Abstract
Growth factor receptor-bound protein 14 (Grb14) is an adapter protein implicated in receptor tyrosine kinase signaling. Grb14−/− studies highlight both the positive and negative roles of Grb14 in receptor tyrosine kinase signaling in a tissue-specific manner. In this study, we made a novel finding that Grb14 inhibits the activity of PTP1B, the major negative regulator of insulin receptor (IR) signaling, in a phosphorylation-regulated manner. Phosphorylation of Tyr-347 in the BPS domain of Grb14 is critical for interaction with PTP1B, resulting in the competitive inhibition of PTP1B activity. We also found that rhodopsin-regulated Src kinase activation in retina leads to the phosphorylation of Grb14. Further, ablation of Grb14 resulted in significantly elevated retinal PTP1B activity in vivo. PTP1B is known to be regulated by oxidation, glutathionylation, phosphorylation, and SUMOlyation, and our study for the first time demonstrates the inhibition of PTP1B activity in vivo by protein molecule Grb14 in a tissue-specific manner.
INTRODUCTION
Growth factor receptor-bound protein 14 (Grb14) is a member of an emerging family of noncatalytic adapter proteins, including Grb7 and Grb10 (11, 20). It is a multidomain protein that possesses several intracellular signaling modules, including a Ras-associating (RA) domain, a pleckstrin homology (PH) domain, a BPS (between PH and SH2) domain, and a C-terminal SH2 domain, as well as a conserved N-terminal proline-rich motif (NPR) (12). The BPS domain contains a region called PIR (phosphorylated insulin receptor [IR]-interacting region) that mediates the binding of Grb14 to the activated IR (13, 23). The RA domain of Grb14 has recently been shown to bind to activated N-Ras (14). We recently reported that Grb14 is a novel modulator of photoreceptor-specific cyclic nucleotide gated channel and that this effect is mediated through its RA domain (19). The crystal structure of the tyrosine kinase domain of the IR in complex with the IR-interacting domain of Grb14 revealed that Grb14 acts as a pseudosubstrate inhibitor of IR kinase by interacting with its substrate binding groove and thus functions as a selective inhibitor of insulin signaling (13). There is convincing evidence for a negative role of Grb14 in insulin signaling in that Grb14-deficient mice show enhanced glucose tolerance and insulin sensitivity (9).
Grb14−/− mouse studies also reveal the positive effects of Grb14 on receptor tyrosine kinase signaling in a tissue-specific manner. A high expression of Grb14 in myocardial tissue activates the phosphoinositide 3-kinase (PI3K)-Akt pathway, and ablation of Grb14 resulted in myocardial infarction and decreased PI3K/Akt activation (28). In the retina, light induces activation of the IR, and ablation of Grb14 results in the loss of light-dependent IR activation (31). The IR activation is essential for photoreceptor neuron survival (29, 32). These studies suggest that Grb14 promotes the IR signaling in vivo in a tissue-specific manner. In vitro experiments have shown that Grb14 impairs the tyrosine kinase activity of the IR toward exogenous substrates and protects the phosphorylated tyrosine residues from protein tyrosine phosphatase 1B (PTP1B) activity (5). In liver, Grb14 deletion resulted in decreased IR phosphorylation due to increased dephosphorylation of the IR by PTPs (9). However, there are no studies available on the interaction between Grb14 and PTP1B.
In this study, we found that the BPS domain of Grb14 inhibits retinal PTP1B activity. Phosphorylation of Tyr-347 in the BPS domain of Grb14 is responsible for its interaction with PTP1B and inhibits its activity. A novel finding in this study was that the state of Grb14 phosphorylation may determine its affinity toward either IR or PTP1B. We have also found that rhodopsin-regulated Src kinase activation in retina leads to the phosphorylation of Grb14. Further, ablation of Grb14 resulted in significantly elevated retinal PTP1B activity in vivo. This study identified for the first time that Grb14 is an endogenous physiological inhibitor of PTP1B in the retina.
MATERIALS AND METHODS
Chemicals.
Anti-PTP1B antibody was obtained from Epitomics (Burlingame, CA), and the PTP1B substrate RRLIEDAEPYAARG and the phosphatase assay reagents were obtained from Upstate Biotechnology (Lake Placid, NY). The monoclonal PY99 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The β-actin antibody was obtained from Affinity BioReagents (Golden, CO). A QuikChange site-directed mutagenesis kit was obtained from Stratagene (La Jolla, CA). Anti-His, anti-Src, and anti-phospho-Src-family (Y416) antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA). The Src inhibitors PP2 and PP3 were obtained from Calbiochem (La Jolla, CA). Anti-glutathione S-transferase (anti-GST) antibody and glutathione-Sepharose 4B matrix were obtained from Amersham Biosciences Corp. (Piscataway, NJ). Generation of a polyclonal Grb14 antibody was described earlier (31). All other reagents were of analytical grade from Sigma (St. Louis, MO).
Animals.
All animal work was performed in strict accordance with the Association for Research in Vision and Ophthalmology statement on the “Use of Animals in Ophthalmic and Vision Research.” All protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute. A breeding colony of albino Sprague-Dawley (SD) rats is maintained in our vivarium in cyclic light (5 lx; 12 h on/12 h off). Experiments were carried out on both male and female rats (150 to 200 g). Photoreceptor-specific conditional PTP1B-knockout (KO) mice were born and raised in 60-lux cyclic light (12 h on/off) in our animal facility.
Generation of photoreceptor-specific PTP1B-knockout mice.
PTP1B floxed mice were generated as described previously (4). The PTP1B floxed homozygous mice were bred with opsin-driven Cre mice, who have an 0.2-kb opsin-Cre promoter. The resultant mice were genotyped for Cre and floxed PTP1B and were heterozygous for the PTP1B floxed allele. To create photoreceptor-specific PTP1B−/−, the floxed PTP1B mice carrying the Cre transgene were bred with the PTP1B floxed homozygous mice (backcross). The genotype of the photoreceptor-specific PTP1B−/− mouse line (i.e., animals carrying the Cre transgene and homozygous for the PTP1B floxed allele) was confirmed by using PCR analysis of tail DNA. To identify rhodopsin-Cre, PCR was done with 1 μl of genomic DNA and the sense primer 5′-AGG TGT AGA GAA GGC ACT TAG C-3′ and the antisense primer 5′-CTA ATC GCC ATC TTC CAG CAG G-3′ to amplify a 411-bp product. To identify PTP1B floxed mice, we used the sense primer 5′-TGC TCA CTC ACC CTG CTA CAA-3′ and the antisense primer 5′-GAA ATG GCT CAC TCC TAC TGG-3′. The wild-type allele generates a 206-bp product, and the floxed allele generates a 327-bp product.
PTP1B activity assay.
The in vitro PTP activity assay was conducted based on a previously published protocol using the peptide RRLIEDAEPYAARG (Upstate Biotechnology) (40). The reaction was carried out in a 60-μl volume in PTP assay buffer (100 mM HEPES [pH 7.6], 2 mM EDTA, 1 mM dithiothreitol, 150 mM NaCl, 0.5 mg/ml bovine serum albumin) at 30°C. At the end of the reaction, 40-μl aliquots were placed in a 96-well plate, 100 μl of malachite green phosphatase reagent (Upstate Biotechnology) was added, and absorbance was measured at 630 nm. The effect of various substrate concentrations in the presence of fixed concentrations of inhibitor (Grb14) was studied. The modes of inhibition and kinetic parameters were evaluated from double reciprocal (Lineweaver-Burk and Eadie-Hofstee) and Dixon plots of the data.
Ex vivo retinal cultures.
Retinas were removed from overnight dark-adapted mice that were born and raised in dim cyclic light (5 lx; 12 h on/off). The retinas were then incubated for 10 min at 27°C in Dulbecco modified Eagle medium (DMEM) (Gibco BRL) in the presence and absence of a 100 μM concentration of either PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine} or a PP2-negative control, PP3 (4-amino-7-phenylpyrazolo[3,4-d]pyramidine), in the dark, and half of the retinas were exposed to light for 30 min. After light exposure, retinas were snap-frozen in liquid nitrogen and lysed for further experiments. In the case of insulin treatment, the retinas were incubated in DMEM containing 1 μM insulin in the dark for 5 min before lysis.
