Abstract
Lipoteichoic acid (LTA) is a crucial cell envelope component in Gram-positive bacteria. In Staphylococcus aureus, the polyglycerolphosphate LTA molecule is synthesized by LtaS, a membrane-embedded enzyme with five N-terminal transmembrane helices (5TM domain) that are connected via a linker region to the C-terminal extracellular enzymatic domain (eLtaS). The LtaS enzyme is processed during bacterial growth, and the eLtaS domain is released from the bacterial membrane. Here we provide experimental evidence that the proteolytic cleavage following residues 215Ala-Leu-Ala217 is performed by the essential S. aureus signal peptidase SpsB, as depletion of spsB results in reduced LtaS processing. In addition, the introduction of a proline residue at the +1 position with respect to the cleavage site, a substitution known to inhibit signal peptidase-dependent cleavage, abolished LtaS processing at this site. It was further shown that the 5TM domain is crucial for enzyme function. The observation that the construction of hybrid proteins between two functional LtaS-type enzymes resulted in the production of proteins unable to synthesize LTA suggests that specific interactions between the 5TM and eLtaS domains are required for function. No enzyme activity was detected upon expression of the 5TM and eLtaS domains as separate fragments, indicating that the two domains cannot assemble postsynthesis to form a functional enzyme. Taken together, our data suggest that only the full-length LtaS enzyme is active in the LTA synthesis pathway and that the proteolytic cleavage step is used as a mechanism to irreversibly inactivate the enzyme.
INTRODUCTION
Lipoteichoic acid (LTA) is a vital cell envelope component of Gram-positive bacteria. In the human pathogen Staphylococcus aureus, LTA is composed of a linear polyglycerolphosphate (PGP) chain that is tethered to the bacterial membrane via the glycolipid diglucosyl-diacylglycerol (Glc2-DAG) (14, 16, 39). In S. aureus, only one enzyme, the lipoteichoic acid synthase LtaS, appears to be required for the production of the PGP backbone chain of LTA (22). In its absence, bacteria are unable to synthesize the polymer, display severe morphological defects, and cease to grow unless the medium is supplemented with high concentrations of salt or sucrose, which are thought to act as osmoprotectants (22, 35). Even under these conditions permissive for growth, S. aureus cells lacking LTA have a drastically altered cell shape (35), altogether demonstrating the importance of the polymer for normal growth, morphology, and cell physiology.
There is strong biochemical evidence that the glycerolphosphate (GroP) subunits of LTA are derived from the head group of the membrane lipid phosphatidylglycerol (PG), and additional experiments suggested that the polymer is extended by the repeated addition of GroP subunits to the distal end of the chain (28, 42). Recently, it was established that the purified enzymatic domain of the S. aureus LtaS protein is sufficient to cleave fluorescently labeled PG to DAG, providing further evidence that this lipid is the physiological substrate of LtaS and is used for the production of LTA (26). Based on all available evidence, it was concluded that the S. aureus LtaS enzyme performs multiple functions, which include recognition and hydrolysis of the membrane lipid PG, selection of the glycolipid anchor Glc2-DAG, transfer of the initial GroP subunit to prime LTA synthesis, and subsequent GroP polymerization to form the PGP backbone chain.
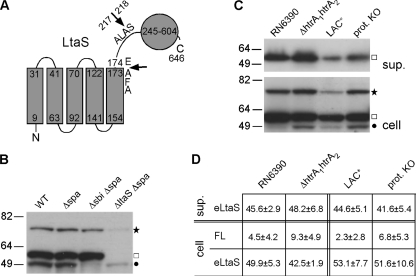
S. aureus LtaS is a predicted membrane protein with five N-terminal transmembrane helices (5TM domain) connected via a linker region to a large C-terminal extracellular enzymatic domain (eLtaS) (Fig. 1A). In previous work, it was shown that a large fraction of the protein is processed in S. aureus and that the eLtaS domain is released into the culture supernatant as well as partially retained within the cell wall fraction (32, 35). Proteomic studies suggested that the processing site is within the linker region following residues 215Ala-Leu-Ala217 (Fig. 1A) (46). The biological significance of this processing step and its effect on LtaS enzyme function are currently not understood and are addressed in this study.
Fig. 1.
LtaS protein and its processing in defined S. aureus protease mutant strains. (A) Predicted membrane topology of S. aureus LtaS. The LtaS protein contains 5 N-terminal TM helices (5TM domain) followed by a 44-amino-acid linker region and the extracellular eLtaS domain. Residues 245 to 604 in the eLtaS fragment align with the PF00884 sulfatase domain, and proposed processing sites are indicated with arrows. (B and C) LtaS protein detection by Western blotting. (B) Strains RN4220 (WT), SEJ1 (Δspa), SEJ1 Δsbi (Δsbi Δspa), and 4S5 (ΔltaS Δspa) were grown to mid-log phase, and cell fractions were prepared and analyzed by Western blotting using a rabbit polyclonal antibody raised against the eLtaS domain. Full-length LtaS- and eLtaS-specific bands are indicated to the right of the panel, with a star and a square, respectively. Note that two unspecific bands are present in the ΔltaS lane. The faint top band is of the same mobility as the full-length LtaS band, and the bottom band is the Sbi protein and is annotated with a filled circle. Sizes of protein standards are given in kDa on the left. (C) S. aureus strain RN6390 and the isogenic mutant strain RN6390 htrA1 htrA2, as well as S. aureus strain LAC* and the isogenic strain LAC* Δaur ΔsspAB ΔscpA spl::erm (prot. KO), were grown to mid-log phase, and equal amounts of supernatant (sup.) and cell fractions were analyzed by Western blotting using LtaS antibody. Full-length LtaS is indicated with a star, eLtaS with a square, and the unspecific Sbi protein with a filled circle. (D) Densitometry analysis. The amounts of the three different LtaS-specific bands (cleaved eLtaS in the supernatant fraction, full-length LtaS in the cell fraction [FL], and cleaved eLtaS in the cell fraction) were quantified by densitometry. The total amount of LtaS protein was set to 100% for each strain, and % values for the three different fractions were calculated. The experiment was performed in triplicate, and the average % values and standard deviations are shown. Two-tailed two-value equal-variance Student's t test was performed, and no statistically significant differences were detected.
One or more LtaS orthologues are found in the genomes of those other members of the Firmicutes that produce a PGP-type LTA polymer (22, 38). These proteins are also predicted to assemble as membrane proteins, with an N-terminal 5TM domain and a linker region that is followed by a large extracellular C-terminal enzymatic domain. All of these proteins appear to be involved in the LTA synthesis process, and in some instances, distinct enzymatic activities can be ascribed to different proteins (41, 44, 45). For instance, LtaP, one of the two Listeria monocytogenes LtaS family proteins, and YvgJ, one of the four Bacillus subtilis LtaS family proteins, promote the production of a GroP-glycolipid intermediate by transferring the initial GroP subunit onto the lipid anchor, but these proteins lack polyglycerolphosphate synthesis activity (44, 45). Hence, these enzymes were named LTA primases to distinguish them from the processive LTA synthase enzymes, which are capable of producing polyglycerolphosphate chains.
The protein processing step is not unique to the S. aureus LtaS enzyme, and cleaved products of the B. subtilis orthologues YflE (LtaSBS) and YfnI were detected in culture supernatants, with N-terminal sequencing locating the cleavage sites after the fifth TM helix, following residues 213Ala-Leu-Ala215 and 212Ala-Tyr-Ala214, respectively (23). Similarly, processed forms of LtaS-type enzymes were found in the supernatants of L. monocytogenes, Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Staphylococcus epidermidis cultures (2, 18, 37, 44). In all cases where the cleavage site of LtaS-type enzymes has been mapped, it occurs after an Ala-X-Ala motif, and the location of the motif within the protein is conserved in LtaS-type enzymes. Well-studied proteases that are known to cleave after an Ala-X-Ala motif are type I signal peptidases (43). Typical signal peptidase substrates are secreted proteins that contain an N-terminal signal peptide, which consists of a few positively charged amino acids, a single hydrophobic helix, and the cleavage site. In contrast, processing of polytopic membrane proteins by signal peptidases seems to be rare (6, 43). However, two pieces of evidence indicate that LtaS-type proteins might be such exceptions and substrates of signal peptidases. A proteomic study of B. subtilis showed that processing of the LtaS-type enzyme YfnI was diminished in the combined absence of two signal peptidases, SipT and SipV (1). More recently, it was reported that addition of the signal peptidase-specific inhibitor arylomycin to S. epidermidis cultures led to a reduction in the amount of a processed LtaS fragment in the culture supernatant (37). However, in the same study, an alternative LtaS processing site, located after residues 171Ala-Phe-Ala173 and closer to the 5TM domain, was proposed based on the results of a computer prediction program (Fig. 1A) (37).
In this work, we provide further evidence that LtaS-type enzymes are cleaved by type I signal peptidase enzymes, as depletion of the single S. aureus signal peptidase SpsB resulted in severely reduced LtaS processing, while inactivation of any of the other known S. aureus proteases did not affect protein cleavage. Through mutant and mass spectrometry analysis, we confirmed that, as initially suggested, the predominant LtaS processing site is after the conserved 215Ala-Leu-Ala217 sequence, located 44 amino acids after the end of the last TM helix, and implications for signal peptidase-mediated cleavage are discussed. Furthermore, we show that the 5TM domain of LtaS is essential for enzyme function in vivo and that the 5TM and eLtaS domains cannot function in the LTA synthesis process once they are split. Therefore, we suggest that the LtaS processing step serves as a mechanism to irreversibly inactivate the enzyme.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in this study are listed in Table 1. Escherichia coli and S. aureus strains were grown at 37°C with shaking in Luria-Bertani (LB) medium and tryptic soy broth (TSB), respectively. Where appropriate, the medium was supplemented with antibiotics, 0.5 to 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), or 200 to 300 ng/ml anhydrotetracycline (Atet).
Table 1.
