Abstract
Self-sampling by cervicovaginal lavage could be an attractive method to detect high-risk human papillomavirus (hr-HPV) infections to identify women with a risk of cervical precancer. The objective of our study was to use self-sampling for the first time in a cross-sectional approach to determine HPV prevalence and genotype distribution. We evaluated participants' acceptance and laboratory results from self-obtained samples versus endocervical brush samples obtained by gynecologists. To determine the sensitivity of both sampling methods in presumed high- and low-prevalence settings, two groups of women 20 to 30 years of age with (n = 55) and without (n = 101) a recent suspicious cytological smear were compared. Overall, 76% (95% confidence interval [95% CI], 65 to 88) of women with and 40% (95% CI, 30 to 49) of women without a recent suspicious cytological smear tested HPV positive. The prevalences of high-risk HPV strains were 71% (95% CI, 59 to 83) and 32% (95% CI, 22 to 41), respectively, for these two groups. The agreement for hr-HPV between the two sampling methods for women with and without suspicious cytology was 84% (κ = 0.65; 95% CI, 0.44 to 0.86) and 91% (κ = 0.78; 95% CI, 0.64 to 0.92), respectively. Participants rated the user-friendliness of the self-sampling method on a visual analog scale from 0 (easy) to 100 (difficult) with a median of 12. In conclusion, self-sampling by cervicovaginal lavage is a reliable method to determine hr-HPV prevalence and is well accepted by young adult females.
INTRODUCTION
Cervical cancer is the second most common cancer in women worldwide, with globally approximately 500,000 new cases and 250,000 deaths each year (http://www.who.int/reproductivehealth/topics/cancers/en/). The strong correlation between cervical cancer and a preceding persistent infection of the cervix with human papillomavirus (HPV) is beyond dispute (2, 18). More than 130 genotypes of HPV have been classified (6), of which about 40 genotypes can infect the anogenital tract (14). Of these 40 genotypes, nearly 20 are thought to be carcinogenic and are classified as high-risk HPV (hr-HPV). Genotypes 16 and 18 alone are associated with approximately 50% of high-grade cervical intraepithelial neoplasia (CIN) and 70% of cervical cancers (4, 20). Anogenital HPV infections are predominantly sexually transmitted, and nearly all sexually active women will be infected at some point during their lifetime (1). Most HPV infections are transient, but persistent infections can progress over years to high-grade CIN or cervical cancer.
Vaccines against HPV genotypes 16 and 18 have been available since 2006. Results from clinical trials indicate high effectiveness against high-grade CIN when HPV-naïve girls and women are vaccinated (7, 16, 17). Numerous industrial countries have implemented vaccination programs to protect girls against infections with HPV types 16 and 18. Target groups for the vaccination are mainly adolescent women preceding their sexual debut. To monitor the impact of the vaccine and the implemented vaccination strategy, it is essential to assess HPV prevalence and type distribution before and after implementation. Population-level studies are mainly based on analysis of cytological samples obtained from opportunistic or organized cervical cancer screening (12, 13). Such prevalence estimates are prone to bias if participation in screening programs is low. To access women in countries with low screening participation or without organized screening programs, alternative approaches are needed to determine HPV prevalence and genotype distribution.
Self-sampling by cervicovaginal lavage has been shown to be useful in previous studies to detect high-grade CIN (3, 8, 21) and was highly accepted in the Netherlands (11). The objective of our study was to evaluate if self-sampling by cervicovaginal lavage is a useful and valid approach to determine HPV prevalence in 20- to 30-year-old females and if the method is accepted in this target group in Germany.
MATERIALS AND METHODS
Study population.