SDS-PAGE and Western blotting.
Proteins were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The blots were washed twice for 10 min with TTBS (20 mM Tris-HCl [pH 7.4], 100 mM NaCl, and 0.1% Tween 20) and blocked with either 5% bovine serum albumin or nonfat dry milk powder (Bio-Rad) in TTBS for 1 h at room temperature. Blots were then incubated with anti-Grb14 (1:1,000), anti-PTP1B (1:1,000), antiactin (1:1,000), anti-phospho-Src (Tyr-416) (1:1,000), anti-Src (1:1,000), anti-His (1:1,000), anti-PY99 (1:1,000), or anti-GST (1:5,000) either for 1 h (GST and actin) at room temperature or overnight (for other antibodies) at 4°C. Following primary antibody incubations, immunoblots were incubated with horseradish peroxidase (HRP)-linked secondary antibodies (mouse or rabbit) and developed by enhanced chemiluminescence (ECL) according to the manufacturer's instructions.
Statistical methods.
Data were analyzed and graphed using GraphPad Prism software (GraphPad Software, San Diego, CA). The data were expressed as means ± standard deviations and compared by Student's t test for unpaired data. The significance was set at P < 0.05.
RESULTS
Grb14 inhibits PTP1B in a light-dependent manner.
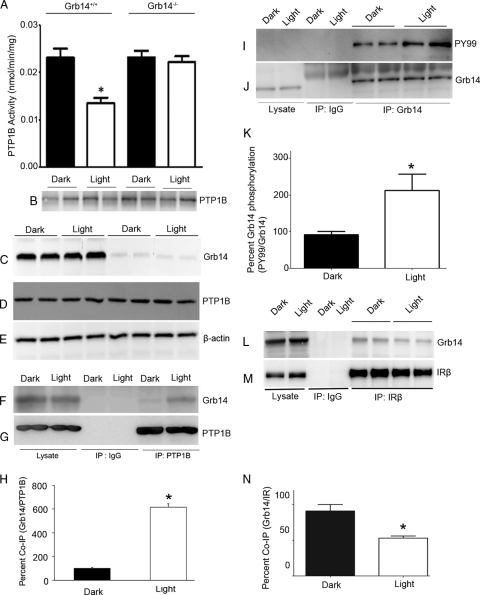
To determine whether the light-induced inhibition of PTP1B activity is mediated by Grb14, we examined the retinal PTP1B activity in Grb14−/− mice. PTP1B was immunoprecipitated (Fig. 1B) from dark- and light-adapted wild-type and Grb14−/− mouse retinas and assayed for the PTP1B activity. The PTP1B activity was significantly lower in the light-adapted retinas than in the dark-adapted retinal controls from the wild-type mice (Fig. 1A). This light-dependent inhibition of PTP1B activity was lost in Grb14−/− mice (Fig. 1A), indicating that Grb14 modulates the PTP1B activity in the light-adapted retinas. The difference in PTP1B activity between dark- and light-adapted conditions was not due to the difference in protein levels of PTP1B, as its expression levels were comparable among the groups (Fig. 1D). Immunoblots of Grb14 further confirm the absence of Grb14 in Grb14−/− mice (Fig. 1C). These results indicate that the loss of light-dependent inhibition of PTP1B activity in Grb14−/− mice is due to the ablation of Grb14.
Fig. 1.
Grb14 regulates the PTP1B activity in vivo. PTP1B activity was measured in the retinas harvested from the dark- and light-adapted wild-type and Grb14−/− mice. (A and B) Retinal proteins from each mouse were immunoprecipitated with the anti-PTP1B (B) antibody and measured for PTP1B activity (A). The immunoblots shown are representative of five retinas examined from each group (dark and light) of wild-type and Grb14−/− mice (B). Data are means ± standard deviations (n = 5). *, P < 0.05. (C to E) Equal amounts of retinal proteins used for PTP1B activity were immunoblotted with anti-Grb14 (C), anti-PTP1B (D), and anti-β-actin (E) antibodies. (F and G) Retinal lysates from dark- and light-adapted rats were immunoprecipitated with either IgG control or anti-PTP1B antibody and immunoblotted with anti-Grb14 (F) and reprobed with anti-PTP1B (G) antibodies. (H) Densitometric analysis of Grb14 binding normalized to PTP1B. Data are means ± standard deviations (n = 2). The dark control was set as 100%. *, P < 0.001 compared to dark-adapted control. (I and J) The dark- and light-adapted retinal lysates from rats were subjected to immunoprecipitation with either IgG control or anti-Grb14 antibody and immunoblotted with anti-PY99 (I) and reprobed with anti-Grb14 (J) antibodies. (K) Densitometric analysis of Grb14 phosphorylation normalized to Grb14. Data are means ± standard deviations (n = 2). The dark control was set as 100%. *, P < 0.001 compared to dark-adapted control. (L and M) Dark- and light-adapted retinal lysates were subjected to immunoprecipitation using the anti-IRβ antibody followed by immunoblotting with anti-Grb14 (L) and reprobed with anti-IRβ (M) antibodies. (N) Densitometric analysis of Grb14 immunoblots was performed in the linear range of detection, and absolute values were then normalized to IRβ. Data are means ± standard deviations (n = 2). The dark-adapted control was set as 100%. *, P < 0.05.
Grb14 interacts with PTP1B in vivo.
To investigate the in vivo interaction between Grb14 and PTP1B, the retinal lysates from dark- and light-adapted rats were subjected to immunoprecipitation with PTP1B antibody. The immunoprecipitates were subjected to immunoblot analysis with Grb14 antibody. The results indicate a significantly higher binding of Grb14 to PTP1B in light-adapted than in dark-adapted retinas (Fig. 1F and H). The same blot was reprobed with PTP1B antibody to ensure the presence of PTP1B under both dark- and light-adapted conditions (Fig. 1G). These results suggest that Grb14 binds to PTP1B in vivo and inhibits PTP1B activity. These results also indicate that Grb14 may undergo some kind of posttranslational modification in light, preferably tyrosine phosphorylation, that may be responsible for its interaction with PTP1B. PTP1B is known to interact with phosphorylated peptide regions in the substrates as well as with synthetic peptide mimetics (8, 18).
In vivo phosphorylation of Grb14.
To determine whether Grb14 undergoes a light-dependent tyrosine phosphorylation in vivo, the retinal lysates from dark- and light-adapted rats were subjected to immunoprecipitation with normal IgG or anti-Grb14 antibody. The immunoprecipitates were subjected to immunoblot analysis with anti-PY99 antibody and reprobed with anti-Grb14 antibody. The results indicate a significantly increased tyrosine phosphorylation on Grb14 in light-adapted retinas compared to the dark-adapted retinas (Fig. 1I to K). These results suggest that Grb14 undergoes a light-dependent tyrosine phosphorylation in vivo.
Increased binding of Grb14 to IR in dark-adapted retinas.
To determine the binding levels of Grb14 to IR, dark- and light-adapted retinal lysates were subjected to immunoprecipitation using anti-IRβ antibody followed by immunoblot analysis with anti-Grb14 and reprobed with anti-IRβ antibody. The results indicate a significantly higher binding of Grb14 to IR in dark-adapted than in light-adapted retinas (Fig. 1L to N). The lesser binding of Grb14 with IR in light suggests that Grb14 may also bind to other binding partners in light, which results in less binding to the IR.
Light-induced activation of Src mediates the phosphorylation of Grb14 in retina.
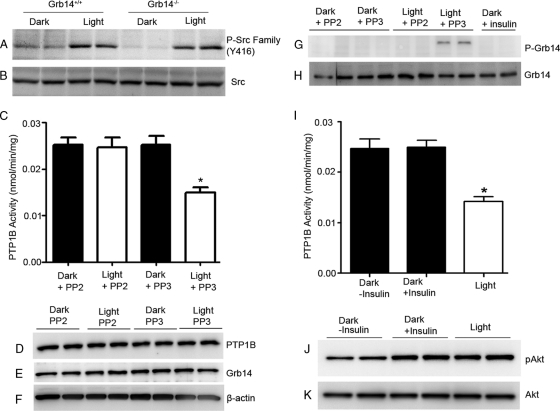
Light induces an association of Src with bleached photoreceptor outer segment membranes (17); however, Src kinase activation under dark- and light-adapted conditions has never been reported. To determine Src kinase activation, we subjected the retinal lysates from dark- and light-adapted wild-type and Grb14−/− mice to immunoblotting with anti-phospho-Src-family (Y416) and anti-Src antibodies. Activation of Src occurred under the light-adapted conditions in both wild-type and Grb14−/− mice (Fig. 2A). Total Src levels were comparable between wild-type and Grb14−/− mice under both dark- and light-adapted conditions (Fig. 2B). These data suggest that the activation of Src kinase in light may mediate the phosphorylation of Grb14 in retina.