Bacterial strains used in this study
| Strain | Relevant featuresa | Reference or source |
|---|---|---|
| Escherichia coli strains | ||
| XL1 Blue | Cloning strain; Tetr; ANG127 | Stratagene |
| CLG190 | Cloning strain which reduces plasmid copy number; Tetr; ANG1141 | Dana Boyd; 20 |
| ANG126 | pTS1 in DH5α; vector with temperature-sensitive replication in S. aureus; Ampr | 34 |
| ANG201 | pCN34 in E. coli; E. coli-S. aureus shuttle vector; Kanr Ampr | 12 |
| ANG243 | pCL55 in XL1 Blue; S. aureus single-site integration vector; Ampr | 30 |
| ANG284 | pitet in XL1 Blue; pCL55 containing Atet-inducible promoter; Ampr | 21 |
| ANG374 | pCL55-ypfP/ltaA in XL1 Blue; Ampr | 21 |
| ANG474 | pMutin-HA in E. coli; Ampr | Bacillus Genetic Stock Center |
| ANG483 | pOK-ltaS in XL1 Blue; Kanr | 22 |
| ANG503 | pCL55-ltaS in XL1 Blue; Ampr | 45 |
| ANG505 | pCL55-yflE in CLG190; Ampr | This study |
| ANG584 | pitet-ltaS-his in XL1 Blue; ltaS with C-terminal His tag under Atet-inducible promoter control; Ampr | This study |
| ANG590 | pitet-sec-eltaS in XL1 Blue; protein A signal peptide fused to eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1112 | pitet-ltaS-T300A in XL1 Blue; ltaS(T300A) allele under Atet-inducible promoter control; Ampr | 32 |
| ANG1211 | pitet-TMsrtA-eltaS in XL1 Blue; sortase TM fused to eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1212 | pitet-1TM-eltaS in XL1 Blue; first TM of ltaS fused to linker plus eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1213 | pitet-3TM-eltaS in XL1 Blue; first three TM of ltaS fused to linker plus eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1214 | pitet-5TMltaA-eltaS in XL1 Blue; first five TM of ltaA fused to linker plus eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1221 | pCN34-5TM in XL1 Blue; 5TM of ltaS under native promoter control; Kanr Ampr | This study |
| ANG1222 | pCN34-ltaS in XL1 Blue; ltaS under native promoter control; Kanr Ampr | This study |
| ANG1242 | pOK-ltaSS218P in XL1 Blue; ltaS(S218P) allele under native promoter control; Kanr | This study |
| ANG1244 | pitet-ltaSS218P in XL1 Blue; ltaS(S218P) allele under Atet-inducible promoter control; Ampr | This study |
| ANG1336 | pitet-ltaSTM-eyflE-his in CLG190; 5TM of ltaS fused to linker plus eyflE domain with C-terminal His tag under Atet-inducible promoter control; Ampr | This study |
| ANG1337 | pitet-yflETM-eltaS in XL1 Blue; 5TM of yflE fused to linker plus eltaS domain under Atet-inducible promoter control; Ampr | This study |
| ANG1368 | pitet-ltaSS218P-his in XL1 Blue; ltaS(S218P) allele with C-terminal His tag under Atet-inducible promoter control; Ampr | This study |
| ANG1577 | pALC2073 in XL1 Blue; Ampr; ANG1577 | 5 |
| ANG1631 | pCN34itetH in XL1 Blue; pCN34 containing Atet-inducible promoter from pALC2073; Kanr Ampr | This study |
| ANG1689 | pCN34itet-ltaST300A in XL1 Blue; ltaS(T300A) allele under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG1811 | pCN34itetH-spsB in XL1 Blue; spsB under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG1815 | pTS1-ΔspsB::erm in XL1 Blue; Ampr | This study |
| ANG1963 | pCN34itetH-spsB-T114C in XL1 Blue; spsB with point mutation T114C under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG1964 | pMutinHA-spsB-T114C in XL1 Blue; spsB with point mutation T114C under IPTG-inducible promoter control; Ampr | This study |
| ANG2007 | pCL55spac-spsB-T114C in XL1 Blue; spsB with point mutation T114C under IPTG-inducible promoter control; Kanr Ampr | This study |
| ANG2158 | pCN34itet-sec-eltaS in XL1 Blue; protein A signal peptide fused to eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2159 | pCN34itet-TMsrtA-eltaS in XL1 Blue; sortase TM fused to eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2160 | pCN34itet-1TM-eltaS in XL1 Blue; first TM of ltaS fused to linker plus eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2161 | pCN34itet-3TM-eltaS in XL1 Blue; first three TM of ltaS fused to linker plus eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2162 | pCN34itet-5TMltaA-eltaS in XL1 Blue; first five TM of ltaA fused to linker plus eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2163 | pCN34itet-ltaSTM-eyflE-his in XL1 Blue; 5TM of ltaS fused to linker plus eyflE domain containing C-terminal His tag under Atet-inducible promoter control; Kanr Ampr | This study |
| ANG2164 | pCN34itet-yflETM-eltaS in XL1 Blue; 5TM of yflE fused to linker plus eltaS domain under Atet-inducible promoter control; Kanr Ampr | This study |
| Staphylococcus aureus strains | ||
| RN4220 | Transformable laboratory strain; ANG113 | 29 |
| SEJ1 | RN4220 Δspa; ANG314 | 21 |
| ANG499 | RN4220 itaS; strain with IPTG-inducible ltaS expression; Ermr; IPTG added | 22 |
| ANG514 | pitet-ltaS integrated into strain ANG499; Ermr Camr; IPTG added | 22 |
| ANG513 | pitet integrated into strain ANG499; Ermr Camr; IPTG added | 22 |
| ANG587 | pitet-ltaS-his integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG595 | pitet-sec-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1130 | ANG499 with pCN34; Ermr Kanr; IPTG added | 45 |
| ANG1217 | pitet-TMsrtA-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1218 | pitet-1TM-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1219 | pitet-3TM-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1220 | pitet-5TMltaA-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1226 | ANG595 with pCN34; Ermr Camr Kanr; IPTG added | This study |
| ANG1227 | ANG595 with pCN34-5TM; Ermr Camr Kanr; IPTG added | This study |
| ANG1228 | ANG595 with pCN34-ltaS; Ermr Camr Kanr; IPTG added | This study |
| ANG1246 | pitet-ltaSS218P integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1346 | pitet-ltaSTM-eyflE-his integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1347 | pitet-yflETM-eltaS integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1370 | pitet-ltaSS218P-his integrated into strain ANG499; Ermr Camr; IPTG added | This study |
| ANG1571 | ANG499 with pCN34itet-ltaS; Ermr Kanr; IPTG added | 45 |
| ANG1658 | ANG499 with pCN34itet-yvgJ; Ermr Kanr; IPTG added | 45 |
| ANG1690 | ANG595 with pCN34itet-ltaST300A; Ermr Kanr; IPTG added | This study |
| ANG1755 | SEJ1 Δsbi | Lab strain collection |
| ANG1786 | 4S5; SEJ1 ΔltaS; LTA-negative suppressor strain that can grow without LTA | R. Corrigan |
| ANG1816 | SEJ1 with pCN34itet-spsB; Kanr | This study |
| ANG1820 | SEJ1 spsB::erm with pCN34itet-spsB; Ermr Kanr | This study |
| ANG2008 | SEJ1 Pspac-spsB; pCL55spac-spsB-T114C integrated into strain SEJ1; Camr | This study |
| ANG2009 | SEJ1 spsB::erm Pspac-spsB; spsB::erm transduced from strain ANG1820 into strain ANG2008; Ermr Camr; IPTG added | This study |
| ANG2165 | ANG499 with pCN34itet-sec-eltaS; Ermr Kanr; IPTG added | This study |
| ANG2166 | ANG499 with pCN34itet-TMsrtA-eltaS; Ermr Kanr; IPTG added | This study |
| ANG2167 | ANG499 with pCN34itet-1TM-eltaS; Ermr Kanr; IPTG added | This study |
| ANG2168 | ANG499 with pCN34itet-3TM-eltaS; Ermr Kanr; IPTG added | This study |
| ANG2169 | ANG499 with pCN34itet-5TMltaA-eltaS; Ermr Kanr; IPTG added | This study |
| ANG2170 | ANG499 with pCN34itet-ltaSTM-eyflE-his; Ermr Kanr; IPTG added | This study |
| ANG2171 | ANG499 with pCN34itet-yflETM-eltaS; Ermr Kanr; IPTG added | This study |
| RN6390 | RN6390; virulent laboratory strain derived from NCTC 8325; ANG2153 | 36 |
| RN6390 htrA1htrA2 | RN6390 htrA1htrA2; Camr Specr; ANG1649 | 40 |
| AH1263 | LAC*; Erm-sensitive community-acquired methicillin-resistant S. aureus LAC strain; ANG1575 | 9 |
| AH1919 | LAC* Δaur ΔsspAB ΔscpA spl::erm (LAC* protease KO); ANG2038 | This study |
| Bacillus subtilis strain | ||
| 168 | Transformable lab strain; trpC2; ANG1691 | 11 |
Antibiotics were used at the following concentrations: for E. coli cultures, ampicillin (Amp) at 100 μg/ml and kanamycin (Kan) at 30 μg/ml; for S. aureus cultures, erythromycin (Erm) at 10 μg/ml, chloramphenicol (Cam) at 7.5 to 10 μg/ml, kanamycin (Kan) at 90 μg/ml, spectinomycin (Spec) at 100 μg/ml, and IPTG at 1 mM. Note that an Atet-inducible promoter was used in S. aureus.
Strain and plasmid construction.
Primers used in this study are listed in Table S1 in the supplemental material. Plasmid pitet-sec-eltaS was constructed for the expression of a secreted version of eLtaS. This plasmid was obtained by amplifying the protein A signal peptide sequence from S. aureus RN4220 chromosomal DNA with primers 401 and 404 and the eltaS sequence from plasmid pCL55-ltaS by use of primers 405 and 319. The resulting products were fused by splice overlap extension PCR (SOE PCR) (24) using primers 401 and 319. The final PCR product was cut with AvrII/BglII and ligated with plasmid pitet, which had been cut with the same enzymes. Plasmid pitet-sec-eltaS was initially obtained in E. coli XL1 Blue (strain ANG590) and subsequently integrated into the geh lipase gene of S. aureus ANG499, yielding strain ANG595(pitet-sec-eltaS).
Plasmids pitet-TMsrtA-eltaS and pitet-5TMltaA-eltaS were constructed for the expression of LtaS variants with altered staphylococcal membrane domains. The srtA TM sequence (TMsrtA) was amplified from S. aureus RN4220 chromosomal DNA by using primers 427 and 432, and the eltaS coding sequence was amplified from plasmid pCL55-ltaS by using primers 433 and 319. The ltaA 5TM sequence (5TMltaA) was amplified from plasmid pCL55-ypfP/ltaA by using primers 260 and 438, and the linker-plus-eltaS sequence was amplified from plasmid pCL55-ltaS by using primers 439 and 319. Appropriate PCR fragments were fused by SOE PCR with primers 427 and 319 (TMsrtA-eltaS) or 260 and 319 (5TMltaA-eltaS). The final PCR products were cloned with AvrII/BglII into plasmid pitet, as described above, and recovered in E. coli XL1 Blue (giving strains ANG1211 and ANG1214). The plasmids were subsequently electroporated into S. aureus ANG499, yielding strains ANG1217(pitet-TMsrtA-eltaS) and ANG1220(pitet-5TMltaA-eltaS).