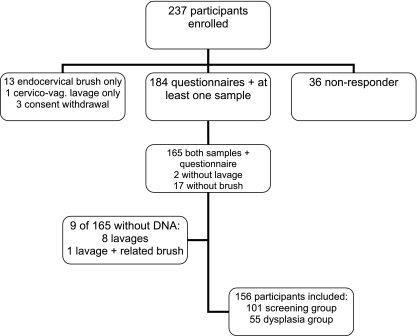
Women aged 20 to 30 years who made an appointment for follow-up examination due to abnormal cytological smears or for routine cervical cancer screening were recruited between April 2009 and January 2010. The aim of the study was to enroll 50 women with a recent abnormal cytological smear in a presumed high-prevalence group and 100 women without a history of abnormal cytological smears in a low- or average-prevalence group. The study protocol was approved by ethics committees of Charité-Universitätsmedizin, Berlin, Germany. After initial informed consent was obtained by a telephone interview, a self-sampling kit, an informed consent form, and a questionnaire were sent to the participants. The questionnaire covered information on demographics, sexual behavior, medical history, and acceptability of the self-sampling device. Within 3 weeks after enrollees had received the package at home, a gynecologist took a cervical smear for cytology (colposcopy-directed for the women who came for follow-up for an abnormal smear) followed by a second sample with a new endocervical brush for HPV testing. The smear was analyzed by local cytologists, and the second sample for HPV testing was sent by mail to the Robert Koch Institute (RKI). A total of 156 women were included in the final analysis (Fig. 1). For the group with presumed high HPV prevalence, 55 women were recruited, and for the group with low or average HPV prevalence, 101 women were recruited. Participants of both groups were comparable in median age (27 and 25 years, respectively; P value of 0.07 [Mann-Whitney test]) and education level (e.g., 52.7% of women in the high-HPV-prevalence group and 52.5% in the low-prevalence group had achieved the highest level of education; P value of 0.98 [chi-square test]; data not shown).
Fig. 1.
Participant disposition.
Sampling and HPV testing.
The self-sampling device used in this study (Delphi Screener, Delphi Bioscience, BV Scherpenzeel, the Netherlands) is a sterile, syringe-like device containing 5 ml of buffered saline. The women plunged the handle, releasing the saline into the vagina, held the handle down for the count of 5, and released it, automatically retrieving the fluid back into the device due to the negative pressure of the retreating plunger. Next, they plunged their lavage specimens into prelabeled coded tubes. The participants were instructed to send the container within 24 h of collection by mail at ambient temperature to the RKI, where it was stored up to 7 days at 4°C. Samples for HPV testing taken from gynecologists were sent under the same mail conditions. Laboratory staff members at Charité were blinded for paired samples. Samples were analyzed by general primer GP5+/GP6+-based PCR and genotyped by luminex-based multiplexed genotyping (MPG) for detection of 19 high-risk (HPV16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -68, -26, -53, -66, -70, -73, and -82) and 7 low-risk (HPV6, -11, -42, -43, -54, -57, and -72) HPV genotypes.
The samples taken from gynecologists were soaked for 10 min in 2 ml of phosphate-buffered saline (PBS) and vortexed vigorously. These cyto brush extracts and lavage solutions (2 ml) were centrifuged at 6,000 rpm for 5 min, the supernatant was discharged, and the sediments were resuspended in 400 μl of PBS. For DNA extraction, 200-μl portions were processed according to the protocol by Qiamp Mini Kit (Qiagen, Hilden, Germany). Purified DNA was eluted 2 times with 80 μl, and 10 μl was used per 50-μl PCR. HPV-specific general primer GP5+/GP6+Bio PCR and genotyping by Luminex-based MPG was performed as described by Schmitt et al. (19). As a control, a PCR was done for β-globin to exclude any negative samples not containing sufficient amounts of DNA. PCR products were analyzed by 2% agarose gel electrophoresis. Samples were run on a Luminex flow cytometer (Bio-Rad). Results were expressed as mean fluorescence intensity (MFI). The mean background MFI of negative control samples was calculated. Positive samples had MFIs at least 3 times higher than those of controls; borderline reactive samples were defined as negative. Samples were analyzed consecutively.
Statistical analysis.
Participants were considered positive for HPV if one of the two samples tested positive. The cumulative number of all positive results from both sampling techniques was set as the gold standard for calculating sensitivity. Thus, false-positive results were missing and specificity could not be determined.
Kappa statistics were used to assess the agreement between HPV results obtained from endocervical brush samples and self-obtained cervicovaginal lavage samples. A kappa value of more than 0.6 was considered substantial agreement. A chi-square test was used to assess the sensitivity for HPV detection of both sampling methods. Overall prevalence, hr-HPV-specific prevalence, and HPV16-specific prevalence and their 95% confidence intervals (95% CIs) were calculated for all participants and stratified by risk group. Statistical analyses were conducted using STATA 11.0 (StataCorp LP, College Station, Texas).