Fig. 2.
Light-induced activation of Src mediates the phosphorylation of Grb14 in retina. (A and B) Retinal lysates from dark- and light-adapted wild-type and Grb14−/− mice were subjected to immunoblotting with anti-phospho-Src-family (Y416) (A) and anti-Src (B) antibodies. (C) Ex vivo retinal explants (n = 5) from dark-adapted rats were placed in DMEM containing either 100 μM PP2 or 100 μM PP3 and incubated for 10 min in the dark. Half of the retinas were then exposed to light for 30 min. The inhibitor-treated and untreated retinal ex vivo explants were subjected to immunoprecipitation with anti-PTP1B antibody, and the PTP1B activity was measured. Data are means ± standard deviations (n = 5). *, P < 0.05. (D to F) The lysates were also subjected to immunoblotting with anti-PTP1B (D), anti-Grb14 (E), and anti-β-actin (F) antibodies. (G and H) The inhibitor-treated and untreated retinal ex vivo explants were subjected to immunoprecipitation with anti-Grb14, and the Grb14 phosphorylation (G) and total levels of Grb14 in the immunoprecipitates (H) were measured. (I) The retinal ex vivo cultures were also incubated in the dark in DMEM containing 1 μM insulin for 5 min before lysis. One separate set of retinal ex vivo cultures was kept in light for 30 min. The dark (insulin-treated and untreated cultures)- and light-adapted retinal ex vivo explants were subjected to immunoprecipitation with anti-PTP1B antibody for activity measurements. Data are means ± standard deviations (n = 5). *, P < 0.05. (J and K) The lysates (duplicate) were subjected to immunoblotting with anti-pAkt (J) and anti-Akt (K) antibodies.
To determine whether light-induced phosphorylation of Grb14 is mediated through the light-induced activation of Src kinase, the ex vivo retinal explants from dark-adapted mice were incubated with either the Src family kinase inhibitor PP2 or the Src family kinase inhibitor control PP3 for 10 min in the dark before light adaptation for 30 min or maintained in the dark for 30 min. Ex vivo retinal explants were also incubated in DMEM containing 1 μM insulin for 5 min in the dark before lysis. PTP1B immunoprecipitates from the dark- and light-adapted PP2- and PP3-treated retinas were assayed for PTP1B activity. Inhibition of Src kinase activity by PP2 resulted in the loss of light-dependent inhibition of PTP1B activity in the retina (Fig. 2C). To confirm that the activity difference between PP2 and PP3 conditions was not due to differences in the protein expression levels of PTP1B and Grb14, the PP2- and PP3-treated lysates were subjected to immunoblotting with the anti-PTP1B and anti-Grb14 antibodies, respectively. The comparable levels of PTP1B (Fig. 2D) and Grb14 (Fig. 2E) were observed under both conditions. In the presence of PP2, we failed to observe the phosphorylation of Grb14 but did so in the presence of PP3 under light-adapted conditions (Fig. 2G). We also did not observe any insulin-mediated phosphorylation of Grb14 in the retina (Fig. 2G). Consistent with this observation, even the insulin treatment failed to inhibit the PTP1B activity ex vivo (Fig. 2I); however, insulin treatment did activate the Akt (Fig. 2J), suggesting that Grb14 is not a substrate of IR and that its phosphorylation is upstream of IR activation. These results suggest that the light-induced activation of Src kinase phosphorylates Grb14 in vivo.
Photobleaching of rhodopsin regulates Src-mediated phosphorylation of Grb14.
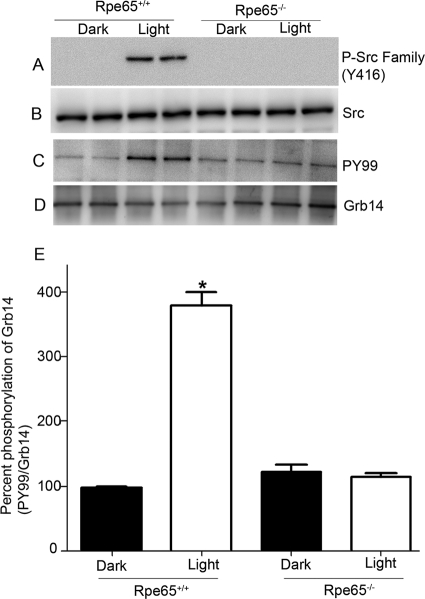
We previously reported significantly higher levels of PTP1B activity in retinal pigment epithelium 65-knockout mice (Rpe65−/−) than in wild-type mice under light-adapted conditions (34). Rpe65−/− mice have opsin in their photoreceptor outer segments but do not form photobleachable rhodopsin (activated form) due to the absence of regeneration of the chromophore 11-cis-retinal (37). To determine whether Src-mediated phosphorylation of Grb14 is signaled through photobleaching of rhodopsin, we subjected the retinal lysates from dark- and light-adapted wild-type and Rpe65−/− mice to immunoblotting with the anti-phospho-Src-family (Y416) and anti-Src antibodies. Activation of Src kinase was found under light-adapted conditions in the wild-type mice but not in the Rpe65−/− mice (Fig. 3A). The levels of total Src were comparable between the wild-type and Rpe65−/− mice (Fig. 3B). These data suggest that photobleaching of rhodopsin is necessary for the activation of Src in the retina. To further confirm whether the rhodopsin-mediated activation of Src is responsible for the phosphorylation of Grb14, we subjected the retinal lysates of the wild-type and Rpe65−/− mice to immunoprecipitation with anti-Grb14 antibody followed by immunoblot analysis with anti-PY99 antibody (Fig. 3C) and reprobing with anti-Grb14 (Fig. 3D) antibody. Loss of light-dependent phosphorylation of Grb14 occurred in the Rpe65−/− mice, but Grb14 phosphorylation occurred in the wild-type mice under light-adapted conditions (Fig. 3C and E). These results suggest that rhodopsin mediates the activation of Src kinase, which in turn phosphorylates the retinal Grb14 in vivo.
Fig. 3.
Photobleaching of rhodopsin regulates Src-mediated phosphorylation of Grb14. (A and B) Retinal lysates from dark- and light-adapted wild-type and Rpe65−/− mice were subjected to immunoblotting with anti-phospho-Src-family (Y416) (A) and anti-Src (B) antibodies. (C and D) The dark- and light-adapted retinal lysates from wild-type and Rpe65−/− mice were subjected to immunoprecipitation with anti-Grb14 antibody and immunoblotted with anti-PY99 (C) and reprobed with anti-Grb14 (D) antibodies. (E) Densitometric analysis of Grb14 phosphorylation normalized to Grb14. Data are means ± standard deviations (n = 2). The dark control was set as 100%. *, P < 0.001 compared to dark-adapted control.
Effect of various domains of Grb14 on PTP1B activity in vitro.
