Abstract
Oxytricha nova is a hypotrichous ciliate containing a transcriptionally active macronucleus and a transcriptionally inactive micronucleus. Two-dimensional gel electrophoresis shows that macronuclei contain a normal complement of inner histones. However, despite extensive efforts, no classical H1-like protein has been detected. Micrococcal nuclease digestion indicates a nucleosome repeat length of approximately 220 bp for macronuclear chromatin. Thermal denaturation profiles of macronuclear chromatin in 0.2 mM EDTA display four transitions at about 46, 57, 64, and 79 degrees C. The lowest of these shifts to higher temperature as the ionic strength is raised to 3-5 mM Na phosphate. These results are consistent with the absence of H1 and a nucleosome repeat of 220-230 bp. Circular dichroism (CD) results agree with these findings. By contrast, micronuclear chromatin displays a much smaller premelt and a more suppressed DNA CD signal at 285 nm, consistent with a micronuclear chromatin repeat of 165-185 bp as determined by micrococcal nuclease digestion.
Full text
PDF





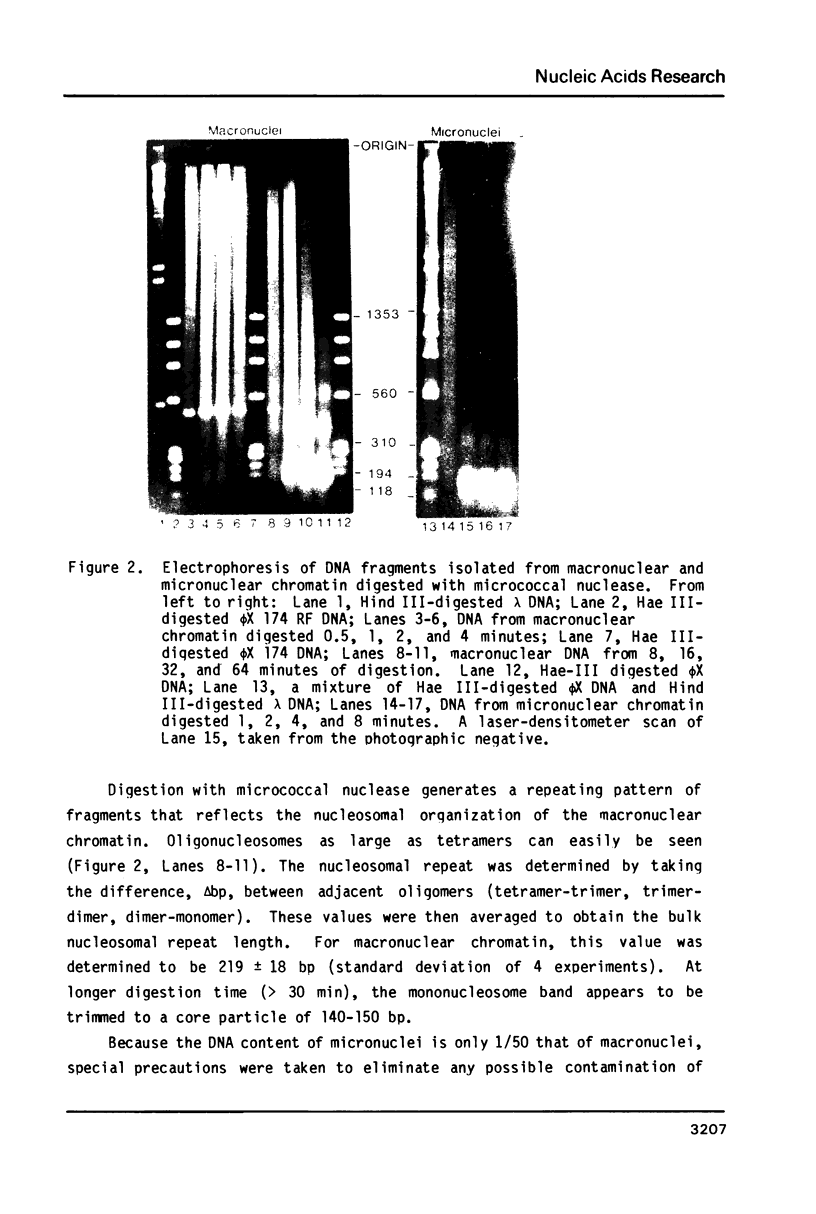

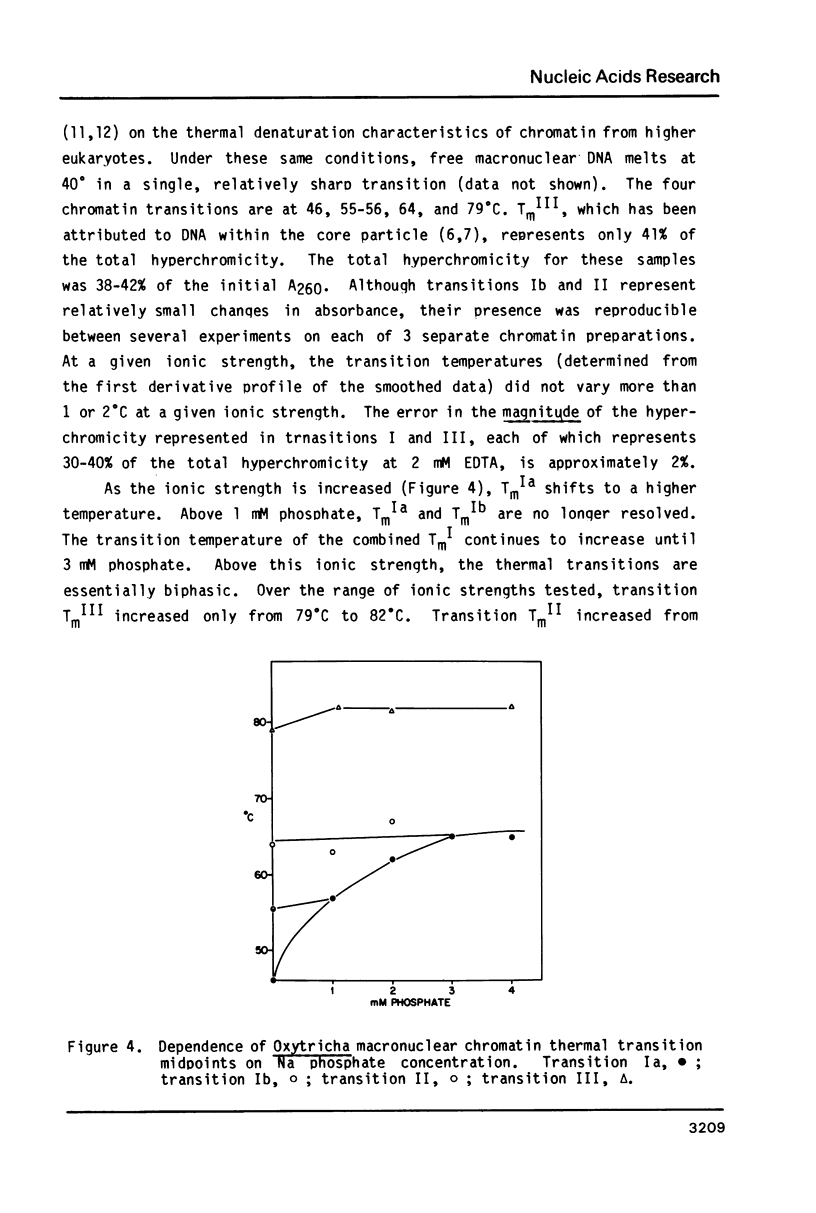
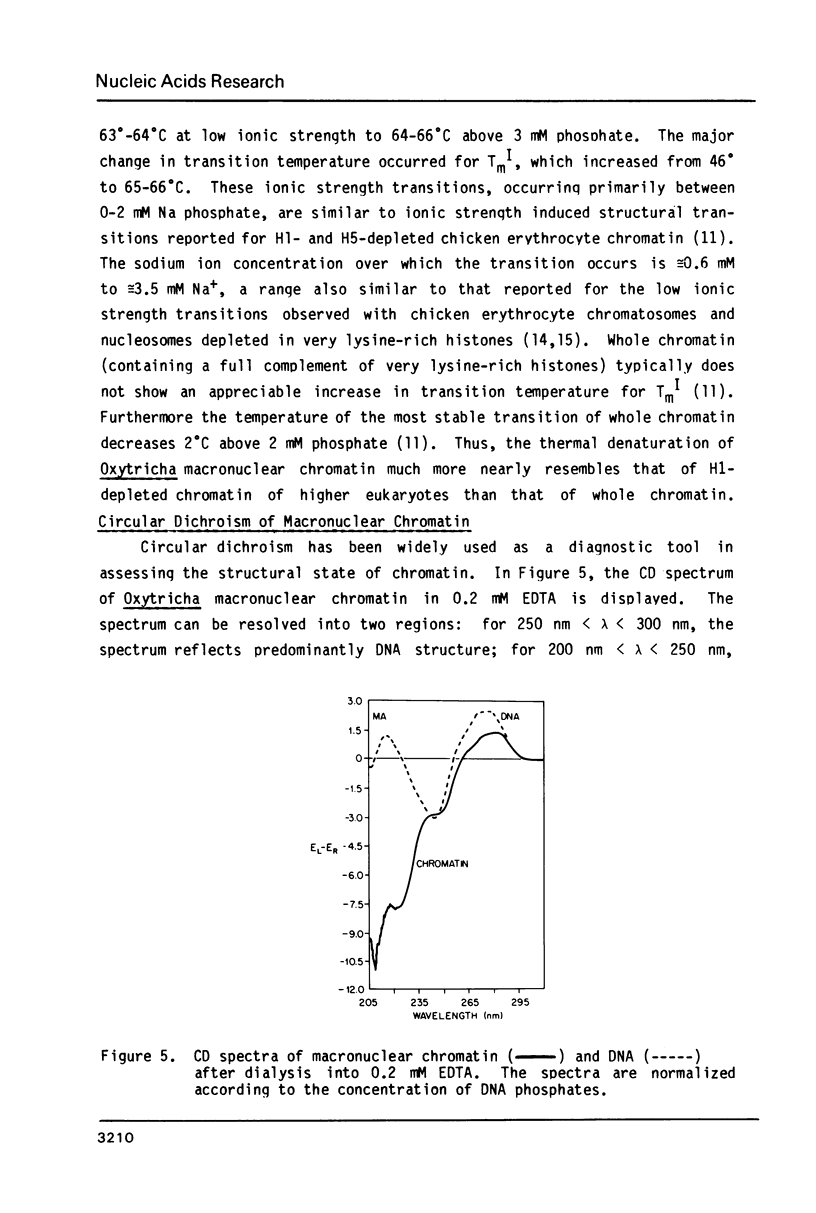







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Gorovsky M. A. Micronuclei of Tetrahymena contain two types of histone H3. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4857–4861. doi: 10.1073/pnas.76.10.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan P. N., Wright E. B., Hsie M. H., Olins A. L., Olins D. E. Physical properties of inner histone-DNA complexes. Nucleic Acids Res. 1978 Oct;5(10):3603–3617. doi: 10.1093/nar/5.