Plasmids pitet-1TM-eltaS and pitet-3TM-eltaS were constructed for the expression of LtaS variants with shortened membrane domains. Gene fragments coding for the first or first three TM helices of ltaS were amplified using primer 318 in combination with primer 434 or 436, respectively, and the linker-plus-eltaS sequence was amplified using primer 319 in combination with primer 435 or 437, respectively. pCL55-ltaS was used as template DNA for all PCRs. The respective PCR products were fused by SOE PCR using primers 318 and 319, and the final PCR fragments were cut with AvrII/BglII and ligated with plasmid pitet. The resulting plasmids were recovered in E. coli XL1 Blue, giving strains ANG1212 and ANG1213, and subsequently introduced into S. aureus ANG499, yielding strains ANG1218(pitet-1TM-eltaS) and ANG1219(pitet-3TM-eltaS).
Plasmids pitet-ltaSTM-eyflE-his and pitet-yflETM-eltaS were constructed for the expression of S. aureus/B. subtilis LtaS hybrid proteins. The pitet-ltaSTM-eyflE-his plasmid was created by amplifying the ltaS TM sequence from plasmid pCL55-ltaS, using primers 318 and 528, and the linker-plus-eyflE sequence from plasmid pCL55-yflE, using primers 529 and 502, and a C-terminal His tag was introduced with the downstream primer. Resulting PCR products were fused using primers 318 and 502, cut with AvrII/BglII, and ligated with pitet as described above. The resulting plasmid was initially obtained in E. coli CLG190 (strain ANG1336) and subsequently electroporated into S. aureus ANG499, yielding strain ANG1346(pitet-ltaSTM-eyflE-his). Plasmid pitet-yflETM-eltaS was constructed by amplifying the yflE TM sequence from plasmid pCL55-yflE, using primers 326 and 526, and the linker-plus-eltaS sequence from plasmid pCL55-ltaS, using primers 527 and 319. PCR products were fused by SOE PCR using primers 326 and 319, and the resulting product was cut and ligated with plasmid pitet as described above. The plasmid pitet-yflETM-eltaS was initially obtained in E. coli XL1 Blue (strain ANG1337) and afterwards introduced into S. aureus ANG499, yielding strain ANG1347(pitet-yflETM-eltaS). Plasmid pCL55-yflE, which was used as the DNA template in PCRs, was constructed as follows. The yflE gene was amplified from B. subtilis 168 chromosomal DNA by using primers 324 and 325. The PCR product and plasmid pCL55 were cut with BamHI/KpnI and ligated. The plasmid pCL55-yflE was recovered in E. coli CLG190, resulting in strain ANG505.
Plasmids pCN34itet-sec-eltaS, pCN34itet-TMsrtA-eltaS, pCN34itet-1TM-eltaS, pCN34itet-3TM-eltaS, pCN34itet-5TMltaA-eltaS, pCN34itet-ltaSTM-eyflE-his, and pCN34itet-yflETM-eltaS were constructed for expression of the corresponding fusion proteins from a multicopy plasmid vector. Respective gene fusions and the pitet promoter region were amplified from plasmids pitet-sec-eltaS, pitet-TMsrtA-eltaS, pitet-1TM-eltaS, pitet-3TM-eltaS, pitet-5TMltaA-eltaS, pitet-ltaSTM-eyflE-his, and pitet-yflETM-eltaS by using primers 159 and 877. The resulting PCR products and vector pCN34 were cut with KpnI/PstI and ligated. Plasmids were initially obtained in E. coli strain XL1 Blue (strains ANG2158 to ANG2164) and subsequently introduced into S. aureus ANG499, yielding strains ANG2165(pCN34itet-sec-eltaS), ANG2166(pCN34itet-TMsrtA-eltaS), ANG2167(pCN34itet-1TM-eltaS), ANG2168(pCN34itet-3TM-eltaS), ANG2169(pCN34itet-5TMltaA-eltaS), ANG2170(pCN34itet-ltaSTM-eyflE-his), and ANG2171(pCN34itet-yflETM-eltaS).
Plasmids pCN34-5TM, pCN34-ltaS, and pCN34itet-ltaST300A were constructed to investigate the functionality of a split enzyme. For the construction of plasmids pCN34-ltaS and pCN34-5TM, the full-length ltaS gene or the 5TM region was amplified, including the ltaS promoter region from plasmid pCL55-ltaS, using primer pair 86/87 or 86/424, respectively. The PCR products and the pCN34 vector were cut with BamHI/SalI and ligated. For construction of plasmid pCN34itet-ltaST300A, ltaS(T300A) and the pitet promoter region were amplified from plasmid pitet-ltaS-T300A by using primers 159 and 800. The PCR product and pCN34 vector were cut with KpnI/SalI and ligated. Plasmids were recovered in E. coli XL1 Blue, resulting in strains ANG1221, ANG1222, and ANG1689. The three plasmids and the empty pCN34 vector were then electroporated into S. aureus ANG595, yielding strains ANG1226(pCN34), ANG1227(pCN34-5TM), ANG1228(pCN34-ltaS), and ANG1690(pCN34itet-ltaST300A), respectively.
Plasmid pitet-ltaSS218P was constructed for the expression of an LtaS variant with a Ser-to-Pro substitution at amino acid position 218. The mutation was initially introduced by QuikChange mutagenesis (Stratagene) in the vector pOK-ltaS, using primers 487 and 488, and the resulting plasmid, pOK-ltaSS218P, was recovered in E. coli XL1 Blue (strain ANG1242). The mutant ltaS allele was then amplified using primers 318 and 319 and cloned with AvrII/BglII into the pitet vector. The plasmid pitet-ltaSS218P was recovered in E. coli XL1 Blue (strain ANG1244) and subsequently electroporated into S. aureus strain ANG499, yielding strain ANG1246(pitet-ltaSS218P).
Plasmids pitet-ltaS-his and pitet-ltaSS218P-his were constructed for the expression of wild-type (WT) LtaS and the LtaSS218P variant fused to a C-terminal His tag and were used for protein purification from supernatants of S. aureus cultures. The respective genes were amplified from plasmids pCL55-ltaS and pitet-ltaSS218P by using primers 318 and 420. The PCR products and the pitet plasmid were cut with AvrII/BglII and ligated. Plasmids were obtained in E. coli XL1 Blue (strains ANG584 and ANG1368) and subsequently electroporated into S. aureus ANG499, yielding strains ANG587(pitet-ltaS-his) and ANG1370(pitet-ltaSS218P-his).
An S. aureus strain with IPTG-inducible spsB expression was constructed to study the effect on LtaS cleavage upon SpsB depletion. For construction of this strain, the plasmid pCN34itetH, which contains an Atet-inducible promoter system, was used. This plasmid was constructed by amplifying the Atet promoter/repressor region from plasmid pALC2073, using primers 909 and 948 in the first round of PCR and primers 908 and 948 in a second PCR to extend the multiple cloning site. The PCR product and vector pCN34 were cut with NarI/XmaI and ligated. The resulting plasmid, pCN34itetH, was recovered in E. coli XL1 Blue, yielding ANG1631. Next, the spsB gene was amplified from S. aureus RN4220 chromosomal DNA by using primers 1007 and 1008. The PCR product and the plasmid pCN34itetH were cut with KpnI/SalI and ligated. The resulting plasmid, pCN34itetH-spsB, was initially recovered in E. coli XL1 Blue (strain ANG1811) and subsequently introduced into S. aureus SEJ1, yielding strain ANG1816(pCN34itetH-spsB). Plasmid pTS1-ΔspsB::erm was constructed for the deletion of the spsB gene. One-kilobase fragments up- and downstream of spsB were amplified from RN4220 genomic DNA with primer pairs 1010/1011 and 1013/1015, respectively, and the erm cassette was amplified from plasmid pMutin-HA by using primers 1012 and 1014. The three purified PCR products were fused by SOE PCR using primers 1010 and 1015, and the final PCR product and vector pTS1 were cut with KpnI/EcoRI and ligated. The plasmid pTS1-ΔspsB::erm was recovered in E. coli XL1 Blue (strain ANG1815) and subsequently introduced into S. aureus [giving ANG1816(SEJ1 pCN34itetH-spsB)] and stably maintained at 30°C in the presence of chloramphenicol (Cam) and kanamycin (Kan). Shifting the temperature to 43°C resulted in a single crossover event and in integration of the pTS1-ΔspsB::erm plasmid into the chromosome. After confirming the chromosomal integration by PCR, the culture was shifted back to 30°C and grown in the absence of Cam but the presence of 50 ng/ml Atet (to induce SpsB expression from the covering plasmid pCN34itetH-spsB), which allowed for a second crossover event and deletion/replacement of the chromosomal spsB gene with an erm marker. Erythromycin (Erm) resistance of the resulting S. aureus strain, ANG1820(SEJ1 spsB::erm pCN34itetH-spsB), was confirmed on selective antibiotic plates, and the replacement of the spsB gene with the erm marker was verified by PCR. The basal expression of SpsB from the covering plasmid pCN34itetH-spsB, even in the absence of Atet, was too high to enable the use of this strain for any further analysis. To this end, S. aureus strain ANG2009, with tightly controlled spsB expression, was constructed by transducing the chromosomal spsB::erm deletion from strain ANG1820 into S. aureus strain ANG2008, which contained a copy of the spsB gene under control of the IPTG-inducible spac promoter control at a different (geh gene) chromosomal location. For this strain construction, an internal BamHI site in spsB needed to be inactivated for cloning purposes. This was done by introducing a T-to-C nucleotide substitution at position 114 within the spsB coding sequence by QuikChange mutagenesis in plasmid pCN34itetH-spsB, using primers 1203 and 1204. This mutation does not alter the protein sequence. Plasmid pCN34itetH-spsB-T114C was recovered in E. coli XL1 Blue, yielding strain ANG1963. Next, the mutated spsB allele was amplified using primers 896 and 1183, and the resulting product and the pMutin-HA vector were cut with HindIII/KpnI and ligated. The plasmid pMutinHA-spsB-T114C was recovered in E. coli XL1 Blue, giving strain ANG1964. Subsequently, the mutated spsB allele, including the IPTG-inducible spac promoter and the lacI repressor region, were excised by cutting plasmid pMutinHA-spsB-T114C with BamHI, and the appropriate DNA fragment was gel purified and ligated with the BamHI-cut S. aureus single-site integration vector pCL55. The plasmid pCL55spac-spsB-T114C was obtained in E. coli XL1 Blue (strain ANG2007) and subsequently electroporated into S. aureus SEJ1, yielding strain ANG2008(SEJ1 Pspac-spsB). The spsB::erm region from strain ANG1820, described above, was then transduced into strain ANG2008 by using phage Φ85, yielding strain ANG2009(SEJ1 spsB::erm Pspac-spsB), with tightly controlled IPTG-inducible spsB expression. Sequences of all inserts were verified by fluorescence automated sequencing at the MRC Clinical Science Center Sequencing Facility at Imperial College London.