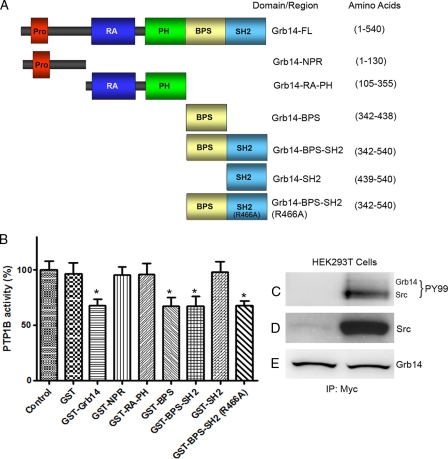
Grb14 has several protein-protein interaction domains. These individual domains/regions span the entire length of the Grb14 protein (Fig. 4A) and include an N-terminal proline-rich region (NPR; amino acids 1 to 130), Grb14-RA-PH (amino acids 105 to 355), Grb14-BPS (amino acids 342 to 438), Grb14-BPS-SH2 (amino acids 342 to 540), Grb14-SH2 (amino acids 439 to 540), and mutant Grb14-BPS-SH2 (R466A) (amino acids 342 to 540). While the RA domain can be expressed in bacteria, it does not fold properly, but the RA-PH domain is stable and properly folds in bacteria (14, 19). We expressed GST and various domains of Grb14 in bacteria and purified these fusion proteins. The purified fusion proteins were incubated with recombinant PTP1B and examined for the dephosphorylation of a synthetic phosphopeptide substrate (RRLIEDAEPYAARG) as a function of the PTP1B activity in vitro (35). A significant Grb14-mediated inhibition of PTP1B activity in vitro was found (Fig. 4B). The GST tag has no effect on the PTP1B activity, and the activity was almost comparable to the activity of the control (no additives) (Fig. 4B). The BPS, BPS-SH2, and mutant BPS-SH2 (R466A) domains of Grb14 were able to inhibit the PTP1B activity in vitro (Fig. 4B). The NPR, RA-PH, and SH2 domains of Grb14 failed to inhibit the PTP1B activity in vitro, and these activities were comparable to those of the control (Fig. 4B). These results suggest that the BPS domain is responsible for the observed inhibition of PTP1B activity by Grb14. As bacteria do not express tyrosine kinases, the observed inhibition of PTP1B activity by the BPS domain represents the inhibition by the nonphosphorylated BPS domain.
Fig. 4.
Effect of Grb14 and its various domains on PTP1B activity. (A) The domain organization of Grb14 and length of each domain/region are depicted. (B) GST, GST-Grb14-FL, GST-NPR (N-terminal proline-rich region), GST-RA-PH, GST-BPS, GST-BPS-SH2, GST-SH2, and GST-BPS-SH2 (R466A) were expressed in E. coli. The fusion proteins were purified and incubated (12 μM) with PTP1B enzyme and examined for the dephosphorylation of a phosphopeptide as a function of PTP1B activity. Data are means ± standard deviations (n = 3). *, P < 0.05; Control, no additives. (C to E) Myc-tagged Grb14 coexpressed with Myc-tagged Src in HEK293T cells. Protein extracts expressing Myc-Grb14 were coimmunoprecipitated with anti-Myc antibody and immunoblotted with anti-PY99 (C) antibody and reprobed with anti-Myc (E) antibody. The presence of Src was tested by immunoblot analysis using anti-Myc (D) antibody.
SRC fails to phosphorylate Grb14 in HEK293T cells.
To study the effect of Grb14 phosphorylation on PTP1B activity, we coexpressed Myc-tagged Grb14 with and without active Src. We have been unable to detect tyrosine phosphorylation of Grb14 by immunoblotting by PY99 antibody (Fig. 4C and D). The autophosphorylation of Src can be detected with PY99 antibody (Fig. 4C). The discrepancy of data between in vivo and HEK cell expression studies reflects cell type-specific effects, for example, the expression of a scaffold in retina that links Grb14 and Src.
Effect of phosphorylated BPS domain on the inhibition of the PTP1B activity.
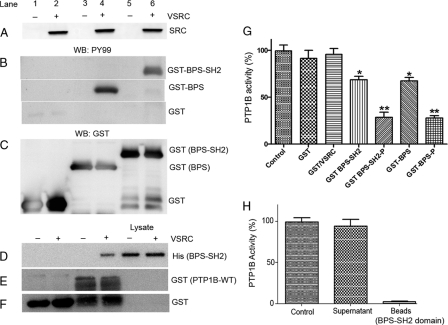
To experimentally analyze the potential role of phosphorylation of BPS-Grb14 on PTP1B inhibition, the GST-tagged BPS (amino acids 342 to 438) or BPS-SH2 (R466A) (amino acids 342 to 540) domain of Grb14 was expressed or coexpressed along with a tyrosine kinase, viral SRC (VSRC), in Escherichia coli (33). GST alone was used as control. The BPS-SH2 (R466A) domain had a significantly higher expression level than that of the BPS domain in E. coli. Further, replacement of Arg466 with Ala in the SH2 domain crippled its ability to interact with the phosphotyrosine peptides, thereby limiting its ability to sequester the phosphopeptide substrate or diluting the interpretation by interacting with other proteins in a biological system. For easy understanding, we have described this mutant in the text as the BPS-SH2 domain. The purified GST fusion proteins were immunoblotted with antiphosphotyrosine (PY99) antibody, and the VSRC expression was verified by anti-SRC antibody (Fig. 5A). To ensure equal amounts of fusion proteins in each sample, the PY99 blot was reprobed with anti-GST antibody (Fig. 5C). A tyrosine kinase-dependent phosphorylation of both the BPS and BPS-SH2 domains of Grb14 occurred in E. coli (Fig. 5B).
Fig. 5.
Grb14 inhibits the PTP1B activity through its BPS domain. (A to F) GST (lanes 1 and 2), GST-BPS (lanes 3 and 4), and GST-BPS-SH2 (lanes 5 and 6) constructs were either expressed alone (lanes 1, 3, and 5) or coexpressed with a kinase, VSRC (lanes 2, 4, and 6), in E. coli. Expressed proteins were subjected to immunoblotting with the anti-Src antibody (A). GST fusion proteins were purified by glutathione-Sepharose beads, and fusions were subjected to immunoblotting with anti-PY99 (B) and reprobed with anti-GST (C) antibodies. The His-tagged BPS domain of Grb14 was either expressed or coexpressed with VSRC, and the proteins were incubated with GST or GST-PTP1B followed by use in a GST pulldown assay. The bound proteins were subjected to immunoblotting with anti-His (D), and the blot was reprobed with the anti-GST (E and F) antibodies. (G) Purified nonphosphorylated and phosphorylated BPS and BPS-SH2 fusion proteins (12 μM) were examined for their effect on PTP1B activity. Data are means ± standard deviations (n = 3). **, P < 0.001; *, P < 0.05; P, phosphorylated; Control, no additives. PTP1B peptide substrate was preincubated with or without the recombinant GST-BPS domain for 30 min. The BPS domain was recovered by the addition of glutathione-Sepharose beads. (H) Following centrifugation, recombinant PTP1B enzyme was added to both the supernatant and the beads and the PTP1B activity was measured. Data are means ± standard deviations (n = 30. Control, no additives (PTP1B + peptide).
To examine the physical interaction between BPS-Grb14 and PTP1B, the His-tagged BPS-SH2 domain was either expressed alone or coexpressed with VSRC in E. coli. The nonphosphorylated or phosphorylated BPS domains were incubated either with GST or with GST-PTP1B and subjected to a GST pulldown assay. The bound proteins were subjected to SDS-PAGE followed by immunoblotting with anti-His antibody. A pulldown of BPS Grb14 by PTP1B was observed only under phosphorylated conditions (Fig. 5D). To ensure equal amounts of fusion proteins in each pulldown, we reprobed the blot with anti-GST antibody (Fig. 5E and F). The band that we observed in the GST (Fig. 5F) under the PTP1B lanes could be due to the degradation of PTP1B fusion.
The phosphorylated and nonphosphorylated fusion proteins were tested for their effect on PTP1B activity, and both BPS and BPS-SH2 domains of Grb14 were able to inhibit the recombinant PTP1B activity in vitro. However, the percentage of inhibition by the BPS domain when it is phosphorylated is significantly higher than that when it is not phosphorylated (Fig. 5G). The control GST or GST/VSRC had no effect on the PTP1B activity (Fig. 5G). The control GST/VSRC also indicates that the observed inhibition is not due to any contaminating phosphotyrosine protein(s) from E. coli. These results suggest that the BPS domain of Grb14 may be an inhibitor of PTP1B activity and that its inhibitory potential is phosphorylation dependent.
To rule out the possibility that BPS-SH2 sequesters the peptide substrate in the PTP1B assay, we preincubated the peptide substrate in the presence or absence of the GST-BPS-SH2 domain for 30 min followed by its recovery by glutathione-Sepharose beads. Following centrifugation, recombinant PTP1B was added to both the supernatant and beads and measured for the PTP1B activity. The PTP1B activity in the supernatant was similar to the activity of the control (absence of BPS domain), and we found no PTP1B activity associated with the beads (Fig. 5H). This experiment suggests that the effect of the BPS domain on PTP1B activity is not due to sequestering the peptide substrate and that the PTP1B inhibition that we described (Fig. 5G) is due to the direct effect of the BPS domain on PTP1B.
