Abstract
Accurate HIV-2 plasma viral load quantification is crucial for adequate HIV-2 patient management and for the proper conduct of clinical trials and international cohort collaborations. This study compared the homogeneity of HIV-2 RNA quantification when using HIV-2 assays from ACHIEV2E study sites and either in-house PCR calibration standards or common viral load standards supplied to all collaborators. Each of the 12 participating laboratories quantified blinded HIV-2 samples, using its own HIV-2 viral load assay and standard as well as centrally validated and distributed common HIV-2 group A and B standards (http://www.hiv.lanl.gov/content/sequence/HelpDocs/subtypes-more.html). Aliquots of HIV-2 group A and B strains, each at 2 theoretical concentrations (2.7 and 3.7 log10 copies/ml), were tested. Intralaboratory, interlaboratory, and overall variances of quantification results obtained with both standards were compared using F tests. For HIV-2 group A quantifications, overall and interlaboratory and/or intralaboratory variances were significantly lower when using the common standard than when using in-house standards at the concentration levels of 2.7 log10 copies/ml and 3.7 log10 copies/ml, respectively. For HIV-2 group B, a high heterogeneity was observed and the variances did not differ according to the type of standard used. In this international collaboration, the use of a common standard improved the homogeneity of HIV-2 group A RNA quantification only. The diversity of HIV-2 group B, particularly in PCR primer-binding regions, may explain the heterogeneity in quantification of this strain. Development of a validated HIV-2 viral load assay that accurately quantifies distinct circulating strains is needed.
INTRODUCTION
The global prevalence of HIV-2 is not well documented, but 1 million to 2 million persons are estimated to be infected (1, 17). HIV-2 is endemic in West Africa, with limited spread to other locales (12, 15, 20, 22, 23, 28–31). Evidence-based management of HIV-2 infection has been hampered by a lack of validated commercially available HIV-2-specific assays for the quantification of HIV-2 viral loads. A limited number of international HIV laboratories use in-house HIV-2 viral load assays (9, 11, 24, 25). In the setting of an international collaboration on HIV-2 infection (the ACHIEV2E collaboration), we previously showed that the results of quantification of the HIV-2 plasma viral RNA load varied considerably between eight participating European laboratories and one African HIV-2 reference laboratory, as only two laboratories were able to yield accurate and reproducible measurements (8). Such heterogeneity in HIV-2 viral load quantification is a considerable issue. At the patient level, difficulties arise in the interpretation of results and may yield inappropriate clinical decisions; at the population level, this may help explain the varied levels of reported response to therapy among different HIV-2-infected cohorts (2, 3, 16, 19, 20, 26). On the basis of our initial results from the first assessment of HIV-2 quantification from collaborating ACHIEV2E laboratories, we hypothesized that the use of a centrally validated and distributed common HIV-2 PCR calibration standard by each participating laboratory would improve the accuracy of HIV-2 RNA viral load measurements.
MATERIALS AND METHODS
The objectives of this second-round validation study of HIV-2 group A and B plasma RNA quantification assays, performed in 12 international laboratories from 11 countries (Table 1), were to compare the overall homogeneity of quantification results and the accuracy and reproducibility of each quantification assay obtained with the HIV-2 calibration standard routinely used in each laboratory and those obtained with a common HIV-2 standard. Four more laboratories have joined the ACHIEV2E study group since the first round in 2006. The 12 virology laboratories which participated in this second round of HIV-2 quality control were from Portugal (n = 2) and Belgium, Canada, France, the Gambia, Germany, Italy, Spain, Switzerland, the United Kingdom, and the United States (one each). The number of HIV-2 plasma viral load assays required in the laboratories participating in this quality control study ranged from 100 to 2,000 per year.
Table 1.