Construction of the extracellular protease mutant strain AH1919 (LAC* Δaur ΔsspAB ΔscpA Δspl::erm) was performed in a series of steps. Plasmids for construction of mutations in sspAB and scpA were made by using pJB38, which is a modified version of plasmid pKOR1 kindly provided by Jeffrey Bose and Kenneth Bayles. To construct pJB38-sspAB, regions flanking the sspAB genes were PCR amplified using oligonucleotide sets CLM459/CLM460 and CLM461/CLM462, with strain LAC* as the template. The two PCR products were spliced together by overlap extension, digested with SacI and SalI, and cloned into pJB38. To construct pJB38-scpA, regions flanking the scpA gene were PCR amplified using oligonucleotide sets CLM455/CLM457 and CLM456/CLM458. The two PCR products were spliced together, digested with EcoRI and KpnI, and cloned into pJB38. To construct the protease mutant strain, the aur gene was inactivated first by using pKOR1-aur (27) and a previously described knockout (KO) protocol (3). Next, the sspAB genes were deleted by using pJB38-sspAB according to the pKOR1 KO protocol, and subsequently the scpA gene was deleted in the same manner. Finally, the ΔsplABCDEF::erm (Δspl::erm) construct was crossed from strain AH750 (8) by using bacteriophage α80. After each construction step, the inactivation of the genes was confirmed using PCR. The final strain was called AH1919 and displayed all of the expected phenotypes for inactivation of the extracellular proteases, such as absence of clearing zones on milk agar plates and failure of supernatant preparations to cleave protease substrates (J. M. Mootz and A. R. Horswill, unpublished observations).
S. aureus growth curves, LTA and protein detection by Western blotting, and densitometry analysis.
S. aureus growth curves and LTA detection by Western blotting were performed as previously described (45). Growth curves using the inducible spsB S. aureus strain ANG2009 were performed with the following slight modifications. Overnight cultures of strain ANG2009 were washed as described previously (45) and then diluted 1:100 into 7 ml TSB medium with and without IPTG and appropriate antibiotics. Cultures were grown at 37°C with shaking, and bacterial growth was monitored by determining the optical density at 600 nm (OD600) every 2 h. At the 4-h time point, cultures were washed and back diluted, and at the 8-h time point, bacteria were back diluted again 1:100 into fresh medium to keep bacteria in the logarithmic growth phase. For the detection of cell-associated LTA and LtaS protein detection by Western blotting, 1-ml culture samples were withdrawn from bacterial cultures at the 4- or 8-h time point, as indicated in the text. Samples were prepared as described previously (21) and suspended in 2% SDS-containing sample buffer normalized to OD600 readings of bacterial cultures, that is, a 1-ml sample from a culture with an OD600 of 1 was suspended in 30 μl sample buffer. Samples were boiled for 20 min and spun at 17,000 × g for 5 min, and 10-μl aliquots were subsequently separated on 15% (LTA detection) or 10% (LtaS detection) SDS-PAGE gels. For detection of the LtaS protein in the culture supernatant, 1-ml culture aliquots were centrifuged at 17,000 × g for 5 min and 900 μl of the supernatant was transferred to a fresh tube containing 90 μl 100% trichloroacetic acid (TCA). Samples were vortexed briefly, incubated on ice for 1 h, and then centrifuged at 17,000 × g for 10 min. The supernatant was aspirated, and the TCA-precipitated protein pellet was washed twice with ice-cold acetone, each time by suspending the pellet in 1 ml ice-cold acetone, incubating the sample on ice for 10 min, and centrifuging it at 17,000 × g for 10 min. After the final centrifugation step, the pellet was air dried and suspended in 2× sample buffer normalized to OD600 readings as described above. Samples were boiled for 5 min and spun at 17,000 × g for 5 min, and 10-μl aliquots were subsequently analyzed by Western blotting.
For densitometry analysis, 2-fold dilutions of purified eLtaS protein were separated in 10% SDS-PAGE gels and the protein detected by Western blotting as described above, using an LtaS-specific antibody (32). Western blot signals were quantified using ImageJ64 software, and the linear signal range was determined in this manner. Next, equal amounts of cell and supernatant fraction samples, normalized based on culture OD600 readings as described above, were loaded on the same gel to ensure equal treatments and Western blot exposure times. The signals for the three different LtaS-specific bands (cleaved eLtaS in the supernatant fraction, full-length LtaS in the cell fraction, and cleaved eLtaS in the cell fraction) were quantified using ImageJ64 software. The total amount of protein (sum of the signals for all three LtaS-specific bands) was set to 100%, and the percentages of the three different LtaS protein bands were calculated accordingly. Experiments and densitometry analysis were performed at least in triplicate, and the average percent values and standard deviations are presented. Two-tailed two-sample equal-variance Student's t test was performed on the data, and statistically significant differences, with P values below 0.01, are indicated with asterisks, or P values are given in the text.
For LTA detection, a polyglycerolphosphate-specific LTA antibody (clone 55; Hycult Biotechnology) and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Cell Signaling Technologies) were used at 1:5,000 and 1:10,000 dilutions, respectively. For LtaS protein detection, a polyclonal eLtaS-specific antibody (32) and HRP-conjugated anti-rabbit antibody (Cell Signaling Technologies) were used at 1:20,000 and 1:10,000 dilutions, respectively. His-tagged proteins were detected with HRP-conjugated anti-His antibody (Sigma) at a 1:10,000 dilution. All experiments were performed at least three times, and representative Western blots are shown.
Purification of His-tagged LtaS fragments from S. aureus culture supernatant.
S. aureus strains ANG587(pitet-ltaS-his) and ANG1370(pitet-ltaSS218P-his) were used for the expression and purification of His-tagged proteins from the respective culture supernatants. To this end, strains ANG587 and ANG1370 were grown overnight at 37°C in 30 to 50 ml TSB medium containing 1 mM IPTG and appropriate antibiotics. The following day, cultures were washed three times with 30 to 50 ml TSB by repeated centrifugation and suspension. Subsequently, 2 to 4 liters of TSB medium containing 300 ng/ml Atet and appropriate antibiotics was inoculated 1:100 with washed bacterial suspensions, and cultures were grown at 37°C overnight. The next day, bacteria were removed by centrifugation for 15 min at 13,700 × g, and the culture supernatants were filtered through 0.2-μm nylon membranes (Whatman). His-tagged proteins were purified from these filtered culture supernatants by gravity flow chromatography. Supernatants were applied to equilibrated Ni-nitrilotriacetic acid (Ni-NTA) columns (1.5-ml column volume) (Qiagen) and washed extensively with buffer A (50 mM Tris, pH 7.5, 150 mM NaCl, 5% glycerol), followed by two additional wash steps with buffer A containing 10 and 50 mM imidazole. Proteins were eluted in four 1-ml fractions with buffer A containing 500 mM imidazole. Fractions containing purified protein were pooled, and protease inhibitors (Roche) were added to the suspension. Next, proteins were concentrated using Amicon Centricon columns (10-kDa cutoff), 20-μl aliquots were separated in 10% SDS-PAGE gels, and proteins were visualized by staining with Coomassie brilliant blue. The main protein band was excised and analyzed by standard tryptic digestion and mass spectrometry at the Taplin Biological Mass Spectrometry Facility at the Harvard Medical School.
S. aureus membrane lipid extraction and glycolipid detection by TLC.
Membrane lipids were isolated and analyzed from S. aureus strains ANG1130(pCN34), ANG1571(pCN34itet-ltaS), ANG1658(pCN34itet-yvgJ), ANG2165(pCN34itet-sec-eltaS), ANG2166(pCN34itet-TMsrtA-eltaS), ANG2167(pCN34itet-1TM-eltaS), ANG2168(pCN34itet-3TM-eltaS), ANG2169(pCN34itet-5TMltaA-eltaS), ANG2170(pCN34itet-ltaSTM-eyflE-his), and ANG2171(pCN34itet-yflETM-eltaS). Bacterial growth, lipid extraction, and glycolipid staining were performed as previously described (45). Experiments were performed at least three times, and a representative thin-layer chromatography (TLC) plate is shown.
RESULTS
LtaS processing is independent of Aur, Ssp, Scp, Spl, and HtrA proteases.