RESULTS
HPV genotyping.
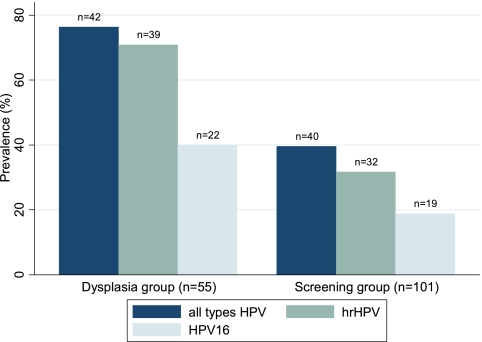
HPV prevalence of any type was highest in women of the high-prevalence group with 76% (95% CI, 65 to 88) (Fig. 2). Among the group of women in the low- or average-prevalence group, a total of 40% (95% CI, 30 to 49) was infected with HPV of any type. The prevalences of hr-HPV differed between the groups, with 71% (95% CI, 59 to 83) in the high-prevalence group and 32% (95% CI, 22 to 41) in the low-prevalence group. HPV16 infections were found in 40% (95% CI, 27 to 53) of women in the high-prevalence group and in 19% (95% CI, 11 to 27) of women in the low- or average-prevalence group. Infections with HPV16 only were the most prevalent infections among women with single-HPV-type detection (n = 21). Identical results for single infections by HPV16 in both samples (self-obtained versus gynecologist-obtained) were found in 16 of 82 HPV-positive women (20%; 95% CI, 12 to 30). In 3 endocervical brush samples versus 2 cervicovaginal lavage samples, HPV16 was found exclusively. Multiple infections with two or more HPV types were observed in 30 of 82 HPV-positive women (37%; 95% CI, 26 to 48).
Fig. 2.
Overall prevalence of HPV (brush and lavage results) in study subgroups.
Overall, 43 of 82 (52%; 95% CI, 41 to 64) positive results were absolutely identical in both sample types, 17 (21%; 95% CI, 13 to 31) were partly identical, and 22 (27%; 95% CI, 18 to 38) did not match.
Concordance of sampling methods.
The agreement between sampling methods (Table 1) in the group of women in the presumed high-prevalence group for any type of HPV and hr-HPV was 84% either, resulting in kappa statistics (κ) of 0.62 (95% CI, 0.40 to 0.85) and 0.65 (95% CI, 0.44 to 0.86). The agreement for HPV16 in the same group was 93% with κ of 0.84 (95% CI, 0.70 to 0.99). In the group of women in the low- or average-prevalence group, the agreement was 87% for any HPV type, 91% for hr-HPV, and 94% for HPV16, resulting in κ of 0.71 (95% CI, 0.57 to 0.86) and 0.78 for hr-HPV (95% CI, 0.64 to 0.92) and HPV 16 (95% CI, 0.61 to 0.95), respectively.
Table 1.
HPV test results and concordance of corresponding self-collected samples and gynecologist-collected samples
| Self-collected sample result | No. of gynecologist-collected samples from participants in group witha: |
|||
|---|---|---|---|---|
| Presumed high prevalence of HPV infection (n = 55) |
Presumed low or avg prevalence of HPV infection (n = 101) |
|||
| HPV negative | HPV positive | HPV negative | HPV positive | |
| HPV any type | ||||
| HPV negative | 13 | 5 | 61 | 4 |
| HPV positive | 4 | 33 | 9 | 27 |
| Kappa (95% CI) | 0.62 (0.40–0.85) | 0.71 (0.57–0.86) | ||
| hr-HPV | ||||
| hr-HPV negative | 16 | 5 | 69 | 4 |
| hr-HPV positive | 4 | 30 | 5 | 23 |
| Kappa (95% CI) | 0.65 (0.44–0.86) | 0.78 (0.64–0.92) | ||
| HPV16 | ||||
| HPV16 negative | 33 | 2 | 82 | 2 |
| HPV16 positive | 2 | 18 | 4 | 13 |
| Kappa (95% CI) | 0.84 (0.70–0.99) | 0.78 (0.61–0.95) | ||
Boldface indicates agreement of results in self-collected and gynecologist-collected samples.