Characteristics of the different quantification assays assessed in the ACHIEV2E collaboration, 2009
| Laboratory | Amplification systema | Primer and probe localization | RNA extraction apparatus | Standard used | Threshold (log10 copies/ml) | Plasma vol required (μl) | HIV-2 group(s) detectable |
|---|---|---|---|---|---|---|---|
| Belgium | LightCycler 2.0 | LTR region (SYBR green detection) | Nuclisens-MiniMag | External, synthetic RNA (ROD sequence) | 1.7 | 1,000 | A, B |
| Canada | Rotor GeneCobert thermocycler | gag gene | QIAamp viral RNA | Internal control | 2.5 | 140 | A, B |
| France | LightCycler | gag gene | Magnapure | External, NIHZ quantified by electron microscopy | 2.0 | 1,000 | A, B, H |
| Gambia | In-house PCR + ELONA | LTR | Boom | CBL23 + internal control | 2.0 | 1,000 | A, B |
| Germany | LightCycler | gag gene | Qiagen, viral RNA | External, NIHZ quantified by electron microscopy | 2.7 | 200 | A, B |
| Italy (Milan) | In-house QRT-PCR | gag gene | Qiagen, viral RNA | Internal control | 2.0 | 1,000 | A |
| Portugal (Coimbra) | LightCycler | gag gene | Qiagen viral RNA | External, NIHZ quantified by electron microscopy | 2.0 | 1,000 | A, B |
| Portugal (Lisboa) | ABI Prism 7000 | LTR | easyMag bioMérieux | External, NIHZ quantified by electron microscopy and brome mosaic virus internal | 2.0 | 1,000 | A, B |
| Spain | Nuclisens EasyQ, version 1.1 | gag gene | Nuclisens | Internal | 2.3 | 1,000 | A, B |
| Switzerland | ABI 7900 HT | gag leader | HIV Monitor | External, ST isolate, only relative quantification | 2.0 | 500 | A, B |
| United Kingdom | ABI Prism 7000 | LTR | Qiagen viral RNA | CBL22 external and brome mosaic virus internal | 2.0 | 200 | A, B |
| United States | ABI 7900 HT | LTR | Qiagen viral RNA | External, NIHZ quantified by electron microscopy | 1.0 | 1,000 | A, B |
ELONA, enzyme-linked oligonucleotide assay; QRT-PCR, quantitative reverse transcription-PCR.
Preparation of the panel.
The sample panel was prepared in a single virology laboratory at Bichat Claude Bernard Hospital (Paris, France) by performing serial dilution in EDTA HIV-negative human plasma of two supernatants originating from a coculture of patient HIV-2 isolates: one HIV-2 group A isolate (GenBank accession number AY688870) and one HIV-2 group B isolate (GenBank accession number AY688889). Those isolate solutions were diluted to obtain aliquots with theoretical concentrations of 2.7 and 3.7 log10 copies/ml.
Preparation of the standard.
The common standard for the group A strain was prepared using stocks of HIV-2 NIHZ counted by electron microscopy (EM) and commercialized by ABI Technologies (catalog number 10-127-000; Advanced Biotechnologies Inc., Columbia, MD). As there is no commercially available group B standard, the common standard for the HIV-2 group B strain (GenBank accession number AY688915) was prepared using a stock of a clinical isolate which had been sent for electron microscopy quantification by the Bichat Claude Bernard Hospital laboratory to ABI Technologies. The theoretical concentrations obtained for the two common standards were 200,000 copies/ml for the group A supernatant and 400,000 copies/ml for the group B supernatant.
Participating laboratories of the ACHIEV2E collaboration are listed in Acknowledgments. Eight of these laboratories took part in the first-round quality control study (8). Four additional laboratories (from Canada, the United States, Portugal, and Italy) took part in the second study.
Each of the 12 participating laboratories had to complete one run (20 aliquots of 1 ml each) of quantification using its own standard and one run (20 aliquots of 1 ml each, at the same theoretical levels) of quantification using the common HIV-2 group A and B standards. Each panel of 20 aliquots was constituted as follows: 10 aliquots of group A (5 at 2.7 log10 copies/ml and 5 at 3.7 log10 copies/ml) and 10 aliquots of group B (5 at 2.7 log10 copies/ml and 5 at 3.7 log10 copies/ml).
Coded aliquots were frozen at −80°C and were sent to the participating laboratories, which were blinded to the HIV-2 concentration and HIV-2 group. The distribution of aliquots was performed by an accredited transporter according to the regulatory standards for the distribution of infected samples.