A large fraction of the S. aureus LtaS protein is cleaved during bacterial growth, and the extracellular eLtaS domain is released into the culture supernatant (17, 32, 46). S. aureus secretes a range of proteases, and to assess their contributions to LtaS processing, we set out to investigate LtaS protein processing in S. aureus strains with defined deficiencies in known extracellular proteases. To this end, a previously described polyclonal antibody raised against the extracellular eLtaS domain was used to detect the LTA synthase protein (32). Protein A and Sbi are two S. aureus proteins known to bind to antibodies. To establish unambiguously which of the bands recognized by the antibody were LtaS specific, Western blot analysis was performed on cell fraction samples isolated from WT RN4220, the isogenic protein A mutant strain SEJ1 (RN4220 Δspa), and an sbi spa mutant strain (RN4220 Δsbi Δspa) (Fig. 1B). In addition, the protein A-negative S. aureus strain 4S5, containing a complete ltaS deletion, was used in the same experiment. The construction of this strain and the reason that this strain can grow in the absence of LTA are described in a different report (R. Corrigan et al., submitted for publication). Two unspecific bands were detected for the ltaS deletion strain, despite the fact that human IgG was used during all antibody incubations to block unspecific antibody binding (Fig. 1B). The faster-migrating unspecific band most likely corresponds to Sbi, since this band was absent in the sample prepared from the sbi mutant strain (Fig. 1B, filled circle). A second, very faint unspecific band, with the same mobility as the full-length LtaS protein, was also observed for this ltaS deletion strain (Fig. 1B, star). However, the intensity of the top (full-length LtaS) band was drastically increased in the LtaS-expressing WT, spa mutant, and spa sbi mutant strains, and the unspecific band should therefore not significantly affect our conclusions, even for later experiments where we performed densitometry analysis. A second very strong band, corresponding to the cleaved eLtaS protein, was observed for all LtaS-expressing strains (Fig. 1B, square). After establishing which bands recognized by the antibody were LtaS specific, protein processing was analyzed in two different protease mutant strains. An S. aureus strain deleted for the two surface serine proteases HtrA1 and HtrA2 (RN6390 htrA1 htrA2) (40) and the isogenic WT RN6390 control strain were grown to mid-log phase. Supernatant and whole-cell protein fractions were prepared and the LtaS protein detected by Western blotting using a polyclonal antibody raised against the extracellular eLtaS domain (32). As seen in Fig. 1C and quantified by densitometry analysis (Fig. 1D), LtaS processing was not impaired in the protease mutant strains, and similar amounts of cleaved protein were detected in the supernatant and cell fractions for the WT and the htrA double mutant strain. Other known S. aureus extracellular proteases are SspA (a serine protease), SplA to SplF (serine protease-like proteins), SspB and ScpA (cysteine proteases), and Aur (a metalloprotease). All of the corresponding genes were inactivated in the S. aureus strain LAC* to create strain AH1919 (LAC* protease KO). Again, no difference in the amount of cleaved LtaS protein was observed in the LAC* protease KO strain compared to the parental LAC* control strain (Fig. 1C and D). This indicates that none of the currently known extracellular S. aureus proteases plays a major role in LtaS processing.
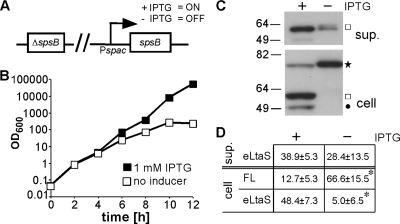
S. aureus signal peptidase SpsB is required for efficient LtaS processing.
Recently, it was found that treatment of S. epidermidis cultures with the signal peptidase-specific inhibitor arylomycin causes a reduction in the amount of cleaved LtaS protein in the culture supernatant (37). In addition, it was reported that processing of the B. subtilis LtaS orthologue YfnI is diminished in the combined absence of the two signal peptidases SipT and SipV (1). S. aureus contains one active type I signal peptidase, which is encoded by spsB and thought to be essential for cell viability (13). To test whether SpsB is required for LtaS processing in S. aureus, the RN4220-derived strain ANG2009, with IPTG-inducible spsB expression, was constructed (Fig. 2A). Strain ANG2009 grew in the presence of IPTG; however, 4 h after removal of the inducer, growth slowed down, and a growth arrest was seen approximately 10 h after removal of IPTG, indicating successful depletion of the signal peptidase (Fig. 2B). To examine the impact of SpsB depletion on LtaS processing, strain ANG2009 was grown for 8 h with or without IPTG. Supernatant and whole-cell protein fractions were prepared from these cultures and analyzed by Western blotting. In the absence of the inducer, a statistically significant accumulation of the full-length LtaS protein was observed, from 12.73% ± 5.32% to 66.62% ± 15.54% (P < 0.01) (Fig. 2C and D). We assume that the detection of the eLtaS protein in the supernatant fraction resulted from residual signal peptidase activity before its complete depletion. Taken together, these data indicate that the S. aureus signal peptidase SpsB is required for efficient LtaS processing.
Fig. 2.
S. aureus signal peptidase SpsB is required for efficient LtaS processing. (A) Schematic representation of S. aureus strain ANG2009, with IPTG-inducible spsB expression. (B) Bacterial growth curves. Washed overnight cultures of S. aureus strain ANG2009, with inducible spsB expression, were back diluted into medium with or without IPTG, and bacterial growth was monitored by determining OD600 readings at the indicated time points. Four hours after the initial dilution, cells were washed and back diluted in fresh medium with or without IPTG, and at the 8-h time point, cultures were diluted a second time to maintain them in the logarithmic growth phase. (C) LtaS detection by Western blotting. At the 8-h time point, supernatant (sup.) and whole-cell (cell) protein samples were prepared, and the LtaS protein was detected by Western blotting using the LtaS-specific antibody. A star indicates the full-length LtaS protein, a square the cleaved eLtaS protein, and a filled circle the unspecific Sbi protein band. Note that the Sbi protein itself is cleaved after its transport across the membrane by the signal peptidase SpsB, and hence the absence of the Sbi band in the spsB depletion strain might be due to its instability if it is not properly processed. (D) Densitometry analysis. Densitometry analysis was performed as described in the legend to Fig. 1D, and the average values and standard deviations for seven experiments are shown. Two-tailed two-value equal-variance Student's t test was performed, and statistically significant differences (P < 0.01) are indicated with an asterisk.
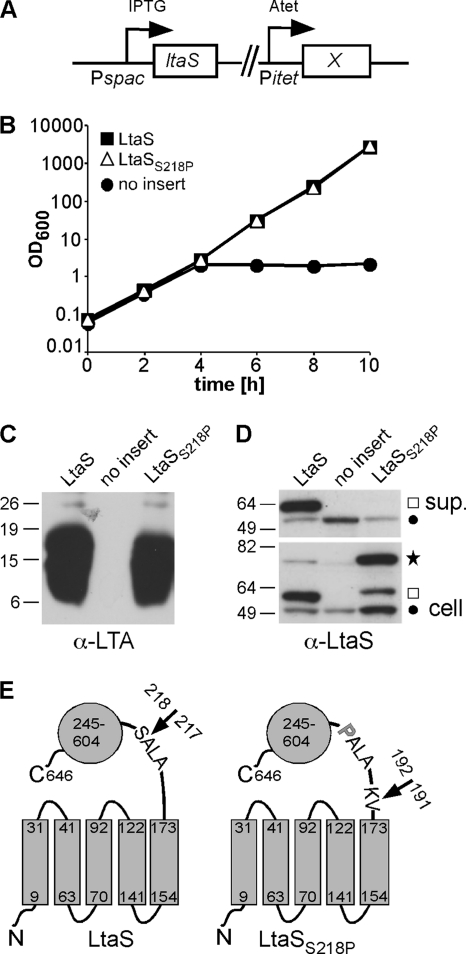
The main processing site in S. aureus LtaS is after Ala217, and a variant with reduced processing retains activity.
Mass spectrometry analysis of secreted S. aureus proteins suggested that the LtaS protein is cleaved following the 5TM domain in the linker region after residues 215Ala-Leu-Ala217 (Fig. 1A) (46). In a recent study, it was suggested that the S. epidermidis LtaS protein is cleaved after residues 171Ala-Phe-Ala173, based on an analysis using the SignalP 3.0 prediction program (7, 37). These residues are also present in the S. aureus protein (Fig. 1A). We wanted to clarify the site of LtaS processing and also investigate if cleavage is required for enzyme function. To determine the processing site, the S. aureus LtaS enzyme was expressed as a C-terminally His-tagged fusion protein from the pitet vector in S. aureus strain ANG499. The processed LtaS protein products were purified from the culture supernatant by nickel-affinity chromatography, and large Coomassie blue-stainable amounts of purified protein were obtained (data not shown) and subjected to standard tryptic digestion and mass spectrometry analysis. A tryptic peptide starting at alanine 215 was detected; however, the first strong signal for the most N-terminal fragment was obtained for a nontryptic peptide starting at serine 218 (see Fig. S1A in the supplemental material), suggesting that the main cleavage site in S. aureus LtaS is between amino acids Ala217 and Ser218 (Fig. 3). For signal peptidase substrates, it has been established that a proline residue in the +1 position relative to the cleavage site prevents processing and converts signal peptidase substrates to competitive inhibitors (4, 10). By analogy, we reasoned that it might be possible to prevent LtaS processing by replacing Ser218 (+1 position) with a proline residue and that this would allow us to determine if LtaS processing is required for function. An LtaSS218P variant was constructed and expressed from an Atet-inducible promoter in S. aureus strain ANG499, which contains the native chromosomal ltaS gene under IPTG-inducible expression control (Fig. 3A). Upon IPTG depletion, strain ANG499 is unable to synthesize LTA and ceases to grow unless a functional ltaS allele is expressed, in this case elsewhere on the chromosome, from the Atet-inducible promoter (Fig. 3A). When this strain is grown in the presence of Atet but absence of IPTG, it can be used to determine both the processing behavior of the LtaSS218P variant and its functionality, based on its ability to complement growth and LTA production. S. aureus ANG499-based strains harboring the empty pitet plasmid and expressing WT LtaS were included as negative and positive controls, respectively. Identical to an S. aureus strain producing WT LtaS, the strain expressing the LtaSS218P variant was able to grow and produce LTA, indicating that the amino acid substitution did not affect the functionality of the protein (Fig. 3B and C). However, compared to WT LtaS, the amount of processed LtaSS218P in the supernatant fraction decreased from 52.18% ± 9.64% to 3.74% ± 4.17% (P < 0.01), and a concomitant accumulation of full-length protein in the cell fraction, from 11.20% ± 4.61% to 59.16% ± 11.45% (P < 0.01), was observed (Fig. 3D). Note that the cleaved LtaSS218P form showed a slightly slower mobility in SDS-PAGE gels than WT LtaS (Fig. 3D), suggesting that this variant is processed at an alternative site closer to the 5TM domain. To investigate the alternative processing further, the LtaSS218P variant was expressed as a C-terminally His-tagged fusion protein, and cleaved protein products were purified from culture supernatant and subjected to tryptic digestion and mass spectrometry analysis as described for WT LtaS. The most N-terminal fragment detected was a nontryptic peptide starting at residue K192 (see Fig. S1B in the supplemental material), suggesting that the LtaSS218P variant is processed between residues Val191 and Lys192 (Fig. 3E). There was no indication that the S. aureus LtaS protein is processed at the computer program-predicted site following residues 171Ala-Phe-Ala173; also, mutating the glutamic acid residue at position 174 to a proline (which would be the +1 position for this cleavage site) did not affect protein processing (data not shown). These findings indicate that mutating the conserved LtaS cleavage site blocks protein processing at the natural site and results in inefficient processing at an alternative site. However, as shown above, efficient processing is not essential for LtaS function.
Fig. 3.