In the high-prevalence group of women with suspicious cytological findings, gynecologist-obtained cervical brush sampling resulted in a sensitivity for the detection of hr-HPV of 87% (34/39; 95% CI, 76 to 97) versus 90% (35/39; 95% CI, 76 to 97) for self-sampling by cervicovaginal lavage. Sensitivity for the detection of hr-HPV in the low- or average-prevalence group was 84% (27/32; 95% CI, 67 to 95) for gynecologist-obtained cervical brush samples and 88% (28/32; 95% CI, 71 to 97) for self-obtained cervicovaginal lavage samples.
Acceptance of self-sampling.
In order to investigate the acceptability of using the self-sampling cervicovaginal lavage method, we evaluated via visual analog data by using a 100-mm scale between 0 (easy) and 100 (difficult or displeasing) for user-friendliness and sensation of the intravaginal application. The participants in the presumed high-prevalence group (n = 54) as well as the participants in the low- or average-prevalence group (n = 99) rated the method as generally easy to use (high-prevalence group: median = 11; interquartile range [IQR], 2 to 25; low- or average-prevalence group: median = 13, IQR, 4 to 25). Participants of both groups voted generally fine for sensation (high-prevalence group: median = 20; IQR, 7 to 40; low- or average-prevalence group: median = 25; IQR, 8 to 40). If the women were offered a choice between collection of the sample by a gynecologist and use of the self-sampling device for HPV testing, 44% (n = 24) of women with a history of abnormal smears and 35% (n = 35) of women without such a history would prefer self-sampling. No preference was held by 11% in the high-prevalence group and 12% in the low- or average-prevalence group.
DISCUSSION
Our results show that self-sampling by cervicovaginal lavage is a reliable method to determine HPV prevalence in women aged 20 to 30 years. Analysis of self-sampled cervicovaginal lavage specimens resulted in comparable numbers of hr-HPV detection and showed similar sensitivity compared to gynecologist-taken samples. These results are in line with previous studies that compared self- and clinician-collected specimens to identify women with a risk of high-grade CIN and cervical precancer (3, 5, 8, 9, 10, 15). In our study, the self-sampling method was used for the first time in a cross-sectional approach to determine HPV prevalence and genotyping and not to determine a clinical outcome. This extends possible applications of the self-sampling method to the field of epidemiology (HPV monitoring) and highlights the public health relevance of this method.
Although the mailing time is not documented for all samples in our study, the high concordance of results between self-obtained and gynecologist-obtained samples suggests that possible different mailing times will not result in statistical differences.
The concordance is good even though the sampled areas differ: the colposcopy-directed endocervical brush sample for HPV testing focuses on transformation zone cells, while the cervicovaginal lavage includes the whole cervical area. The higher prevalence of HPV, hr-HPV, and HPV16 in cervicovaginal lavage samples may be explained by additional infections at extracervical sites. Since these infections may be a reservoir for virus infecting the cervical epithelium at the transformation zone, they are probably epidemiologically relevant. Therefore, cervicovaginal lavage sampling may be superior to cervix-directed sampling for future HPV prevalence studies.
The prevalence of hr-HPV in our study population of women aged 20 to 30 years was high, with 71% in the group of women with abnormal cytological findings and 32% in women attending routine screening. Even though our sample size is small, these prevalences are comparable to findings of HPV positivity in Denmark (12) with hr-HPV prevalence of 45% (95% CI, 43 to 47) in women 20 to 24 years of age. Still, our study population can be regarded as a convenience sample including a considerable proportion of women with previous suspicious cytological findings. Therefore, a study is planned to assess HPV prevalence in the general female population in Germany to monitor the impact of HPV vaccination.
The acceptance of self-sampling in 20- to 30-year-old females is high. Even if half of the participants of our study would prefer to visit a gynecologist than to use a self-sampling device at home, nearly all women declared that the device was easy to use. The participants of our study had already made an appointment for screening before enrolling, so it is not surprising that they have no barrier to going to a gynecologist. Thus, using self-sampling by cervicovaginal lavage could provide safe and low-barrier access to young women.
Conclusion.
In conclusion, self-sampling by cervicovaginal lavage has a broad variety of applications. The easy-to-handle device with its excellent user acceptance, high sensitivity in detecting hr-HPV, robustness against environmental influences, and handy postal shipping conditions makes self-sampling a reliable and valuable method to conduct population-based studies on HPV prevalence. Furthermore, the implementation of self-sampling for hr-HPV testing in screening programs could have the potential to achieve high public health relevance to increase screening coverage.