The types of the different assays used by the laboratories are listed briefly in Table 1, and the locations of the different primers and probes mapped to ROD are shown in Fig. 1. All of the assays were based on a PCR method; thus, the sequences of the primers and the sites of primer binding in the target virus are also listed. The number of mismatches between the primers and test strain target sequence, the in-house standards, and the common standard is listed in Table 2.
Fig. 1.
ACHIEV2E primers and probes mapped to the M15390 HIV-2 ROD genome. The figure depicts the relevant portion of the HIV-2 ROD genome (GenBank accession number M15390) with nucleotide positions indicated. The nucleotide positions of all primers and their binding orientation (>>>, forward strand; <<<, reverse strand) are indicated. Note that the group B primers used by the Swiss group (HIV-2TMFPRB, HIV-2TMRPRB, TMPROBEB) are not shown but map to the comparable positions in HIV-2 group viruses as TMFPR1, TMRPR1, and TMPROBE1, respectively. Some of the LTR-targeted primers have a second target site in the 3′ LTR.
Table 2.
Primer and probe sequences used by the different laboratories and number of mismatches within the ACHIEV2E HIV-2 group A and group B standards and samples
| Region and primer or probe | Sequence | No. of hits in 25 HIV-2 genomesa | No. of mismatches |
|||
|---|---|---|---|---|---|---|
| HIV-2 sample A | HIV-2 sample B | HIV-2 standard A | HIV-2 standard B | |||
| LTR | ||||||
| Belgium For | TCGCCGCCTGGTCATTC | 22 | 1 | 2 | 1 | 0 |
| Belgium Rev | GCCGCCCTTACTGCCTTCA | 24 | 1 | 1 | 0 | 0 |
| Gambia For | ATTGAGCCCTGGGAGGTTCTCTCCA | 22 | —b | — | — | — |
| Gambia Rev | TTCGGGCGCCAACCTGCTAGGGATTTT | 23 | 1 | 0 | 0 | 0 |
| Switzerland TMFPR1 | AACAAACCACGACGGAGTGC | 17 | 0 | 0 | ||
| Switzerland TMRPR1 | CCACACGCTGCCTTTGGTA | 11 | 1 | 1 | ||
| Switzerland TMPROBE1 | TCGGCCCGCGCITTTCTAGG | 13 | 1 | 0 | ||
| Switzerland TMFPRB | AATCAACCACGACGGAGAGC | 10 | 0 | 0 | ||
| Switzerland TMRPRB | CTCCTCACGCTGCCTGGT | 11 | 0 | 0 | ||
| Switzerland TMPROBEB | CCGGCCTGCGCTTTTACAGG | 9 | 1 | 0 | ||
| UK HIV-2 For | GCAGGTAGAGCCTGGGTGTTC | 23 | — | — | — | — |
| UK HIV-2 Rev | CTTGCTTCTAAYTGGCAGCTTTATT | 18 | 3 | 2 | 0 | 1 |
| UK HIV-2 probe | TGGGCAGAYGGCTCCACGC | 22 | 0 | 1 | 0 | 0 |
| USA For | GCGGAGAGGCTGGCAGAT | 22 | — | — | — | — |
| USA Rev | GAACACCCAGGCTCTACCTGCTA | 22 | — | — | — | — |
| USA probe | AGAGAACCTCCCAGG | 22 | — | — | — | — |
| gag | ||||||
| France F3 For | GCGCGAGAAACTCCGTCTTG | 21 | 2 | 2 | ||
| France L140 Rev | TCCAACAGGCTCTCTGCTAATCC | 24 | 3 | 0 | 0 | 0 |
| France probe | TAGGTTACGGCCCGGCGGAAAGA | 25 | 1 | 0 | 0 | 1 |
| France R1 Rev | AACATATTGTGTGGGCAGCGAA | 15 | 0 | 4 | 0 | 6 |
Number of primer targets with 2 mismatches or less in 25 full HIV-2 genomes listed in GenBank. Only mismatches in the first half of the genome are included.
—, target outside sequenced region.