Kinetic characterization of BPS domain effect on PTP1B activity.
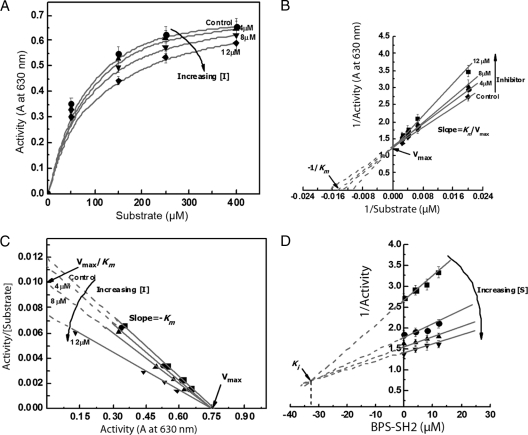
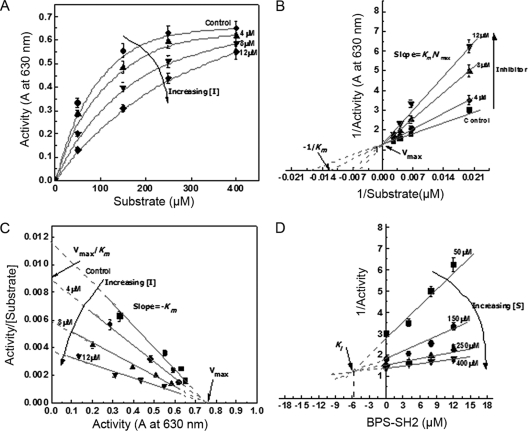
To assess the effect of both nonphosphorylated (Fig. 6) and phosphorylated (Fig. 7) BPS-SH2 Grb14 on PTP1B activity, kinetic parameters Vmax and Km and the inhibition constant (Ki) were calculated from initial velocities (Fig. 6A and 7A) and Eadie-Hofstee (Fig. 6C and 7C) and Dixon (Fig. 6D and 7D) plots. The mode of inhibition of PTP1B was evaluated from the double reciprocal plots titrated with different concentrations of substrate in the presence of various concentrations of the BPS-SH2 domain. The apparent Michaelis constant (Km) of PTP1B for its substrate increased from 61 ± 10 to 70 ± 10, 76 ± 10, and 94 ± 10 at nonphosphorylated BPS-SH2 domain concentrations of 0, 4.0, 8.0, and 12.0 μM, respectively (Fig. 6B and C). The Km of PTP1B for its substrate increased from 61 ± 10 to 83 ± 10, 140 ± 15, and 192 ± 15 at phosphorylated BPS-SH2 domain concentrations of 0, 4.0, 8.0, and 12.0 μM, respectively (Fig. 7B and C). Our data indicate that BPS-Grb14 interacts with PTP1B and inhibits its activity in a competitive manner in both phosphorylated and nonphosphorylated states. However, the Dixon plots (Fig. 6D and 7D) reveal that the phosphorylated BPS has a 5.4-fold stronger inhibition effect as indicated by lower Ki (6 μM) than does the nonphosphorylated state (Ki, 32.5 μM). These results indicate that phosphorylated BPS-Grb14 has a stronger inhibitory effect on PTP1B activity than does its nonphosphorylated counterpart.
Fig. 6.
Kinetic characterization of the nonphosphorylated BPS domain of Grb14 on PTP1B. (A) The effect of various concentrations of PTP peptide substrate on PTP1B activity in the presence of various concentrations (0, 4, 8, and 12 μM) of GST-BPS-SH2 domain in the nonphosphorylated state. Data are means ± standard deviations (n = 3). (B) Double reciprocal plot of the data shows the competitive nature of inhibition. (C) Eadie-Hofstee plot of the data further indicating the competitive nature of inhibition. (D) Dixon plot (to calculate Ki) with various concentrations of GST-BPS-SH2 as abscissa and reciprocal of PTP1B activity as ordinate. A, absorbance at 630 nm.
Fig. 7.
Kinetic characterization of phosphorylated BPS domain of Grb14 on PTP1B. (A) The effect of various concentrations of PTP peptide substrate on PTP1B activity in the presence of various concentrations (0, 4, 8, and 12 μM) of GST-BPS-SH2 domain in the phosphorylated state. Data are means ± standard deviations (n = 3). (B) Double reciprocal plot of the data shows the competitive nature of inhibition. (C) Eadie-Hofstee plot of the data further indicating the competitive nature of inhibition. (D) Dixon plot (to calculate Ki) with various concentrations of GST-BPS-SH2 as abscissa and reciprocal of PTP1B activity as ordinate. A, absorbance at 630 nm.
Molecular determinants of BPS-Grb14 involved in interaction with PTP1B activity.
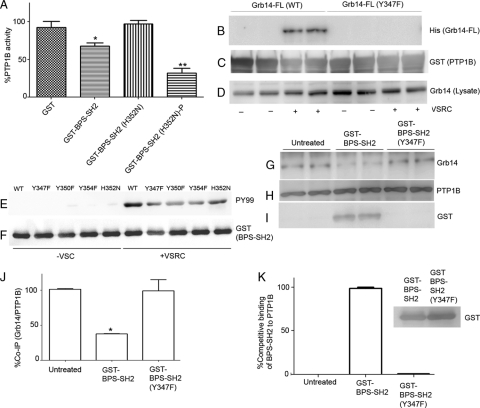
The molecular modeling of Grb14 peptide (amino acids 345 to 357) with the PTP1B active site revealed a potential interaction of His352 of Grb14 with Asp48 of PTP1B due to close proximity leading to possible ionic salt bridge formation (data not shown). The replacement of His352 with Asn resulted in the complete loss of the nonphosphorylated BPS-SH2 domain-induced inhibitory effect on PTP1B (Fig. 8A), but the PTP1B activity was significantly inhibited when it was phosphorylated (Fig. 8A). The inhibition may be through a salt bridge formation with Asp-48, which probably leads to blocking or interfering with the PTP1B active site. This interaction may be sufficient to dictate the association of Grb14 with the active site of PTP1B, rendering it a competitive inhibitor, even in a nonphosphorylated state. Several studies have also suggested that a potent and selective inhibitor may be designed by targeting the region containing Arg47 and Asp48 (1, 2). The apparent lack of binding of BPS-Grb14 with PTP1B under nonphosphorylated conditions (coimmunoprecipitation [co-IP] or GST pulldown) could be due to a weak interaction through His352 between PTP1B and Grb14, and this transient interaction could not be easily detected. However, in solution, the nonphosphorylated BPS domain is able to inhibit the PTP1B activity.
Fig. 8.
Identification of molecular determinants in BPS domain of Grb14 that inhibit the PTP1B activity under nonphosphorylated and phosphorylated conditions. (A) GST, GST-BPS-SH2, and nonphosphorylated and phosphorylated histidine mutant (H352N) were tested for their effect on PTP1B activity. Data are means ± standard deviations (n = 3). **, P < 0.001; *, P < 0.05; P, phosphorylated. (B to D) His-tagged full-length Grb14 and Grb14-Y347F mutant were either expressed alone or coexpressed with a kinase, VSRC, in E. coli. Expressed proteins were incubated with GST or GST-PTP1B followed by use in a GST pulldown assay. The bound proteins were subjected to immunoblotting with anti-His (B), the blot was reprobed with anti-GST (C), and the lysates were reprobed with anti-Grb14 antibodies (D). (E and F) GST-BPS-SH2 (WT) and the respective tyrosine and histidine mutants (Y347F, Y350F, H352N, and Y354F) were either expressed alone or coexpressed with VSRC, and the purified proteins were subjected to immunoblotting with anti-PY99 (E) and reprobed with anti-GST (F) antibodies. (G to I) In vivo tyrosine phosphorylation on Tyr347 residue in Grb14. Retinal lysates from light-adapted rats were immunoprecipitated with anti-PTP1B antibody, and the immune complexes were incubated with phosphorylated, purified soluble fusion proteins of either GST-BPS-SH2 or GST-BPS-SH2-Y347F mutant or a control without any treatment for 30 min. The immune complexes were washed and subjected to immunoblot analysis with anti-Grb14 (G) and reprobed with anti-PTP1B (H) and anti-GST (I) antibodies. (J and K) Densitometric analysis of Grb14 normalized to PTP1B (J) and GST-BPS-SH2 or GST-BPS-SH2-Y347F mutant normalized to PTP1B (K). Data are means ± standard deviations (n = 2). The wild-type BPS-SH2 binding was set as 100% (K). Inset, immunoblot analysis of GST-BPS-SH2 or GST-BPS-SH2-Y347F mutant with anti-GST antibody.