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan P. N., Wright E. B., Olins D. E. Core nucleosomes by digestion of reconstructed histone-DNA complexes. Nucleic Acids Res. 1979 Apr;6(4):1449–1465. doi: 10.1093/nar/6.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Martinson H. G. The roles of H1, the histone core and DNA length in the unfolding of nucleosomes at low ionic strength. Nucleic Acids Res. 1980 Nov 11;8(21):4969–4987. doi: 10.1093/nar/8.21.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman M. K., Fasman G. D. Dependence of mononucleosome deoxyribonucleic acid conformation on the deoxyribonucleic acid length and H1/H5 content. Circular dichroism and thermal denaturation studies. Biochemistry. 1980 Feb 5;19(3):532–541. doi: 10.1021/bi00544a022. [DOI] [PubMed] [Google Scholar]
- Fulmer A. W., Fasman G. D. Ionic strength-dependent conformational transitions of chromatin. Circular dichroism and thermal denaturation studies. Biopolymers. 1979 Nov;18(11):2875–2891. doi: 10.1002/bip.1979.360181115. [DOI] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., Chan A., Hanlon S. Mixed conformations of deoxyribonucleic acid in intact chromatin isolated by various preparative methods. Biochemistry. 1972 Nov 7;11(23):4347–4358. doi: 10.1021/bi00773a023. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Heumann J. M., Herrick G., Prescott D. M. The gene-size DNA molecules in Oxytricha. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):483–492. doi: 10.1101/sqb.1978.042.01.051. [DOI] [PubMed] [Google Scholar]