LtaSS218P variant with mutated cleavage site shows reduced processing but retains activity. (A) S. aureus strains used for complementation analysis contained the chromosomal copy of the ltaS gene under IPTG-inducible spac promoter control, and the LtaS variant under investigation (X) was expressed from an Atet-inducible promoter at an ectopic chromosomal location. (B) Bacterial growth curves. Expression of WT LtaS and the LtaSS218P variant was induced after removal of IPTG by the addition of Atet. As a negative control, an S. aureus strain containing the empty pitet vector (no insert) was used, and growth of all cultures was monitored by determining OD600 readings at the indicated time points. Cultures were back diluted 1:100 in fresh medium at the 4-h time point to maintain cultures in the logarithmic growth phase. (C) Detection of LTA by Western blotting. At the 4-h time point, 1-ml culture aliquots were removed and samples prepared and analyzed by Western blotting. A mouse monoclonal anti-LTA antibody (clone 55; Hycult Biotechnology) was used for the detection of LTA in the cell-associated fraction. (D) Detection of LtaS by Western blotting. At the 4-h time point, 1-ml culture samples were removed and supernatant (sup.) and whole-cell (cell) samples prepared as described in the legend to Fig. 1C. A star indicates the full-length LtaS protein, a square the cleaved eLtaS protein, and a filled circle the unspecific Sbi protein band. (E) Schematic representation of WT LtaS and the LtaSS218P variant. Numbers refer to amino acid positions, and arrows indicate main cleavage sites identified by mass spectrometry analysis. The proline substitution in the LtaSS218P mutant is shown as a bold gray letter. S. aureus strains ANG513, ANG514, and ANG1246 were used for this experiment.
The 5TM domain of LtaS is required for in vivo function.
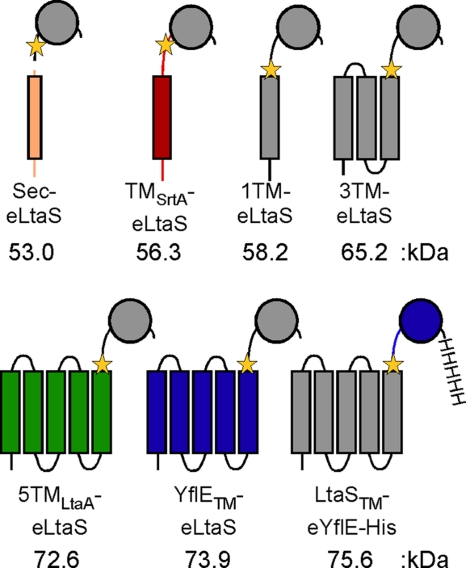
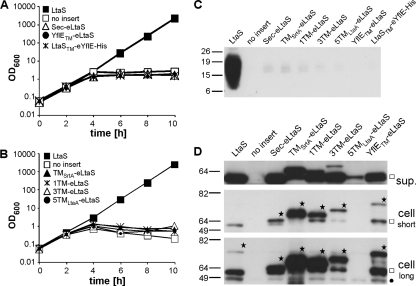
Recently, it was shown that the recombinant eLtaS domain retains enzymatic activity in an in vitro assay system and is capable of hydrolyzing fluorescently labeled PG lipid to DAG (26). To investigate if the eLtaS domain is sufficient for LTA production and to determine the contribution and specificity of the 5TM domain for in vivo function, a series of eLtaS fusion proteins with different membrane domains were constructed (Fig. 4). A secreted eLtaS variant (Sec-eLtaS) was constructed by replacing the 5TM domain and linker region with the conventional signal sequence of protein A. A single TM helix derived from S. aureus sortase A was fused to eLtaS (TMSrtA-eLtaS) in an attempt to anchor the eLtaS domain to the membrane. Multiple TM helices derived from the S. aureus LtaA membrane protein were fused to the linker region and the eLtaS domain (5TMLtaA-eLtaS), and two constructs with shortened LtaS-derived membrane domains were made, with the last four (1TM-eLtaS) or last two (3TM-eLtaS) TM helices of LtaS removed. Finally, domain swaps were performed between the S. aureus LtaS enzyme and the B. subtilis LtaS orthologue YflE (YflETM-eLtaS and LtaSTM-eYflE-His) (Fig. 4). In a previous study, it was shown that B. subtilis YflE (LtaSBS) complements growth and LTA production in an S. aureus LtaS depletion strain, suggesting that these two enzymes have similar functional requirements (22). To test the functionality of these seven fusion proteins, the proteins were expressed as described above for the LtaSS218P variant in S. aureus strain ANG499, which is unable to synthesize LTA and ceases to grow upon IPTG depletion unless a functional LtaS protein is expressed from the Atet-inducible promoter (Fig. 3A). In contrast to the case for WT LtaS, expression of the seven fusion proteins did not restore bacterial growth or LTA production, and identical to the case for the negative-control strain containing the empty pitet vector, growth of all strains ceased after 4 h and no LTA-specific signal was detected on Western blots (Fig. 5A to C). Expression of all seven fusion proteins was confirmed by Western blotting using LtaS-specific antibody (Fig. 5D) or a His-tag-specific antibody for the detection of the LtaSTM-eYflE-His fusion construct (see Fig. S2 in the supplemental material). Bands of the expected size for the full-length fusion proteins were detected for all constructs (Fig. 5D, stars), with the exception of the 5TMLtaA-eLtaS protein, which was poorly expressed, with only a small amount of the cleaved eLtaS fragment detected in the supernatant fraction. eLtaS fragments could also be detected in the supernatant fraction for all other fusion proteins. With the exception of the TMStrA-eLtaS protein, these cleaved fragments were of similar size to the eLtaS fragment derived from the native protein, suggesting that processing occurred at the same site or a similar site to that in wild-type LtaS. The exact cleavage site could not be determined based on the Western blot analysis. However, it seems unlikely that the fusion proteins were nonfunctional because of incorrect processing, considering that the LtaSS218P variant was processed at an alternative site but remained functional. Also, although the protein levels differed greatly between the different fusion proteins, we do not believe that insufficient protein quantity was the reason for the lack of complementation, as nondetectable amounts of WT LtaS expressed from the weak spac promoter are sufficient to promote growth and LTA synthesis (see Fig. S3 in the supplemental material) (22). Taken together, these experiments demonstrate that the 5TM domain of LtaS is essential for enzyme function and polyglycerolphosphate backbone chain production in vivo. Furthermore, the observation that domain swaps between two functional LTA synthases (S. aureus LtaS and B. subtilis YflE) did not yield a functional protein suggests a specific interaction between the 5TM and eLtaS domains.
Fig. 4.
Schematic representation of the different LtaS fusion proteins used for complementation analysis. The protein A signal peptide was fused to eLtaS to produce a secreted eLtaS variant (Sec-eLtaS). The eLtaS domain was fused to the sortase A TM helix (TMSrtA-eLtaS) or the first 5 TM helices of LtaA (5TMLtaA-eLtaS), and shortened LtaS versions with 1 or 3 TM helices were also constructed (1TM-eLtaS and 3TM-eLtaS). In addition, two S. aureus LtaS and B. subtilis YflE hybrid proteins (YflETM-eLtaS and LtaSTM-eYflE-His) were made. The LtaSTM-YflE-His construct contained a C-terminal His tag for protein detection purposes. Protein portions shown in gray were derived from the S. aureus LtaS protein, colored fragments were derived from other S. aureus proteins, and the fusion sites are indicated by yellow stars. The number below each fusion protein refers to the molecular mass of the full-length protein. Details on the construction of these fusion proteins can be found in Materials and Methods.
Fig. 5.
Complementation of growth and LTA production upon expression of different fusion proteins. (A and B) Bacterial growth curves. The fusion proteins indicated in the legend and depicted in Fig. 4 were expressed in S. aureus strain ANG499 by the addition of Atet after removal of IPTG. A strain expressing WT LtaS served as a positive control, and a strain containing the empty pitet vector (no insert) served as a negative control. Bacterial growth was monitored by determining OD600 readings at the indicated time points. All cultures were back diluted 1:100 at the 4-h time point, and the culture with the WT LtaS-expressing control strain was back diluted a second time at the 8-h time point, to maintain the cultures in the logarithmic growth phase. LTA (C) and LtaS protein (D) were detected by Western blotting. At the 4-h time point, 1-ml aliquots were removed from the S. aureus cultures described above, LTA in the cell-associated fraction was detected using an LTA-specific antibody, and the LtaS protein in the supernatant (sup.) and cell fractions was detected using the LtaS-specific antibody. Proteins expressed are indicated above each lane, and the assumed full-length LtaS fusion proteins are indicated with stars. The cleaved eLtaS fragments and the unspecific Sbi protein band are indicated by a square and a filled circle, respectively. Note that two different exposure times (short and long) are shown for the cell fraction sample in order to visualize the full-length wild-type LtaS protein. Exposure times and protein loading differed between supernatant and cell samples and are therefore not comparable with each other. S. aureus strains ANG513, ANG514, ANG595, ANG1217, ANG1218 ANG1219, ANG1220, ANG1347, and ANG1346 were used for this experiment.
The 5TM domain is also needed for the LTA primase reaction.
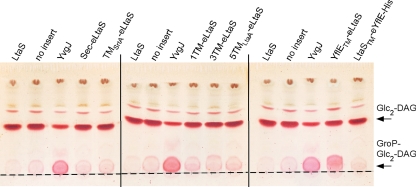
S. aureus LtaS initiates LTA synthesis by the transfer of the first GroP subunit to the glycolipid anchor. Usually, this GroP-Glc2-DAG intermediate does not accumulate in S. aureus, as it is further extended by the repeated addition of GroP subunits to form the LTA backbone chain. From previous studies, it is known that some LtaS-type enzymes are unable to polymerize an LTA chain but are still capable of producing the GroP-glycolipid intermediate (44, 45). These enzymes were named LTA primases, and as an example, the expression of the B. subtilis LTA primase YvgJ in S. aureus from the Atet promoter and a multicopy plasmid leads to a clear accumulation of the GroP-Glc2-DAG glycolipid intermediate (45). This intermediate would not have been detected by the Western blot analysis described above. To test if the eLtaS domain alone or any of the other fusion proteins with altered TM domains retained the ability to synthesize the GroP-glycolipid intermediate, membrane glycolipids needed to be analyzed. To this end, all seven fusion proteins were expressed in S. aureus ANG499 from the same plasmid system as that used for the B. subtilis YvgJ control protein. Expression of all fusion proteins from this plasmid system was confirmed by Western blotting (see Fig. S4 in the supplemental material), but again, none of them restored bacterial growth or LTA production to the S. aureus LtaS depletion strain (data not shown). Next, total lipids were extracted from mid-log-phase cultures of S. aureus strains either expressing the fusion proteins, B. subtilis YvgJ, or S. aureus WT LtaS or containing empty pCN34 as a control. Next, the lipids were separated by TLC and glycolipids visualized by staining with α-naphthol and sulfuric acid (Fig. 6). A glycolipid band corresponding to Glc2-DAG was detected in all samples. In addition, as described previously, a clear accumulation of the slower-migrating glycolipid GroP-Glc2-DAG was observed in the samples isolated from the YvgJ-expressing strain (Fig. 6) (45). Similarly, accumulation of this slower-migrating glycolipid band was seen upon expression of the YflETM-eLtaS fusion protein. However, no signal above the empty vector (no insert) control was seen upon expression of all six other LtaS fusion proteins (Fig. 6). While these data show that one of the B. subtilis/S. aureus LtaS hybrid proteins retained some in vivo enzyme activity, they also suggest that the eLtaS domain alone is not sufficient to initiate LTA synthesis. Therefore, the 5TM domain is critical in vivo for both the LTA priming and the polymerization reaction.