Comparisons of homogeneity of HIV-2 quantifications obtained with the in-house standards (Table 3) and with the common HIV-2 group A and B standards were conducted on the quantification results at the theoretical concentration levels of 2.7 log10 copies/ml and 3.7 log10 copies/ml. For each of these, three types of variance were estimated and compared: the intralaboratory variance, the interlaboratory variance, and the overall variance (intralaboratory plus interlaboratory). Variances were compared using the F test based on the Snedecor-Fisher distribution.
Table 3.
Comparison of interlaboratory variabilities for HIV-2 group A and B RNA quantifications
| Group and RNA load (log10 copies/ml) | Variance |
Variance ratio | F value | Statistical significance (P value) | |
|---|---|---|---|---|---|
| In-house standards | Common standard | ||||
| Group A | |||||
| 2.7 | 2.72 | 0.54 | 5.0 | 4.39 | 0.04 |
| 3.7 | 1.58 | 0.95 | 1.7 | 2.85 | 0.21 |
| Group B | |||||
| 2.7 | 1.80 | 1.68 | 1.1 | 3.87 | 0.46 |
| 3.7 | 2.24 | 2.45 | 0.92 | 3.18 | 0.55 |
The accuracy of each quantification assay was estimated separately for HIV-2 group A and B strains at both theoretical concentration levels of 2.7 log10 and 3.7 log10 copies/ml. A quantification assay was defined as accurate if 5/5 of the measurements fell within an expected interval, considered to be clinically acceptable and defined as [(observed median viral load/3) − (observed median viral load × 3)]. Only the data from laboratories which were able to successfully detect and quantify the samples were used to calculate the observed median viral load.
The reproducibility of quantification assays was estimated separately for HIV-2 groups A and B at both theoretical concentration levels of 2.7 log10 copies/ml and 3.7 log10 copies/ml. The reproducibility of the assays was evaluated using the coefficient of variation (CV) and the intralaboratory coherence coefficient (ILCC).
RESULTS
HIV-2 group A quantification results.
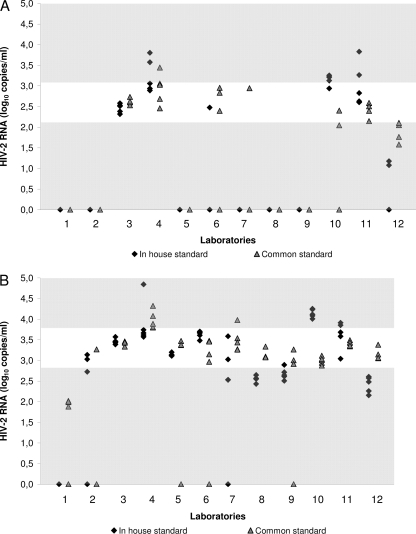
At the theoretical level of 2.7 log10 copies/ml, the observed median viral load was 2.6 log10 copies/ml. The accuracy interval was 2.1 to 3.1 log10 copies/ml. Five laboratories reported undetectable RNA values for at least 9 out of 10 aliquots whatever standard was used. Moreover, only 23% and 32% of the quantification results fell within the accuracy interval with the in-house standards and the common standard, respectively (P = 0.31) (Fig. 2A). Interlaboratory variance was 5 times lower when using the common standard (P = 0.04) (Table 3). Intralaboratory variability was not significantly lower when using the common standard (P = 0.19), but the overall variability (intralaboratory plus interlaboratory) was higher with the in-house standards than with the common standard (P = 0.0001). The coefficients of variation for reproducibility varied from 4.0% to 17.1% for the in-house standards and from 2.8% to 12.9% for the common standard.
Fig. 2.
Accuracy of HIV-2 group A RNA quantification assays evaluated by the ACHIEV2E collaboration in 2009. Quantification results are reported for each participating laboratory. The accuracy interval is represented by the white area for each of the three theoretical viral loads used. (A) Theoretical viral load of 2.7 log10 copies/ml; (B) theoretical viral load of 3.7 log10 copies/ml.