ACKNOWLEDGMENTS
We thank Stefanie Germer, Gerhard Falkenhorst, Heidi Jones, Merle M. Böhmer, and Ole Wichmann for their technical support, comments, and assistance in preparation of the manuscript. We also thank the lab staff at Charité Tumorimmunologie and the colleagues at Robert Koch Institute for their continuous support. Last but not least, many thanks go to Rene Hol and Marloes Voll for helpful discussion and great support.
This study was exclusively financed by internal funds of RKI and Charité. We declare no conflict of interests.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Baseman J. G., Koutsky L. A. 2005. The epidemiology of human papillomavirus infections. J. Clin. Virol. 32S:S16–S24 [DOI] [PubMed] [Google Scholar]
- 2. Bosch F. X., Lorincz A., Muñoz N., Meijer C. J., Shah K. V. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brink A. A., et al. 2006. High concordance of results of testing for human papillomavirus in cervicovaginal samples collected by two methods, with comparison of a novel self-sampling device to a conventional endocervical brush. J. Clin. Microbiol. 44:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clifford G. M., Smith J. S., Plummer M., Muñoz N., Franceschi S. 2003. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 88:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dannecker C., et al. 2004. Primary cervical cancer screening by self-sampling of human papillomavirus DNA in internal medicine outpatient clinics. Ann. Oncol. 15:863–869 [DOI] [PubMed] [Google Scholar]
- 6. de Villiers E.-M., Fauquet C., Broker T. R., Bernard H.-U., zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27 [DOI] [PubMed] [Google Scholar]
- 7. FUTURE II Study Group 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 10:1915–1927 [DOI] [PubMed] [Google Scholar]
- 8. Gök M., et al. 2010. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ 340:c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hillemanns P., et al. 1999. Screening for cervical neoplasia by self-assessment for human papillomavirus DNA. Lancet. 354:1970. [DOI] [PubMed] [Google Scholar]
- 10. Jones H. E., Altini L., de Kock A., Young T., van de Wijgert J. H. 2007. Home-based versus clinic-based self-sampling and testing for sexually transmitted infections in Gugulethu, South Africa: randomised controlled trial. Sex. Transm. Infect. 83:552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones H. E., Wiegerinck M. A., Nieboer T. E., Mol B. W., Westhoff C. L. 2008. Women in the Netherlands prefer self-sampling with a novel lavaging device to clinician collection of specimens for cervical cancer screening. Sex. Transm. Dis. 35:916–917 [DOI] [PubMed] [Google Scholar]
- 12. Kjaer S. K., et al. 2008. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int. J. Cancer 123:1864–1870 [DOI] [PubMed] [Google Scholar]
- 13. Klug S. J., et al. 2007. Prevalence of human papillomavirus types in women screened by cytology in Germany. J. Med. Virol. 79:616–625 [DOI] [PubMed] [Google Scholar]
- 14. Munoz N., Castellsague X., Berrington de Gonzalez A., Gissmann L. 2006. Chapter 1: HPV in the etiology of human cancer. Vaccine 24(Suppl. 3):S1–S10 [DOI] [PubMed] [Google Scholar]
- 15. Nobbenhuis M. A., et al. 2002. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J. Clin. Pathol. 55:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paavonen J., et al. 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314 [DOI] [PubMed] [Google Scholar]
- 17. Rambout L., Hopkins L., Hutton B., Fergusson D. 2007. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 177:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schiffman M., Castle P. E., Jeronimo J., Rodriguez A. C., Wacholder S. 2007. Human papillomavirus and cervical cancer. Lancet 370:890–907 [DOI] [PubMed] [Google Scholar]
- 19. Schmitt M., Dondog B., Waterboer T., Pawlita M. 2008. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J. Clin. Microbiol. 46:1050–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith J. S., et al. 2007. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer 121:621–632 [DOI] [PubMed] [Google Scholar]
- 21. Szarewski A., et al. 2011. HPV self-sampling as an alternative strategy in non-attenders for cervical screening - a randomised controlled trial. Br. J. Cancer 104:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]