Fig. 6.
TLC analysis of glycolipids produced by S. aureus strains expressing different LtaS fusion proteins. The LtaS fusion protein indicated above each lane was expressed from a multicopy plasmid by the addition of Atet after removal of IPTG in the S. aureus LtaS depletion strain ANG499. Strains expressing S. aureus LtaS and B. subtilis YvgJ and a strain containing the empty plasmid pCN34 (no insert) were used as controls. Cultures were grown to mid-log phase, and lipids were extracted and separated by TLC as described in Materials and Methods. Glycolipids were visualized by staining with α-naphthol and sulfuric acid. The origin is marked with a dashed line, and the positions of the glycolipids Glc2-DAG (top band) and GroP-Glc2-DAG (bottom band) are indicated with arrows on the right. S. aureus strains ANG1130, ANG1571, ANG1658, and ANG2165 to ANG2171 were used for this experiment.
The 5TM and eLtaS domains cannot be expressed separately without loss of function.
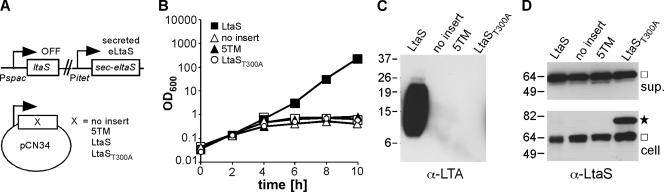
LtaS is processed efficiently during bacterial growth, and the experiments described above have shown that the 5TM domain is essential for in vivo function. To investigate if the LtaS enzyme retains any activity once it is split into the 5TM and eLtaS domains, an S. aureus strain was constructed in which the two domains were expressed as separate proteins. S. aureus strain ANG595 carries the native ltaS gene under IPTG-inducible promoter control, and the Sec-eLtaS protein is expressed from the Atet-inducible promoter (Fig. 7A). A plasmid containing the 5TM domain under native LtaS promoter control was introduced into this strain, allowing for the expression of the 5TM domain and the secreted eLtaS domain as two separate fragments in the absence of IPTG and presence of Atet (Fig. 7A). The empty plasmid pCN34 and a plasmid for expression of the full-length LtaS protein were also introduced into strain ANG595, and the resultant strains served as controls. No growth complementation or LTA production was observed upon expression of the two domains as separate fragments, in contrast to the case with expression of the full-length protein (Fig. 7B and C). Western blot analysis of supernatant and whole-cell fractions confirmed the expression and secretion of the eLtaS domain in all strains tested (Fig. 7D). However, we were unable to verify the expression of the 5TM domain fragment by Western blotting when it was expressed as either a C- or N-terminally His-tagged fusion protein (data not shown). To overcome this problem, the LtaST300A variant, in which the active site threonine is mutated to an alanine residue, was expressed in place of the 5TM domain. The LtaST300A variant is inactive but stably expressed (32), and once cleaved, the 5TM domain will be present at least temporarily in the membrane. To this end, plasmid pCN34itet-ltaST300A was int roduced into S. aureus ANG595 (Fig. 7A), and addition of Atet to this strain resulted in the expression of both the LtaST300A variant and the secreted eLtaS domain (Fig. 7D). However, their coexpression also did not promote bacterial growth or LTA production (Fig. 7B and C). These results suggest that the 5TM and eLtaS domains cannot be assembled postsynthesis to form a functional enzyme, and consequently, the LtaS processing step seems to irreversibly inactivate the enzyme.
Fig. 7.
LtaS functions as a full-length enzyme but not as a split enzyme. (A) Schematic representation of S. aureus strains used for separate expression of the 5TM domain and the eLtaS domain. The secreted eLtaS variant (Sec-eLtaS) was expressed from a chromosomal integration vector by the addition of Atet, and the 5TM domain of LtaS (5TM), an inactive full-length LtaS variant (LtaST300A), or WT LtaS (LtaS; positive control) was expressed from a multicopy plasmid. A strain containing the empty plasmid vector pCN34 (no insert) was used as a negative control. (B) Bacterial growth curves. The growth of the S. aureus strains described in panel A was monitored by determining OD600 readings at the indicated time points. Cultures were back diluted 1:100 in fresh medium at the 4-h time point to maintain them in the logarithmic growth phase. LTA (C) and LtaS (D) were detected by Western blotting. Samples were prepared and analyzed by Western blotting as described in the legend to Fig. 3. The anti-LtaS Western blot with cell samples was exposed twice as long as the Western blot with supernatant samples. A star indicates the full-length LtaS protein, and a square indicates the cleaved eLtaS protein. S. aureus strains ANG1226, ANG1227, ANG1228, and ANG1690 were used for this experiment.
DISCUSSION
Proteolytic cleavage is an essential step for the removal of unfolded or otherwise damaged proteins from cells and is used to adjust protein levels depending on need. Cleavage at defined sites within preproteins is also a well-established mechanism of posttranslational alteration that is used to affect the activity of enzymes. In several instances, this processing leads to enzyme activation, for instance, in the case of the S. aureus extracellular proteases themselves. These proteins are secreted as inactive zymogens and cleaved by signal peptidase to release them from the membrane, and subsequent additional processing steps at defined sites finally lead to the full activation of these enzymes (33). Through previous work, it has been established that the S. aureus LTA synthase enzyme LtaS is efficiently processed at a defined site during bacterial growth (32, 35, 46). The data presented in the current study suggest that this processing step is most probably accomplished by the signal peptidase SpsB and serves as a mechanism to irreversibly inactivate the enzyme so that it can no longer function in the LTA synthesis pathway.
In this study, we confirm that the main processing site in the S. aureus LtaS protein is following residues 215Ala-Leu-Ala217 within the linker region between the 5TM and eLtaS domains (Fig. 3). Further experimental evidence is provided that the essential S. aureus type I signal peptidase SpsB is the protease responsible for this processing step, as depletion of SpsB led to an accumulation of the full-length protein, while inactivation of any of the other currently known extracellular proteases did not affect LtaS processing (Fig. 1 and 2). In addition, introduction of a proline residue at the +1 position with respect to the cleavage site, which is known to inhibit signal peptidase-dependent cleavage, prevented LtaS processing at this site (Fig. 3). However, it should be noted that LtaS is a very atypical substrate for this type of protease. Usually, signal peptidase substrates contain an N-terminal signal peptide that consists of a positively charged N terminus, a central hydrophobic region, and a polar extracellular C-terminal region which contains the actual cleavage site, often ending with an Ala-X-Ala sequence at the −3 to −1 positions, with the alanine at position −1 being especially important (43). Typically, the cleavage sites are placed 3 to 7 residues after the hydrophobic core, and this spacing is critical, as signal peptidases are integral membrane enzymes with an active site in close proximity to the bacterial membrane (43). LtaS is cleaved after an Ala-X-Ala motif, and this motif is quite conserved among LtaS-type enzymes, particularly the alanine at the −1 position, which is a typical feature for a signal peptidase substrate. However, the motif is not located after an N-terminal signal peptide but following multiple TM helices, and furthermore, the processing site is more than 40 amino acids after the end of the last hydrophobic region. Therefore, we must assume that the binding of the eLtaS domain to its PG substrate in the bacterial membrane or, alternatively, an interaction between the eLtaS domain and the 5TM domain (discussed below) retains the cleavage site in close enough proximity to the membrane for signal peptidase to act upon. Usually, it is thought that signal peptides are removed either during the translocation step or shortly afterwards (43). However, LtaS needs to stay long enough intact to perform its function in the LTA synthesis pathway, so a mechanism might be in place that determines the exact timing of this processing step.
The E. coli MdoB protein is a Mn2+-dependent metal enzyme that performs a reaction similar to that of LtaS (19, 25). MdoB catalyzes the transfer of GroP subunits from the membrane lipid PG onto periplasmic oligosaccharides under low-osmolarity conditions. Interestingly, similar to LtaS, MdoB consists of an N-terminal domain with multiple TM helices and a C-terminal extracellular enzymatic domain (31). The enzymatic domain of MdoB is also cleaved during bacterial growth, by an as yet unknown protease also speculated to be a signal peptidase and released from the membrane into the periplasmic space (31). Recently, a model was proposed in which the full-length MdoB enzyme transfers GroP from the lipid PG onto nascent oligosaccharide molecules, while the cleaved protein is thought to swap GroP subunits from one oligosaccharide molecule to another (31). Thus, it is thought that MdoB processing leads to a switch in substrate specificity but that both the full-length and cleaved forms are necessary for proper substitution of the membrane-derived oligosaccharides. It is not assumed that GroP subunits are transferred between different LTA chains in S. aureus or onto a different acceptor molecule. However, S. aureus LtaS is thought to perform two slightly different functions: LtaS primes LTA synthesis by the addition of a GroP subunit to a hydroxyl group of the glycolipid anchor and subsequently polymerizes the LTA chain by the addition of GroP subunits to the hydroxyl group of the terminal GroP subunits, presumably using PG as a substrate for both reactions. However, in contrast to the case for MdoB, our data suggest that the cleaved eLtaS fragment does not retain any activity relevant for the synthesis of LTA, as expression of the eLtaS domain alone results in neither polyglycerolphosphate chain formation nor the production of the GroP-glycolipid intermediate (Fig. 5 and 6). We cannot formally exclude that the eLtaS domain retains another in vivo activity which was not observed because of the types of assays we performed. It is possible that eLtaS is still able to hydrolyze the membrane lipid PG without performing a transfer reaction. Alternatively, the eLtaS domain might indeed be involved in the transfer of GroP subunits between chains or from the LTA chain onto other molecules or in the release and degradation of LTA chains, but all of these possibilities are highly speculative. However, what seems to be clear is that in the absence of a functional full-length LtaS protein, the eLtaS domain alone is not sufficient to promote LTA synthesis and growth of S. aureus.