- Levinger L., Barsoum J., Varshavsky A. Two-dimensional hybridization mapping of nucleosomes. comparison of DNA and protein patterns. J Mol Biol. 1981 Mar 5;146(3):287–304. doi: 10.1016/0022-2836(81)90389-2. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Nock A., Riewe M., Steinbrück G. Chromatin structure in the macronucleus of the ciliate Stylonychia mytilus. Nucleic Acids Res. 1978 Dec;5(12):4699–4709. doi: 10.1093/nar/5.12.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary A. R., Fasman G. D. The effect of H1 on mononucleosome conformation. Arch Biochem Biophys. 1980 May;201(2):603–614. doi: 10.1016/0003-9861(80)90550-0. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. The number of charge-charge interactions stabilizing the ends of nucleosome DNA. Nucleic Acids Res. 1980 Jun 25;8(12):2751–2769. doi: 10.1093/nar/8.12.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G., Bostock C. J. Genetic apparatus of Stylonychia sp. Nature. 1973 Apr 27;242(5400):576, 597-600. doi: 10.1038/242576a0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Mechanism of a reversible, thermally induced conformational change in chromatin core particles. J Biol Chem. 1979 Oct 25;254(20):10123–10127. [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of DNA in chromatin core particles containing poly(dAdT)-poly(dAdT) studied by 31 P NMR spectroscopy. Nucleic Acids Res. 1979 Sep 25;7(2):481–492. doi: 10.1093/nar/7.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Iso K. Quantitative analysis of chromatin structure by circular dichroism. J Mol Biol. 1981 Sep 5;151(1):143–163. doi: 10.1016/0022-2836(81)90225-4. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Tatchell K., Van Holde K. E., Klump H. Thermal denaturation of nucleosomal core particles. Nucleic Acids Res. 1978 Jan;5(1):139–160. doi: 10.1093/nar/5.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]




