Abstract
Brachyspira pilosicoli is an etiological agent of human intestinal spirochetosis. Bloodstream infection due to this microorganism is rare. We report a case of B. pilosicoli bacteremia in a 70-year-old patient who presented with multiorgan failure.
CASE REPORT
A 70-year-old Spanish man with a history of high blood pressure, concentric hypertrophy of the left ventricle, and chronic obstructive pulmonary disease (COPD) with emphysema was referred from a regional hospital to the intensive care unit (ICU) at our center for severe dyspnea. He arrived intubated and oxygen saturation was 75%. On examination he presented with multiorgan failure, hypothermia, tachycardia, and oliguria. Laboratory tests revealed a leukocyte count of 16.5 × 109/liter with 93.7% polymorphonuclear (PMN) cells. A chest X ray showed diffuse emphysema and an upper lobe image suggesting old tuberculosis. Blood and urine specimens for culture were collected on arrival. As respiratory infection was suspected to have exacerbated his COPD, empirical treatment with amoxicillin-clavulanic acid was started. Stool cultures were not performed as the patient had no previous or present signs of gastrointestinal disorders. Initial urine culture was negative. Culture of the bronchoalveolar lavage (BAL) fluid specimen collected on the second day revealed nonsignificant counts of Staphylococcus aureus and scarce colonies of Aspergillus fumigatus, initially considered colonization of the respiratory tract. The patient was hemodynamically unstable for several days due to persistence of rapid atrial fibrillation. Noradrenalin was administered for 3 days, and continuous hemodiafiltration was required. As the patient's condition remained critical, empirical amoxicillin-clavulanic was changed to voriconazole and cloxacillin on the fifth day and continued for 10 days.
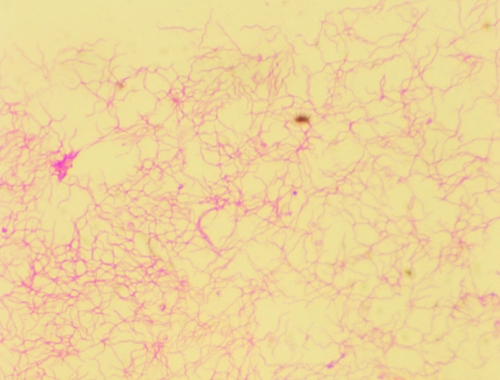
The anaerobic bottle (SN anaerobic medium) from the blood culture set taken on arrival was positive after 6.5 days of incubation in a BacT/Alert automated system (bioMérieux). Gram staining revealed Gram-negative, spiral-shaped bacteria, initially suggesting either a member of the Campylobacter genus or a spirochete (Fig. 1). A subculture of the isolate on Schaedler agar plates (bioMérieux) supplemented with sheep blood (5%) at 37°C yielded growth as a thin, smooth, and beta-hemolytic film under anaerobic conditions only. Catalase, oxidase, and indole test results were negative while the result for hippurate hydrolysis was positive. The isolate was identified as Brachyspira pilosicoli by 16S rRNA gene sequencing and compared to sequences available from GenBank using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The nucleotide sequence of the isolate displayed 100% identity to B. pilosicoli strains ATCC 51139T and P43/6/78 (GenBank accession numbers AY155458 and U14927, respectively).
Fig. 1.
Gram stain of the isolate growing on Schaedler agar.
Antibiotic susceptibility of the strain was studied by Etest according to the manufacturer's instructions for anaerobes (AB Biodisk, Sweden). Susceptibility assays were performed on Schaedler agar plates and incubated at 37°C under anaerobic conditions for 3 days. The moxifloxacin MIC (2 μg/ml) was the highest displayed by the strain. MICs of amoxicillin-clavulanic acid, meropenem, ceftriaxone, and metronidazole were 0.032 μg/ml, 0.032 μg/ml, 0.125 μg/ml, and <0.016 μg/ml, respectively. Etests for ampicillin, penicillin, and piperacillin all showed a MIC of <0.016 μg/ml. β-Lactamase production was negative according to results from a BBL Cefinase nitrocefin disk (Becton Dickinson).
After 40 days in the ICU, the patient was transferred to the respiratory unit. He presented no other respiratory symptoms during his stay and was discharged 2 weeks later.