Our data clearly indicate that the 5TM domain of LtaS plays an essential, as yet unknown role in LtaS enzyme function (Fig. 5 and 6). Possibly this domain recruits and “prepares” the PG lipid substrate for efficient hydrolysis by the active site located in the eLtaS domain. Interestingly, two enzymes, produced by fusing the 5TM and eLtaS domains of two functional LtaS-type enzymes, resulted in the formation of hybrid proteins that were unable to produce LTA (Fig. 5). This indicates that very specific protein-protein interactions might occur between the 5TM and eLtaS domains, and perhaps only through these interactions will a fully functional active site be formed. Ongoing structural studies on the full-length LtaS protein will hopefully provide further insight in the future. Moreover, we found that expression of the N- and C-terminal LtaS domains as one protein is crucial for enzyme function. It was not possible to reconstitute a functional enzyme by expressing the two domains separately as was recently reported for MprF, a different S. aureus enzyme (15). This membrane protein is composed of two distinct domains that can be separated physically, but the split enzyme remains functional (15).
In summary, our data provide strong evidence that only the full-length LtaS enzyme is functional and active in the LTA synthesis pathway. We speculate that the LtaS processing step has a regulatory role and provides a mechanism for the cell to control LtaS activity. This might be of importance during the bacterial cell cycle and division process or when bacteria enter the stationary phase and no longer divide. One can assume that under nondividing conditions, a halt in LTA synthesis is essential for bacteria to maintain a normal membrane composition and integrity. The proteolytic cleavage of LtaS-type enzymes appears to be a more general phenomenon for this class of proteins, as processed forms have now been detected in a range of Gram-positive bacteria (2, 18, 23, 37, 44). Our findings, combined with the results of previously published studies (1, 37), provide more and more evidence that this processing step is performed by type I signal peptidases. These proteases are key enzymes in bacterial cells and are essential for life and therefore unlikely to be inactivated or lost with time (43). In future studies, it will be interesting to further investigate the timing and exact cues that lead to LtaS processing in order to better understand how LTA synthesis is controlled in the bacterial cell.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alexandra Gruss for providing strain RN6390 and the htrA mutant strain RN6390 (htrA1 htrA2) and Ross Tomaino and colleges at the Taplin Biological Mass Spectrometry Facility for mass spectrometry analysis. We also thank Kenneth Bayles and Jeffrey Bose for providing the pJB38 plasmid and Rebecca Corrigan for construction of the sbi and ltaS deletion strains.
This work was supported by Wellcome Trust grant WT084483 to A.G. and by National Institute of Allergy and Infectious Diseases award AI078921 to A.R.H.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Antelmann H., et al. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 11:1484–1502 [DOI] [PubMed] [Google Scholar]
- 2. Antelmann H., et al. 2005. The extracellular and cytoplasmic proteomes of the non-virulent Bacillus anthracis strain UM23C1-2. Proteomics 5:3684–3695 [DOI] [PubMed] [Google Scholar]
- 3. Bae T., Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63 [DOI] [PubMed] [Google Scholar]
- 4. Barkocy-Gallagher G. A., Bassford P. J., Jr 1992. Synthesis of precursor maltose-binding protein with proline in the +1 position of the cleavage site interferes with the activity of Escherichia coli signal peptidase I in vivo. J. Biol. Chem. 267:1231–1238 [PubMed] [Google Scholar]
- 5. Bateman B. T., Donegan N. P., Jarry T. M., Palma M., Cheung A. L. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 69:7851–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beltzer J. P., Wessels H. P., Spiess M. 1989. Signal peptidase can cleave inside a polytopic membrane protein. FEBS Lett. 253:93–98 [DOI] [PubMed] [Google Scholar]
- 7. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 8. Boles B. R., Horswill A. R. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boles B. R., Thoendel M., Roth A. J., Horswill A. R. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruton G., et al. 2003. Lipopeptide substrates for SpsB, the Staphylococcus aureus type I signal peptidase: design, conformation and conversion to alpha-ketoamide inhibitors. Eur. J. Med. Chem. 38:351–356 [DOI] [PubMed] [Google Scholar]
- 11. Burkholder P. R., et al. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 33:345–348 [PubMed] [Google Scholar]
- 12. Charpentier E., et al. 2004. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 70:6076–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cregg K. M., Wilding I., Black M. T. 1996. Molecular cloning and expression of the spsB gene encoding an essential type I signal peptidase from Staphylococcus aureus. J. Bacteriol. 178:5712–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duckworth M., Archibald A. R., Baddiley J. 1975. Lipoteichoic acid and lipoteichoic acid carrier in Staphylococcus aureus H. FEBS Lett. 53:176–179 [DOI] [PubMed] [Google Scholar]
- 15. Ernst C. M., et al. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer W. 1994. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. (Berlin) 183:61–76 [DOI] [PubMed] [Google Scholar]
- 17. Gatlin C. L., et al. 2006. Proteomic profiling of cell envelope-associated proteins from Staphylococcus aureus. Proteomics 6:1530–1549 [DOI] [PubMed] [Google Scholar]
- 18. Gohar M., et al. 2005. A comparative study of Bacillus cereus, Bacillus thuringiensis and Bacillus anthracis extracellular proteomes. Proteomics 5:3696–3711 [DOI] [PubMed] [Google Scholar]
- 19. Goldberg D. E., Rumley M. K., Kennedy E. P. 1981. Biosynthesis of membrane-derived oligosaccharides: a periplasmic phosphoglyceroltransferase. Proc. Natl. Acad. Sci. U. S. A. 78:5513–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gründling A., Gonzalez M. D., Higgins D. E. 2003. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J. Bacteriol. 185:6295–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gründling A., Schneewind O. 2007. Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J. Bacteriol. 189:2521–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gründling A., Schneewind O. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 104:8478–8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirose I., et al. 2000. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146:65–75 [DOI] [PubMed] [Google Scholar]
- 24. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68 [DOI] [PubMed] [Google Scholar]
- 25. Jackson B. J., Bohin J. P., Kennedy E. P. 1984. Biosynthesis of membrane-derived oligosaccharides: characterization of mdoB mutants defective in phosphoglycerol transferase I activity. J. Bacteriol. 160:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karatsa-Dodgson M., Wörmann M. E., Gründling A. 2010. In vitro analysis of the Staphylococcus aureus lipoteichoic acid synthase enzyme using fluorescently labeled lipids. J. Bacteriol. 192:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kavanaugh J. S., Thoendel M., Horswill A. R. 2007. A role for type I signal peptidase in Staphylococcus aureus quorum sensing. Mol. Microbiol. 65:780–798 [DOI] [PubMed] [Google Scholar]
- 28. Koch H. U., Haas R., Fischer W. 1984. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur. J. Biochem. 138:357–363 [DOI] [PubMed] [Google Scholar]
- 29. Kreiswirth B. N., et al. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 30. Lee C. Y., Buranen S. L., Ye Z. H. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103:101–105 [DOI] [PubMed] [Google Scholar]
- 31. Lequette Y., Lanfroy E., Cogez V., Bohin J. P., Lacroix J. M. 2008. Biosynthesis of osmoregulated periplasmic glucans in Escherichia coli: the membrane-bound and the soluble periplasmic phosphoglycerol transferases are encoded by the same gene. Microbiology 154:476–483 [DOI] [PubMed] [Google Scholar]
- 32. Lu D., et al. 2009. Structure-based mechanism of lipoteichoic acid synthesis by Staphylococcus aureus LtaS. Proc. Natl. Acad. Sci. U. S. A. 106:1584–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nickerson N. N., Prasad L., Jacob L., Delbaere L. T., McGavin M. J. 2007. Activation of the SspA serine protease zymogen of Staphylococcus aureus proceeds through unique variations of a trypsinogen-like mechanism and is dependent on both autocatalytic and metalloprotease-specific processing. J. Biol. Chem. 282:34129–34138 [DOI] [PubMed] [Google Scholar]
- 34. O'Connell C., Pattee P. A., Foster T. J. 1993. Sequence and mapping of the aroA gene of Staphylococcus aureus 8325-4. J. Gen. Microbiol. 139:1449–1460 [DOI] [PubMed] [Google Scholar]
- 35. Oku Y., et al. 2009. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol. 191:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powers M. E., et al. 2011. Type I signal peptidase and protein secretion in Staphylococcus epidermidis. J. Bacteriol. 193:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rahman O., Dover L. G., Sutcliffe I. C. 2009. Lipoteichoic acid biosynthesis: two steps forwards, one step sideways? Trends Microbiol. 17:219–225 [DOI] [PubMed] [Google Scholar]
- 39. Reichmann N. T., Gründling A. 2011. Location, synthesis and function of glycolipid and polyglycerolphosphate lipoteichoic acid in gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol. Lett. 319:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rigoulay C., et al. 2005. Comparative analysis of the roles of HtrA-like surface proteases in two virulent Staphylococcus aureus strains. Infect. Immun. 73:563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sutcliffe I. C. 2011. Priming and elongation: dissection of the lipoteichoic acid biosynthetic pathway in Gram-positive bacteria. Mol. Microbiol. 79:553–556 [DOI] [PubMed] [Google Scholar]
- 42. Taron D. J., Childs W. C., III, Neuhaus F. C. 1983. Biosynthesis of d-alanyl-lipoteichoic acid: role of diglyceride kinase in the synthesis of phosphatidylglycerol for chain elongation. J. Bacteriol. 154:1110–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Roosmalen M. L., et al. 2004. Type I signal peptidases of Gram-positive bacteria. Biochim. Biophys. Acta 1694:279–297 [DOI] [PubMed] [Google Scholar]
- 44. Webb A. J., Karatsa-Dodgson M., Gründling A. 2009. Two-enzyme systems for glycolipid and polyglycerolphosphate lipoteichoic acid synthesis in Listeria monocytogenes. Mol. Microbiol. 74:299–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wörmann M. E., Corrigan R. M., Simpson P. J., Matthews S. J., Gründling A. 2011. Enzymatic activities and functional interdependencies of Bacillus subtilis lipoteichoic acid synthesis enzymes. Mol. Microbiol. 79:566–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ziebandt A. K., et al. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480–493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.