Examination of BPS domain using the phosphorylation site prediction program (7) indicated a putative tyrosine phosphorylation site on Tyr347. To determine that phosphorylated Tyr347 is a prerequisite for the association of BPS-Grb14 with PTP1B, His-tagged full-length Grb14 and mutant Grb14-Y347F were either expressed alone or coexpressed with VSRC in E. coli. The nonphosphorylated or phosphorylated Grb14 was incubated with GST-PTP1B and subjected to a GST pulldown assay (Fig. 8C). The bound proteins were subjected to SDS-PAGE followed by immunoblotting with the anti-His antibody (Fig. 8B). To ensure the expression of wild-type and mutant Grb14, the lysates were subjected to immunoblotting with the anti-His antibody (Fig. 8D). Replacement of Tyr347 with Phe abolished the binding of Grb14 to PTP1B, whereas the binding of wild-type Grb14 was unaffected (Fig. 8B), suggesting that Tyr347 in the BPS domain plays a critical role in its physical interaction with the PTP1B. We also found that the mutant Grb14-Y347F failed to inhibit the PTP1B activity in vitro (data not shown).
The mutants of BPS-SH2 Grb14 (Y347F, Y350F, Y354F, and His352N) were either expressed alone or coexpressed with VSRC as GST fusion proteins. The purified fusion proteins were immunoblotted with anti-PY99 (Fig. 8E) and reprobed with anti-GST (Fig. 8F) antibody to ensure equal amounts of fusion proteins. All mutant proteins were tyrosine phosphorylated (Fig. 8E). However, the tyrosine mutants had less phosphorylation than did the wild type and His352 mutant (Fig. 8E). We have also examined other tyrosine mutants in the BPS region (Y350F and Y354F), and these mutants were still able to associate with PTP1B and inhibit its activity in vitro (data not shown).
In vivo tyrosine phosphorylation on Tyr347 residue in Grb14.
Since we do not have a phosphospecific Tyr347 antibody, we adapted an alternative competitive approach to demonstrate the in vivo tyrosine phosphorylation on residue 347 of Grb14. Retinal lysates from light-adapted rats were immunoprecipitated with anti-PTP1B antibody, and the immune complexes were incubated with phosphorylated purified soluble fusion proteins of either GST-BPS-SH2 or GST-BPS-SH2-Y347F mutant or a control without any treatment for 30 min. The immune complexes were washed and subjected to SDS-PAGE followed by immunoblot analysis with anti-Grb14 and reprobed with anti-PTP1B and anti-GST antibodies. The results indicate a significant reduction in the binding interaction between Grb14 and PTP1B under GST-BPS-SH2 fusion protein treatment conditions compared to that under conditions of no treatment and GST-BPS-SH2-Y347F mutant treatment (Fig. 8G and J). This experiment suggests that wild-type phosphorylated BPS domain competes with endogenous Grb14 for the same binding site on PTP1B. Consistent with this hypothesis, we found the association of GST-BPS-SH2 fusion protein with PTP1B, but not GST-BPS-SH2-Y347F mutant (Fig. 8I and K), even though we added the same amounts of wild-type and mutant fusion proteins (Fig. 8K, inset). Collectively, these findings suggest that in retina Grb14 undergoes light-dependent tyrosine phosphorylation on Tyr347.
PTP1B does not regulate Src activation in the retina.
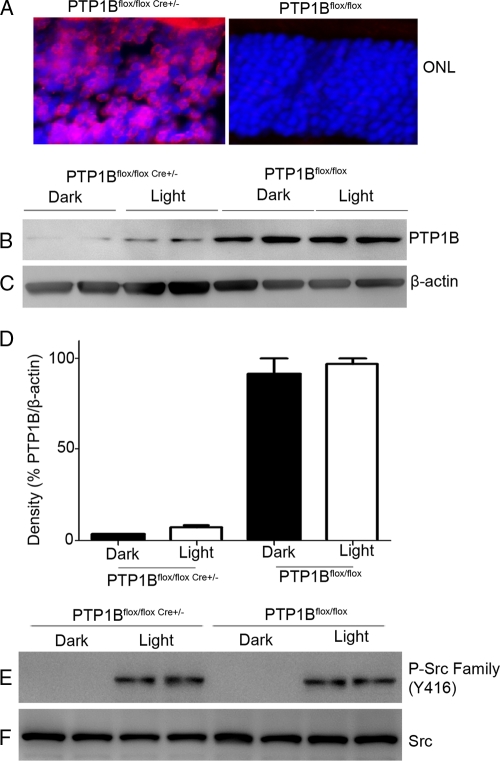
To investigate the functional significance of PTP1B on Src activation in rods, we generated a rod photoreceptor-specific conditional PTP1B−/− model. For this purpose, mice expressing the floxed PTP1B (4) allele were bred with a mouse line that expresses Cre recombinase under the control of the mouse rod opsin promoter (25). We assessed Cre recombinase cellular localization in the retinas of wild-type and knockout (KO) littermates by immunofluorescence microscopy using an anti-Cre antibody. Cre expression was localized to rod photoreceptor nuclei in the conditional PTP1B KO retinas, while Cre recombinase staining was not detected in wild-type controls (Fig. 9A). These experiments suggest that Cre expression was present only in rod photoreceptors. To evaluate the efficiency of Cre-mediated deletion of the PTP1B in rod photoreceptors, retinal lysates from the conditional PTP1B KO mice and wild-type controls were subjected to immunoblot analysis with anti-PTP1B, and the results indicate that KO mice had approximately 2 to 4% of PTP1B protein content compared to that from wild-type controls (Fig. 9B and D). β-Actin expression was used as loading control (Fig. 9C). These results indicate that mouse opsin-driven Cre successfully deleted most of the PTP1B gene expression in rod photoreceptor cells.
Fig. 9.
PTP1B does not regulate Src activation. Rod photoreceptor-specific deletion of PTP1B was done by cross-breeding floxed PTP1B mice to mice that express Cre recombinase under the control of the mouse rod opsin promoter. (A) Immunohistochemical analysis of Cre recombinase expression in knockout and wild-type control retinas harvested from littermates. (B and C) Knockout and wild-type control retinal extracts from dark- and light-adapted mice were subjected to immunoblot analysis with anti-PTP1B (B) and anti-β-actin (C) antibodies. (D) Densitometric analysis of PTP1B normalized to β-actin. Data are means ± standard deviations (n = 2). The dark control was set as 100%. (E and F) Retinal lysates from dark- and light-adapted wild-type and PTP1B−/− mice were subjected to immunoblotting with anti-phospho-Src-family (Y416) (E) and anti-Src (F) antibodies.
To determine whether PTP1B contributes to Src kinase activation, we subjected the retinal lysates from dark- and light-adapted wild-type and PTP1B−/− mice to immunoblotting with anti-phospho-Src-family (Y416) and anti-Src antibodies. Activation of Src occurred under light-adapted conditions for both wild-type and PTP1B−/− mice (Fig. 9E). Total Src levels were comparable between wild-type and PTP1B−/− mice under both dark- and light-adapted conditions (Fig. 9F). These data suggest that Src activation is not regulated by PTP1B and the lack of Src activation under dark-adapted conditions is not due to the dephosphorylation of Src by PTP1B.
