 containing 1% fetal calf serum (FCS) rather than 10% FCS, which is the usual concentration for general cell culture and which is used to prepare samples by the PBAS1 method, as well; this has greatly reduced the preparation cost, because FCS is the most expensive reagent used in the preparation of PBAS (14, 15). The total cost for 20 sets of the panel test slides, which are prepared from 4 ml PBAS2, can be set below $150, including all reagents necessary for cell culture and polyacrylamide preparation and the glass slides (below $7.50 for a single set), whereas PBAS1 costs more than $350 for 20 sets. In addition, if the smears are mounted with a cover glass, such sets can be used repeatedly. These cost reductions may enhance the further availability of our PBAS.
With regard to maintaining consistent positivity, we examined the positivity grades of panel test smears prepared with PBAS2 (Table 1). The results demonstrated that the PBAS2 panel test slides provide a precise number of bacilli in each positive grade. Although the results had values a bit higher than the median for each grade compared to the results obtained from PBAS1 (14), it is possible to adjust these values by changing the volume of FFTBS placed on the slide glass before mixing with the negative PBAS.
On the other hand, Toman described the relationship between bacillary counts and the number of microscopic fields observed, and the positivity grades are defined based on the assumption that 1% of the total smear area is examined for each smear (12). Therefore, it is important to pick up 10 μl of a sputum specimen in as exact a volume as possible; to smear a 1- by 2-cm or 2- by 3-cm ellipse precisely; and to examine 1% of the entire smear area, 100 or 300 fields, respectively. In this respect, our PBAS2 smear preparation procedure may contribute to training microscopists to pick up exactly 10 μl sputum. It is also important to confirm that the volume of the sputum specimen, the smear area, and the proportion of the examined area to the entire smear area are consistent in order to decrease variation and to ensure the accurate reporting of positivity. However, because microscopes have evolved since Toman published the document and microscopes equipped with eyepieces that have a field number of 26, which is 1.6 times larger than Toman's calculation (approximately 16), are now commercially available (7), the number of fields examined to cover 1% of the smear area may be reduced using the most recent microscopes. Nevertheless, as shown in Table 1, our PBAS2 can satisfy the criteria for both 100- and 300-field examinations in the number of AFB enumerated for all grades and the number of fields that contain at least 1 AFB and more than 10 AFB in the ++ and +++ grades, respectively.
In conclusion, although the detection of TB cases by smear microscopy requires >10 kCFU/ml sputum, the rapidity and cost-effectiveness of this method are still excellent. Our PBAS2 has evolved in several respects, including further cost reduction in preparation, and it has also been simplified to allow easy preparation, so it can be utilized in many laboratories. Therefore, PBAS2 can play an important role in the training of staff in the entire course of smear microscopy, especially in developing countries with high TB burdens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ida Surjanti and Kasiama Desy for their valuable assistance in preparing the panel test slide sets and blind examination of the slides.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Aziz M. A., et al. 2002. External quality assessment for AFB smear microscopy, p. 1–111 Association for Public Health Laboratories, Washington, DC [Google Scholar]
- 2. Boehme C. C., et al. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Kantor I. N., et al. 1998. Laboratory services in tuberculosis control. Organization and management. Part I, p. 42–43 WHO, Geneva, Switzerland [Google Scholar]
- 4. Lange C., Mori T. 2010. Advances in the diagnosis of tuberculosis. Respirology 15:220–240 [DOI] [PubMed] [Google Scholar]
- 5. Martinez-Guarneros A., et al. 2003. Implementation of proficiency testing in conjunction with a rechecking system for external quality assurance in tuberculosis laboratories in Mexico. Int. J. Tuberc. Lung Dis. 7:516–521 [PubMed] [Google Scholar]
- 6. Nguyen T. N., et al. 1999. Quality control of smear microscopy for acid-fast bacilli: the case for blinded re-reading. Int. J. Tuberc. Lung Dis. 3:55–61 [PubMed] [Google Scholar]
- 7. Olympus Microscope Resource Center 2010. Basic concepts in optical microscopy, eyepieces (oculars), http://www.olympusmicro.com/primer/anatomy/oculars.html
- 8. Pandey B. D., et al. 2008. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. J. Med. Microbiol. 57:439–443 [DOI] [PubMed] [Google Scholar]
- 9. Tanaka H., Hirose H., Kato Y., Kida S., Miyajima E. 2010. Clinical evaluation of TRCRapid M.TB for detection of Mycobacterium tuberculosis complex in respiratory and nonrespiratory specimens. J. Clin. Microbiol. 48:1536–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tuberculosis Division of the International Union Against Tuberculosis and Lung Disease 2005. Tuberculosis bacteriology-priorities and indications in high prevalence countries: position of the technical staff of the Tuberculosis Division of the International Union Against Tuberculosis and Lung Disease. Int. J. Tuberc. Lung Dis. 9:355–361 [PubMed] [Google Scholar]
- 11. Wallis R. S., et al. 2010. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375:1920–1937 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization 2004. Toman's tuberculosis: case detection, treatment, and monitoring. Questions and answers, 2nd ed, p. 11–13 WHO, Geneva, Switzerland [Google Scholar]
- 13. World Health Organization 2010. Global tuberculosis control 2010. http://www.who.int/tb/publications/global_report/2010/en/index.html
- 14. Yamada H., Mitarai S., Aguiman L., Matsumoto H., Fujiki A. 2006. Preparation of mycobacteria-containing artificial sputum for TB panel testing and microscopy of sputum smears. Int. J. Tuberc. Lung Dis. 10:899–905 [PubMed] [Google Scholar]
- 15. Yamada H., Matsumoto H., Mitarai S., Fujiki A. 2008. Stability for long-term storage and reproducibility of positivity in the panel test slide prepared with the polyacrylamide-based artificial sputum. Kekkaku 83:65–71 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.