At the theoretical level of 3.7 log10 copies/ml, the observed median viral load was 3.3 log10 copies/ml. The accuracy interval was 2.8 to 3.8 log10 copies/ml. Only two laboratories reported a majority of undetectable RNA values (6 out of 10 measurements). Forty-five percent and 70% of the quantification results were in the accuracy interval with the in-house standard and the common standard, respectively (P = 0.007) (Fig. 2B). Interlaboratory variability was not significantly lower when using the common standard (P = 0.21). However, the intralaboratory variability was 3 times lower (P = 0.0008) and the overall variability was 2 times lower (P = 0.03) when using the common standard. The coefficients of variation for reproducibility varied from 1.1% to 17.2% for the in-house standards and from 1.4% to 8.4% for the common standard.
HIV-2 group B quantification results.
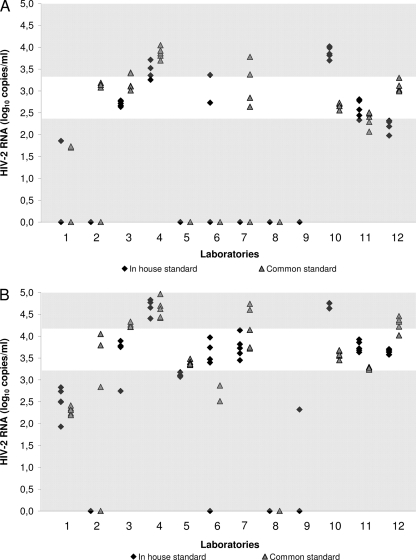
At the theoretical level of 2.7 log10 copies/ml, the observed median viral load was 2.8 log10 copies/ml. The accuracy interval was 2.4 to 3.3 log10 copies/ml. Irrespective of the standard used, 7 laboratories reported undetectable RNA values for at least 6 aliquots out of 10. Eighteen percent and 35% of the quantification results were in the accuracy interval with the in-house standard and the common standard, respectively (P = 0.04) (Fig. 3A). Interlaboratory, intralaboratory, and overall variabilities were not significantly lower when using the common standard than when using the in-house standards (P = 0.46, P = 0.78, and P = 0.43, respectively). The coefficients of variation for reproducibility varied from 2.2% to 14.7% for the in-house standards and from 0.8% to 16.4% for the common standard.
Fig. 3.
Accuracy of HIV-2 group B RNA quantification assays evaluated by the ACHIEV2E collaboration in 2009. Quantification results are reported for each participating laboratory. The accuracy interval is represented by the white area for each of the three theoretical viral loads used. (A) Theoretical viral load of 2.7 log10 copies/ml; (B) theoretical viral load of 3.7 log10 copies/ml.
At the theoretical level of 3.7 log10 copies/ml, the observed median viral load was 3.7 log10 copies/ml. The accuracy interval was 3.2 to 4.2 log10 copies/ml. One laboratory reported 100% of undetectable RNA values. Thirty-eight percent and 35% of the quantification results were in the accuracy interval with the in-house standards and the common standard, respectively (P = 0.93) (Fig. 3B). Interlaboratory, intralaboratory and overall variabilities were not significantly lower when using the common standard than when using the in-house standards (P = 0.55, P = 0.53, and P = 0.61, respectively). The coefficients of variation for reproducibility varied from 1.2% to 14.0% for the in-house standards and from 0.8% to 17.9% for the common standard.
DISCUSSION
This second HIV-2 viral load quality control assessment among the laboratories in the ACHIEV2E network showed that the homogeneity of HIV-2 RNA group A quantification can be significantly improved by using a centrally validated and distributed common standard. The accuracy of the results evaluated at the theoretical level of 3.7 log10 copies/ml is better than that at the level of 2.7 log10 copies/ml, as we had previously shown in the first HIV-2 study (8) and as has been observed in an HIV-1 viral load quality control study (21). Six laboratories reported between 90 and 100% of undetectable RNA values whatever the theoretical concentrations were. The threshold of quantification obtained by these laboratories varied from 1.7 to 2.7 log10 copies/ml and could explain these results for all the laboratories but one.