DISCUSSION
The BPS domain of Grb14 was previously shown to have negative insulin signaling function, and the important question that remains to be addressed is the spatial and temporal aspect of this regulatory mechanism and the interplay with protein tyrosine phosphatases. Recently, several studies indicated that Grb14 plays an important role in various tissues, including a protective role against cardiac dysfunction (28), and our recent study also indicated that Grb14 is a physiological modulator of cyclic nucleotide gated channel function in rod photoreceptor cells (19). In the retina, Grb14 protects the retinal IR activation, which is an important element of photoreceptor neuroprotection (31). Our current study provides the molecular evidence that the BPS domain of Grb14 directly interacts with PTP1B and inhibits its activity, and this may be the mechanism underlying the Grb14-mediated protective role in growth factor signaling.
Based on our experimental evidence, we propose that the state of phosphorylation on Grb14 may determine its affinity toward either IR or PTP1B; nonphosphorylated form toward IR and phosphorylated form toward PTP1B. Consistent with this idea, in retina significantly increased tyrosine phosphorylation of IR and the activation of its downstream effectors were observed in light compared to dark-adapted conditions (27, 30). Under these conditions, we found decreased PTP1B activity in light compared to dark-adapted conditions (34), and the decreased PTP1B activity in light correlates with the higher Grb14 phosphorylation observed in the current study. The phosphorylation-dependent affinity shift of Grb14 toward PTP1B is further evident from our finding that an increased amount of Grb14 associates with IR in the dark. In light, significantly less binding of Grb14 to IR was observed; presumably, the phosphorylated form may have higher affinity/recognition toward PTP1B than IR, which results in the inhibition of PTP1B activity and subsequent activation of IR signaling. The kinetic characterization studies show that the phosphorylated BPS domain has a 5.4-fold-lower Ki than does the nonphosphorylated form and that the established Ki is in the μM range. The apparently high Ki not only is achievable locally in a microcosm physiologically but also indicates that Grb14-induced inhibition is not a complete inhibition and arises more from the fact that the interaction of the protein itself is altered between PTP1B and IR, depending on the phosphorylation status. These observations further suggest that Grb14 phosphorylation may increase or decrease its association with other proteins which can affect PTP1B activity in cells. We also found that the pool of Grb14 that coimmunoprecipitates with PTP1B is tyrosine phosphorylated, as light-adapted retinal PTP1B immunoprecipitates treated with calf intestinal phosphatase resulted in the loss of Grb14 association with PTP1B (data not shown). The phosphorylation switch has also been observed in Grb10. Tyr67 has been identified as the major phosphorylation site in Grb10, and mutation of Tyr67 to Gly increased the affinity of Grb10 for IR (24), further confirming our study result that the nonphosphorylated form has a higher affinity for IR.
Grb14 has several potential tyrosine phosphorylation sites, but we found that phosphorylation of Tyr347 is critical for its interaction with PTP1B and the resultant inhibition of PTP1B activity. The functional role of other tyrosine residues in Grb14 is currently unknown. The tyrosine residue that we identified in bovine Grb14 (Tyr347; Tyr350 in human) is highly conserved in Grb7 and Grb10 but not Caenorhabditis elegans F10E9.6 (12); however, we have not tested Grb7 and Grb10 effects on PTP1B inhibition. On the basis of the conserved nature, they are highly likely to inhibit the PTP1B activity. Importantly, the corresponding site in mouse Grb10 is recorded as being phosphorylated on Phosphosite (www.phosphosite.org). A recent study shows that Tyr347 is present in the helical extension to the PH domain involved in dimerization of Grb14 (14). It is possible that a nonphosphorylated Grb14 is involved in dimerization, and dimerization itself may be disrupted upon phosphorylation, thus making the protein more available and exposed. The activity of PTP1B is generally regulated in vivo by oxidation and reduction reactions involving the invariant cysteine in the active site (38). In addition, the activity of PTP1B is also shown to be regulated by glutathionylation of the active site cysteine (3) and posttranslational modifications by SUMOlyation (10) and Akt-mediated phosphorylation (36). Most of the inhibitors of PTP1B are small molecules, and so far, no in vivo protein inhibitor(s) has been identified. This is, to the best of our knowledge, the first molecular evidence that Grb14 is a physiological endogenous inhibitor of PTP1B activity in vivo. The effect of Grb14 on PTP1B may have further implications in the PTP1B involvement in leptin (41), STAT (16), and Ras signaling (15) pathways (Fig. 10).
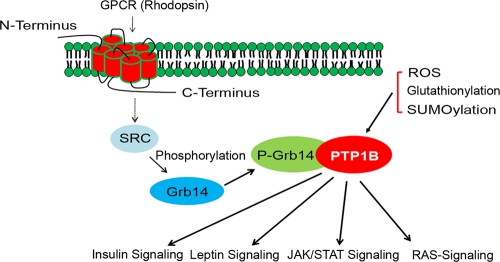
Fig. 10.
Working model for the Grb14-mediated inhibition of PTP1B activity. Known PTP1B regulators are reactive oxygen species (ROS), glutathionylation, and SUMOylation. The novel PTP1B inhibitory pathway is activated through G-protein-coupled receptor rhodopsin-mediated activation of a nonreceptor tyrosine kinase Src, which in turn phosphorylates Grb14. Phosphorylated Grb14 binds to PTP1B and inhibits its activity through a competitive mode of inhibition. Grb14-mediated inhibition of PTP1B may have significance in insulin, leptin, Jak/Stat, and Ras signaling pathways. P-Grb14, phosphorylated Grb14.
It is interesting that in vivo Grb14 phosphorylation is a retina-specific phenomenon, as we failed to observe this phosphorylation in other tissues such as heart and liver (data not shown). Further, we also failed to observe Grb14 phosphorylation in HEK293T cells coexpressed with tyrosine kinase Src. These observations suggest the expression of a specific scaffold/adapter in retina which is able to associate or bring Grb14 and Src together and perhaps also suggest the absence of this scaffold in other tissues. Grb14 acts as a pseudosubstrate inhibitor that binds to the substrate binding groove of IR kinase and thus functions as a selective inhibitor of insulin signaling (13). Convincing evidence for a negative role of Grb14 in insulin signaling exists in that Grb14-deficient mice show enhanced glucose tolerance and insulin sensitivity (9). Identification and characterization of the scaffold that is specially expressed in retina may help us to understand the regulation of Grb14 in other tissues.
We previously reported the importance of an endogenous IR/PI3K/Akt pathway in providing neuroprotection to rod and cone photoreceptors under normal and stressed conditions (22, 26, 32, 42). Our earlier studies suggest that PTP1B is a negative regulator of IR in photoreceptors (34). Our current study on Grb14 suggests that it plays a key role in the signaling pathways in vision or in the maintenance of cells involved in the vision process. Although our current study did not directly address the neuroprotective role of Grb14 in the retina, it clearly describes the mechanism of a novel rhodopsin-mediated inhibition of PTP1B activity by Grb14. These studies suggest that rhodopsin may have additional previously uncharacterized signaling functions in photoreceptor neurons. It is interesting that mice with ablation of IR (32) and its downstream effectors, Akt2 (26) and Bcl-xl (42), in rod photoreceptors did not exhibit any detectable functional phenotypes when they were raised under normal lighting conditions. However, significant photoreceptor degeneration was observed when the mice were exposed to bright-light stress. In future, we will challenge Grb14 KO mice with light stress and examine whether Grb14-deficient mice are more susceptible to light stress-induced photoreceptor degeneration in the presence and absence of phosphomimetics of Grb14 peptides. Consistent with this hypothesis, we recently reported that intravenous injection of an allosteric inhibitor of PTP1B protects rats against light stress-induced retinal degeneration through the protection of IR phosphorylation (34). These studies would further advance our understanding of the functional role of Grb14 in photoreceptor neuroprotection.
Expression of the Src family tyrosine kinases cSrc (39) and p59fyn (21) was observed in retina. The expression of other Src family members has not been studied in the retina. In the retina, Src activation is under the control of rhodopsin, and this finding may have clinical relevance, as we have previously reported elevated levels of retinal PTP1B in STZ-induced diabetes (35), Rpe65−/− mice deficient in photobleaching of rhodopsin (a mouse model for Leber congenital amaurosis [LCA] type 2), and a mouse model of retinitis pigmentosa (34). We recently reported that PTP1B activity negatively regulated the neuroprotective survival signaling in the retina. It has been reported previously that PTP1B catalyzed the dephosphorylation of Src phosphotyrosine 527 and contributes to the activation of Src (6, 43). In retina, deletion of PTP1B did not influence light-dependent activation of Src, suggesting that Src activation is not mediated through PTP1B. Our current study suggests that light-dependent activation of Src mediates the phosphorylation of the BPS region of Grb14, which in turn inhibits the PTP1B activity in vivo. Activators of Src or agents that induce the phosphorylation of Grb14 or inhibitors of PTP1B may serve as potential therapeutic agents to treat retinal degeneration.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (EY016507-05; EY00871; EY12190).