Although anaerobic spirochetes from the genus Brachyspira (derived from the unification with the former Serpulina genus) colonize the intestinal tracts of animals and humans (12, 13), blood infection is rarely described. Two species of these slow-growing, fastidious bacteria have been found in humans. Brachyspira aalborgi had been detected only in humans until very recently, while B. pilosicoli has a wide host range (mainly swine and poultry) (11, 13). Both species are the etiologic agents of human intestinal spirochetosis (HIS) (13), which is defined histologically by the presence of spirochetes forming a dense fringe on the colonic epithelium (8, 17). Colonization of the large intestine is usually followed by the attachment of spirochetes to the epithelial apical membrane, but penetration and cell damage are uncommon (5, 17). The clinical significance of HIS is still controversial (6, 15), since most cases are asymptomatic, though some individuals may present abdominal cramps, diarrhea, and rectal bleeding (15, 17).
Water supplies have been suggested as the possible means of transmission and are considered to play a role in the high prevalence of human fecal carriers in certain regions of the world (10, 16). While HIS is quite common in developing areas, in western countries it is mainly seen in men who have sex with men (13), HIV-infected individuals, and certain populations, such as Australian aborigines (3). HIS seems to be relatively uncommon in our area (Catalonia, Spain), according to a prospective 10-year study using specific PCR detection on colonic biopsy specimens from patients with chronic watery diarrhea (6). No stool cultures were taken to confirm spirochete fecal carriage in our patient since he did not display diarrhea and the possibility of HIS was not suspected at any point.
Bacterial translocation from the intestine to the bloodstream occurs frequently in patients with impaired immune defense and/or injury of the gut mucosa barrier, or it may be the result of bacterial overgrowth (16). Nevertheless, spirochetemia seems to be a rather rare phenomenon even in those human populations in which fecal carriage has often been observed (3). Only nine cases of B. pilosicoli bacteremia have been reported to date in the English literature, corresponding to immunocompromised or critically ill patients (1, 7, 9, 16). The low number of reports about B. pilosicoli bloodstream infections is likely due to a low incidence of transient spirochetemia and technical problems in isolating these microorganisms (3). The main drawbacks in their recovery are the low growth rate, fastidious growth requirements, and detection difficulties with some automatic blood culture systems (3, 16). Brooke et al. (4) evaluated several blood culture systems for detection of B. pilosicoli strains and concluded that Bactec performed better than BacT/Alert. The BacT/Alert system detected B. pilosicoli growth in SN anaerobic bottles but not in Ecosorb-containing FAN anaerobic bottles. They suggested that Ecosorb may bind nutrients such as cholesterol, which is essential for growth of the closely related porcine intestinal spirochete Brachyspira hyodysenteriae (14). Brooke et al. (4) also proposed that B. pilosicoli spirochetemia should be considered in febrile patients from populations with high rates of intestinal carriage.
The patient described herein presented with multiorgan failure that was considered the result of severe decompensation of his pulmonary disease, although the etiological agent responsible for the respiratory infection remained uncertain. A positive blood culture for anaerobic spirochetes obtained after nearly 1 week of incubation was unexpected for both clinicians and microbiologists. By then, the pulmonary infection had been treated empirically with amoxicillin-clavulanic acid, which probably correctly treated transient B. pilosicoli bacteremia. There is currently little literature available on the treatment of B. pilosicoli infections, though metronidazole has been proposed for intestinal spirochetosis with persistent symptoms (5, 6, 17) and β-lactams have been used in spirochetemia (9). A high proportion of β-lactamase production has been assessed by positive nitrocefin tests in isolates from certain populations, most likely reflecting exposure to antibiotics (2). Since our strain displayed a negative nitrocefin test and showed similar MICs to penicillin, ampicillin, and β-lactam/inhibitor combinations, the possibility of β-lactamase expression was ruled out.
The clinical significance of B. pilosicoli bacteremia is difficult to determine in this case. Multiorgan shock leads to mesenteric ischemia, which may favor intestinal spirochete translocation into the bloodstream, as previously suggested in the context of cardiogenic shock (1). In conclusion, our patient was probably an unknown B. pilosicoli fecal carrier in whom transient spirochetemia was a chance finding, secondary to multiorgan failure.