As we observed in 2006 in the first round of HIV-2 quality control assessments (8), there is, however, a high heterogeneity of HIV-2 RNA group B quantification results even with the use of a common standard. Several laboratories failed to detect HIV-2 group B, and sequence differences between the primers/probes used by individual labs and the target sequence of the study samples are the suspected cause. The difficulty in accurately detecting and amplifying HIV-2 group B could be due to the significant HIV-2 genetic diversity (14). Indeed, the average genetic diversity between HIV-2 groups A and B is ∼20% in the gag gene, which is higher than that among HIV-1 group M isolates (13). The problem of HIV-2 genetic diversity for accurate viral load quantification is similar to that previously observed for HIV-1, where genetic diversity makes HIV-1 RNA quantification difficult, especially in patients infected by HIV-1 non-B subtypes (5, 10, 27, 32). All of the assay methods involved a PCR step. Primer binding to HIV-2 target sequences is an important determinant of PCR success, and PCR with HIV targets is notoriously susceptible to target site evolution. An attempt was made to determine if the presence of primer mismatches between the primer and the HIV-2 sequence could be a source of variability in the detection and quantitation. Although partial long terminal repeat (LTR) and gag sequences were available for the four virus preparations used in the study (group A standard and sample, group B standard and sample), several of the groups' primer targets fell outside the sequenced regions (Table 2). In these cases, primer identities with the HIV-2 ROD and HIV-2 EHO sequences were used as group A and B surrogates. Furthermore, details of the primer sequences were not available for all of the groups. Nonetheless, an analysis of the available sequence data indicated that there was no association between the modest number of primer target mismatches observed and detection performance. Furthermore, the same primer sets were used by several groups and yielded different levels of detection performance, indicating other sources of variability in the assays, such as extraction methods and the instruments used for PCR signal detection. Future efforts to standardize HIV-2 RNA quantification assays should focus on generating more complete primer and target sequences and examine the variability in other components of the assay systems. As developed with the HIV-1 RNA quantification commercial assay (Cobas AmpliPrep/Cobas TaqMan, version 2.0; Roche Diagnostics), which includes 2 sets of primers and probes located in the LTR and gag regions, one can hypothesize that using a combination of primers and probes located in different regions of the genome could improve HIV-2 RNA quantification (7).
The strengths of this study include the large number of participating HIV-2 laboratories, as well as central processing, standard validation by EM counting, and blinding of HIV-2 samples before distribution. Study limitations include the presence of only one HIV-2 group A isolate, only one HIV-2 group B isolate, and no HIV-2 group C to H isolates in the blinded samples, only two viral load levels (500 and 5,000 copies/ml) in the blinded samples (and no samples with very low levels [50 copies/ml]), and no formal testing of intralaboratory and interassay variation for each HIV-2 group and sample concentration.
In this 2nd international collaboration to validate HIV-2 viral load assays, using a common standard generally improved HIV-2 group A quantification. Unfortunately, no such improvement was observed for group B HIV-2 RNA quantification, and this is probably due to the high diversity of HIV-2 group B isolates and viral assay design directed at HIV-2 group A isolates in most labs. The level of HIV-2 viral diversity, as well as interlab assay heterogeneity, may make it difficult to compare results between the different HIV-2-infected cohorts of the ACHIEV2E collaboration, especially in countries where HIV-2 group B circulates (4, 6, 14, 18). Ultimately, further efforts to standardize, validate, and commercialize simple, low-cost HIV-2 viral load assays are needed. Such efforts will, hopefully, lead to improved care and treatment of HIV-2 infection in both developed and resource-limited locales.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS). The Swiss HIV Cohort Study is supported by the Swiss National Science Foundation.