We appreciate the technical help of Dustin Allen. We thank Roger J. Daly (Garvan Medical Institute, Sydney, Australia) for providing us the Grb14−/− mice. We thank Benjamin Neel (Ontario Cancer Institute, Canada) for providing PTP1B floxed mice and Yun Le (University of Oklahoma Health Science Center) for providing opsin-Cre mice. We acknowledge Jian-xing Ma (University of Oklahoma Health Sciences Center) for providing Rpe65−/− mice.
Footnotes
Published ahead of print on 26 July 2011.
REFERENCES
- 1. Ala P. J., et al. 2006. Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J. Biol. Chem. 281:38013–38021 [DOI] [PubMed] [Google Scholar]
- 2. Asante-Appiah E., et al. 2002. The structure of PTP-1B in complex with a peptide inhibitor reveals an alternative binding mode for bisphosphonates. Biochemistry 41:9043–9051 [DOI] [PubMed] [Google Scholar]
- 3. Barrett W. C., et al. 1999. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38:6699–6705 [DOI] [PubMed] [Google Scholar]
- 4. Bence K. K., et al. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12:917–924 [DOI] [PubMed] [Google Scholar]
- 5. Bereziat V., et al. 2002. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem. 277:4845–4852 [DOI] [PubMed] [Google Scholar]
- 6. Bjorge J. D., Pang A., Fujita D. J. 2000. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J. Biol. Chem. 275:41439–41446 [DOI] [PubMed] [Google Scholar]
- 7. Blom N., Gammeltoft S., Brunak S. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351–1362 [DOI] [PubMed] [Google Scholar]
- 8. Combs A. P. 2010. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J. Med. Chem. 53:2333–2344 [DOI] [PubMed] [Google Scholar]
- 9. Cooney G. J., et al. 2004. Improved glucose homeostasis and enhanced insulin signalling in Grb14-deficient mice. EMBO J. 23:582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dadke S., et al. 2007. Regulation of protein tyrosine phosphatase 1B by sumoylation. Nat. Cell Biol. 9:80–85 [DOI] [PubMed] [Google Scholar]
- 11. Daly R. J. 1998. The Grb7 family of signalling proteins. Cell. Signal. 10:613–618 [DOI] [PubMed] [Google Scholar]
- 12. Daly R. J., Sanderson G. M., Janes P. W., Sutherland R. L. 1996. Cloning and characterization of GRB14, a novel member of the GRB7 gene family. J. Biol. Chem. 271:12502–12510 [DOI] [PubMed] [Google Scholar]
- 13. Depetris R. S., et al. 2005. Structural basis for inhibition of the insulin receptor by the adaptor protein Grb14. Mol. Cell 20:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Depetris R. S., Wu J., Hubbard S. R. 2009. Structural and functional studies of the Ras-associating and pleckstrin-homology domains of Grb10 and Grb14. Nat. Struct. Mol. Biol. 16:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dube N., Cheng A., Tremblay M. L. 2004. The role of protein tyrosine phosphatase 1B in Ras signaling. Proc. Natl. Acad. Sci. U. S. A. 101:1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukada T., Tonks N. K. 2003. Identification of YB-1 as a regulator of PTP1B expression: implications for regulation of insulin and cytokine signaling. EMBO J. 22:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghalayini A. J., et al. 2002. Light-dependent association of Src with photoreceptor rod outer segment membrane proteins in vivo. J. Biol. Chem. 277:1469–1476 [DOI] [PubMed] [Google Scholar]
- 18. Groves M. R., Yao Z. J., Roller P. P., Burke T. R., Jr., Barford D. 1998. Structural basis for inhibition of the protein tyrosine phosphatase 1B by phosphotyrosine peptide mimetics. Biochemistry 37:17773–17783 [DOI] [PubMed] [Google Scholar]
- 19. Gupta V. K., Rajala A., Daly R. J., Rajala R. V. 2010. Growth factor receptor-bound protein 14: a new modulator of photoreceptor-specific cyclic-nucleotide-gated channel. EMBO Rep. 11:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holt L. J., Siddle K. 2005. Grb10 and Grb14: enigmatic regulators of insulin action—and more? Biochem. J. 388:393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ingraham C. A., Cooke M. P., Chuang Y. N., Perlmutter R. M., Maness P. F. 1992. Cell type and developmental regulation of the fyn proto-oncogene in neural retina. Oncogene 7:95–100 [PubMed] [Google Scholar]
- 22. Ivanovic I., et al. 2011. Deletion of the p85alpha regulatory subunit of phosphoinositide 3-kinase in cone photoreceptor cells results in cone photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 52:3775–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasus-Jacobi A., et al. 1998. Identification of the rat adapter Grb14 as an inhibitor of insulin actions. J. Biol. Chem. 273:26026–26035 [DOI] [PubMed] [Google Scholar]
- 24. Langlais P., Dong L. Q., Hu D., Liu F. 2000. Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family. Oncogene 19:2895–2903 [DOI] [PubMed] [Google Scholar]
- 25. Le Y. Z., et al. 2006. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol. Vis. 12:389–398 [PubMed] [Google Scholar]
- 26. Li G., et al. 2007. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 27:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li G., Rajala A., Wiechmann A. F., Anderson R. E., Rajala R. V. 2008. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J. Neurochem. 107:1382–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin R. C., et al. 2010. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler. Thromb. Vasc. Biol. 30:724–732 [DOI] [PubMed] [Google Scholar]
- 29. Punzo C., Kornacker K., Cepko C. L. 2009. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat. Neurosci. 12:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajala A., et al. 2007. G-protein-coupled receptor rhodopsin regulates the phosphorylation of retinal insulin receptor. J. Biol. Chem. 282:9865–9873 [DOI] [PubMed] [Google Scholar]
- 31. Rajala A., et al. 2009. Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation. Biochemistry 48:5563–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajala A., Tanito M., Le Y. Z., Kahn C. R., Rajala R. V. 2008. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 283:19781–19792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajala R. V., McClellan M. E., Chan M. D., Tsiokas L., Anderson R. E. 2004. Interaction of the retinal insulin receptor beta-subunit with the P85 subunit of phosphoinositide 3-kinase. Biochemistry 43:5637–5650 [DOI] [PubMed] [Google Scholar]
- 34. Rajala R. V., Tanito M., Neel B. G., Rajala A. 2010. Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J. Biol. Chem. 285:8894–8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajala R. V., Wiskur B., Tanito M., Callegan M., Rajala A. 2009. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest. Ophthalmol. Vis. Sci. 50:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ravichandran L. V., Chen H., Li Y., Quon M. J. 2001. Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 15:1768–1780 [DOI] [PubMed] [Google Scholar]
- 37. Redmond T. M., et al. 1998. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 20:344–351 [DOI] [PubMed] [Google Scholar]
- 38. Salmeen A., et al. 2003. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423:769–773 [DOI] [PubMed] [Google Scholar]
- 39. Sorge L. K., Levy B. T., Maness P. F. 1984. pp60c-src is developmentally regulated in the neural retina. Cell 36:249–257 [DOI] [PubMed] [Google Scholar]
- 40. Taghibiglou C., et al. 2002. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 277:793–803 [DOI] [PubMed] [Google Scholar]
- 41. Zabolotny J. M., et al. 2002. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2:489–495 [DOI] [PubMed] [Google Scholar]
- 42. Zheng L., Anderson R. E., Agbaga M. P., Rucker III E. B., Le Y. Z. 2006. Loss of BCL-XL in rod photoreceptors: increased susceptibility to bright light stress. Invest. Ophthalmol. Vis. Sci. 47:5583–5589 [DOI] [PubMed] [Google Scholar]
- 43. Zhu S., Bjorge J. D., Fujita D. J. 2007. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 67:10129–10137 [DOI] [PubMed] [Google Scholar]