Acknowledgments
This study was partially supported by the Ministry of Health and Consumer Affairs, Instituto de Salud Carlos III-Feder, Spanish Network for the Research in Infectious Diseases (REIPI/RD06/0008/0013).
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Bait-Merabet L., Thille A., Legrand P., Brun-Buisson C., Cattoir V. 2008. Brachyspira pilosicoli bloodstream infections: case report and review of literature. Ann. Clin. Microbiol. Antimicrob. 7:19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brooke C. J., Hampson D. J., Riley T. V. 2003. In vitro antimicrobial susceptibility of Brachyspira pilosicoli isolates from humans. Antimicrob. Agents Chemother. 47:2354–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brooke C. J., Hampson D. J., Riley T. V., Lum G. 2001. Failure to detect Brachyspira pilosicoli in bloodstream of Australian patients. J. Clin. Microbiol. 39:4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooke C. J., et al. 2000. Evaluation of blood culture systems for detection of the intestinal spirochaete Brachyspira (Serpulina) pilosicoli in human blood. J. Med. Microbiol. 49:1031–1036 [DOI] [PubMed] [Google Scholar]
- 5. Calderaro A., et al. 2007. Infective colitis associated with human intestinal spirochetosis. J. Gastroenterol. Hepatol. 22:1772–1779 [DOI] [PubMed] [Google Scholar]
- 6. Esteve M., et al. 2006. Intestinal spirochetosis and chronic watery diarrhoea: clinical significance and histological response to treatment and long-term follow up. J. Gastroenterol. Hepatol. 21:1326–1333 [DOI] [PubMed] [Google Scholar]
- 7. Fournié-Amazouz E., et al. 1995. Isolations of intestinal spirochaetes from the blood of human patients. J. Hosp. Infect. 30:160–162 [DOI] [PubMed] [Google Scholar]
- 8. Harland W. A., Lee F. D. 1967. Intestinal spirochetosis. Br. Med. J. 3(5567):718–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanavaki S., et al. 2002. Brachyspira (Serpulina) pilosicoli spirochetemia in an immunocompromised patient. Infection 30:175–177 [DOI] [PubMed] [Google Scholar]
- 10. Margawani K. R., Robertson I. D., Brooke C. J., Hampson D. J. 2004. Prevalence, risk factors and molecular epidemiology of Brachyspira pilosicoli in humans on the island of Bali, Indonesia. J. Med. Microbiol. 53:325–332 [DOI] [PubMed] [Google Scholar]
- 11. Munshi M. A., Taylor N. M., Mikosza A. S. J., Spencer P. B. S., Hampson D. J. 2003. Detection by PCR and isolation assays of the anaerobic intestinal spirochete Brachyspira aalborgi from the feces of captive nonhuman primates. J. Clin. Microbiol. 41:1187–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ochiai S., Adachi Y., Mori K. 1997. Unification of the genera Serpulina and Brachyspira, and proposals of Brachyspira hyodysenteriae comb. nov., Brachyspira innocens comb. nov. and Brachyspira pilosicoli comb. nov. Microbiol. Immunol. 41:445–452 [DOI] [PubMed] [Google Scholar]
- 13. Smith J. L. 2005. Colonic spirochetosis in animals and humans. J. Foot. Prot. 68:1525–1534 [DOI] [PubMed] [Google Scholar]
- 14. Stanton T. B., Cornell C. P. 1987. Erythrocytes as a source of essential lipids for Treponema hyodysenteriae. Infect. Immun. 55:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanahashi J., et al. 2008. Human intestinal spirochetosis in Japan; its incidence, clinicopathologic features and genotypic identification. Mod. Pathol. 21:76–84 [DOI] [PubMed] [Google Scholar]
- 16. Trott D. J., et al. 1997. Identification and characterization of Serpulina pilosicoli isolates recovered from the blood of critically ill patients. J. Clin. Microbiol. 35:482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsinganou E., Gebbers J. O. 2010. Human intestinal spirochetosis—a review. GMS Ger. Med. Sci. 8:Doc01 doi:10.3205/000090 [DOI] [PMC free article] [PubMed] [Google Scholar]