Participating laboratories are as follows: in Belgium, Belgian AIDS Reference Laboratories, Louvain University, Patrick Goubau and Jean Ruelle; in Canada, McGill University, Mark Wainberg; in France, Laboratoire de Virologie de l'Hôpital Bichat-Claude Bernard, F. Damond and F. Brun-Vezinet; in the Gambia, Medical Research Council Laboratories in Gambia, Sarah Rowland-Jones; in Germany, Loeffler-Institut für Medizinische Mikrobiologie Universität Greifswald, Bernd Kupfer and Jürgen Rockstroh; in Italy, Department of Clinical Sciences L. Sacco, Section of Infectious Diseases, University of Milan; in Portugal, Hospital de Egas Moniz, Lisboa Perpetua Gomes, Kamal Mansinho and Ricardo Camacho, and Hospital de Santa Maria, Serviço de Doenças Infecciosas, Lisbon, Francisco Antunes and Emilia Valadas; in Spain, Service of Infectious Diseases, Hospital Carlos III, Madrid, Vicente Soriano, Berta Rodés, and Ana Treviño; in Switzerland, Swiss National Center for Retroviruses for the Swiss HIV Cohort Study, Jürg Böni; in the United Kingdom, Centre for Virology, Research Department of Infection, UCL Medical School, London, Bridget Ferns, Jeremy Garson, and Deenan Pillay; in the United States, Division of Allergy and Infectious Diseases, University of Washington School of Medicine, Seattle, WA, Geoffrey Gottlieb, Nancy Kiviat, Steve Hawes, and Papa Salif Sow (Seattle/Senegal); and in Portugal, Departamento de Doenças Infecciosas, Hospitais da Universidade de Coimbra, João Vaz, Vítor Duque, Saraiva da Cunha, and Meliço-Silvestre.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Arien K. K., et al. 2005. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J. Virol. 79:8979–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benard A., et al. 2011. Immunovirological response to triple nucleotide reverse-transcriptase inhibitors and ritonavir-boosted protease inhibitors in treatment-naive HIV-2-infected patients: the ACHIEV2E Collaboration Study Group. Clin. Infect. Dis. 52:1257–1266 [DOI] [PubMed] [Google Scholar]
- 3. Borget M. Y., Diallo K., Adje-Toure C., Chorba T., Nkengasong J. N. 2009. Virologic and immunologic responses to antiretroviral therapy among HIV-1 and HIV-2 dually infected patients: case reports from Abidjan, Cote d'Ivoire. J. Clin. Virol. 45:72–75 [DOI] [PubMed] [Google Scholar]
- 4. Chen Z., et al. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Damond F., Apetrei C., Descamps D., Brun-Vezinet F., Simon F. 1999. HIV-1 subtypes and plasma RNA quantification. AIDS 13:286–288 [DOI] [PubMed] [Google Scholar]
- 6. Damond F., et al. 2001. Variability of human immunodeficiency virus type 2 (HIV-2) infecting patients living in France. Virology 280:19–30 [DOI] [PubMed] [Google Scholar]
- 7. Damond F., et al. Evaluation of an upgraded version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test for HIV-1 load quantification. J. Clin. Microbiol. 48:1413–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damond F., et al. 2008. Quality control assessment of human immunodeficiency virus type 2 (HIV-2) viral load quantification assays: results from an international collaboration on HIV-2 infection in 2006. J. Clin. Microbiol. 46:2088–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damond F., et al. 2005. Improved sensitivity of human immunodeficiency virus type 2 subtype B plasma viral load assay. J. Clin. Microbiol. 43:4234–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damond F., et al. 2007. Human immunodeficiency virus type 1 (HIV-1) plasma load discrepancies between the Roche COBAS AMPLICOR HIV-1 MONITOR version 1.5 and the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 assays. J. Clin. Microbiol. 45:3436–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferns R. B., Garson J. A. 2006. Development and evaluation of a real-time RT-PCR assay for quantification of cell-free human immunodeficiency virus type 2 using a brome mosaic virus internal control. J. Virol. Methods 135:102–108 [DOI] [PubMed] [Google Scholar]
- 12. Fultz P. N., et al. 1988. Seroprevalence of HIV-1 and HIV-2 in Guinea Bissau in 1980. AIDS 2:129–132 [DOI] [PubMed] [Google Scholar]
- 13. Gao F. 2005. Detection of HIV-2 by PCR. Methods Mol. Biol. 304:191–200 [DOI] [PubMed] [Google Scholar]
- 14. Gao F., et al. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao F., Yue L., Sharp P. M., Hahn B. H. 1993. Genetic typing of HIV-2 from a Senegalese/German heterosexual transmission. AIDS Res. Hum. Retroviruses 9:703–704 [DOI] [PubMed] [Google Scholar]
- 16. Gottlieb G. S., et al. 2009. Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource-limited West Africa. Clin. Infect. Dis. 48:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gottlieb G. S., et al. 2008. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS 22:2069–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heredia A., et al. 1998. Phylogenetic analysis of HIV type 2 strains from Portugal. AIDS Res. Hum. Retroviruses 14:471–473 [DOI] [PubMed] [Google Scholar]
- 19. Jallow S., et al. 2009. Virological response to highly active antiretroviral therapy in patients infected with human immunodeficiency virus type 2 (HIV-2) and in patients dually infected with HIV-1 and HIV-2 in the Gambia and emergence of drug-resistant variants. J. Clin. Microbiol. 47:2200–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matheron S., et al. 2003. Factors associated with clinical progression in HIV-2-infected patients: the French ANRS cohort. AIDS 17:2593–2601 [DOI] [PubMed] [Google Scholar]
- 21. Muyldermans G., et al. 2000. Blinded, multicenter quality control study for the quantification of human immunodeficiency virus type 1 RNA in plasma by the Belgian AIDS reference laboratories. Clin. Microbiol. Infect. 6:213–217 [DOI] [PubMed] [Google Scholar]
- 22. Naucler A., Andreasson P. A., Costa C. M., Thorstensson R., Biberfeld G. 1989. HIV-2-associated AIDS and HIV-2 seroprevalence in Bissau, Guinea-Bissau. J. Acquir. Immune Defic. Syndr. 2:88–93 [PubMed] [Google Scholar]
- 23. Ntemgwa M. L., et al. 2009. Nucleoside and nucleotide analogs select in culture for different patterns of drug resistance in human immunodeficiency virus types 1 and 2. Antimicrob. Agents Chemother. 53:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodes B., et al. 2007. Quantitative detection of plasma human immunodeficiency virus type 2 subtype A RNA by the Nuclisens EasyQ Assay (version 1.1). J. Clin. Microbiol. 45:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruelle J., Mukadi B. K., Schutten M., Goubau P. 2004. Quantitative real-time PCR on LightCycler for the detection of human immunodeficiency virus type 2 (HIV-2). J. Virol. Methods 117:67–74 [DOI] [PubMed] [Google Scholar]
- 26. Sarfo F. S., et al. 2009. Inadvertent non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy in dual HIV-1/2 and HIV-2 seropositive West Africans: a retrospective study. J. Antimicrob. Chemother. 64:667–669 [DOI] [PubMed] [Google Scholar]
- 27. Scott L. E., et al. 2009. Evaluation of the Abbott m2000 RealTime human immunodeficiency virus type 1 (HIV-1) assay for HIV load monitoring in South Africa compared to the Roche Cobas AmpliPrep-Cobas Amplicor, Roche Cobas AmpliPrep-Cobas TaqMan HIV-1, and BioMerieux NucliSENS EasyQ HIV-1 assays. J. Clin. Microbiol. 47:2209–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smallman-Raynor M., Cliff A. 1991. The spread of human immunodeficiency virus type 2 into Europe: a geographical analysis. Int. J. Epidemiol. 20:480–489 [DOI] [PubMed] [Google Scholar]
- 29. Soriano V., et al. 2000. Human immunodeficiency virus type 2 (HIV-2) in Portugal: clinical spectrum, circulating subtypes, virus isolation, and plasma viral load. J. Med. Virol. 61:111–116 [PubMed] [Google Scholar]
- 30. Toro C., et al. 2002. Infection with retroviruses other than HIV-1 in Spain: a retrospective analysis for HIV-2, HTLV-I, and/or HTLV-II. HIV Clin. Trials 3:397–402 [DOI] [PubMed] [Google Scholar]
- 31. Valadas E., Franca L., Sousa S., Antunes F. 2009. 20 years of HIV-2 infection in Portugal: trends and changes in epidemiology. Clin. Infect. Dis. 48:1166–1167 [DOI] [PubMed] [Google Scholar]
- 32. Wirden M., et al. 2009. Impact of discrepancies between the Abbott RealTime and Cobas TaqMan assays for quantification of human immunodeficiency virus type 1 group M non-B subtypes. J. Clin. Microbiol. 47:1543–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]