Abstract
The phenomenon of phase variation between yellow and tan forms of Myxococcus xanthus has been recognized for several decades, but it is not known what role this variation may play in the ecology of myxobacteria. We confirm an earlier report that tan variants are disproportionately more numerous in the resulting spore population of a M. xanthus fruiting body than the tan vegetative cells that contributed to fruiting body formation. However, we found that tan cells may not require yellow cells for fruiting body formation or starvation-induced sporulation of tan cells. Here we report three differences between the yellow and tan variants that may play important roles in the soil ecology of M. xanthus. Specifically, the yellow variant is more capable of forming biofilms, is more sensitive to lysozyme, and is more resistant to ingestion by bacteriophagous nematodes. We also show that the myxobacterial fruiting body is more resistant to predation by worms than are dispersed M. xanthus cells.
INTRODUCTION
Phase variations in bacteria are reversible phenotypic changes that occur as a result of DNA sequence alterations like slipped-strand mispairing, DNA methylation, and inversion of DNA sequences. These DNA changes have been studied in pathogenic bacteria that can alter cell surface antigens to evade detection by the immune response (7, 34). However, the role of phase variation as a survival strategy in nonpathogenic, environmental bacteria is less well understood. Myxococcus xanthus is a soil-dwelling bacterial species capable of undergoing phase variation between yellow and tan variants, although the molecular mechanism of this variation is uncharacterized and it is not known how this phenotypic plasticity would allow for greater fitness in the environment.
M. xanthus is a Gram-negative, motile bacterium that survives by hydrolyzing amino acids, often by acting as a predator of other soil-dwelling bacteria. During nutrient deprivation, vegetatively growing M. xanthus cells are capable of a primitive multicellular development into macroscopic fruiting bodies containing hundreds of thousands of stress-resistant spores (31). This developmental process is dependent upon motility on a surface and upon cell-to-cell signaling (13, 16). It is also possible to use glycerol to induce individual M. xanthus cells to sporulate in liquid culture (10). In its vegetatively growing state, M. xanthus can undergo phase variation between two different cell types that can form yellow or tan colonies (6). Tan variants are generally considered to be nonpigmented, while yellow variants produce a polar, acetone-soluble pigment with an absorbance maximum at 379 nm (5, 6). Because tan and yellow cells can interconvert, this is considered an example of true phase variation. In addition to colony pigmentation, the two cell types produce different colony morphologies, with yellow cells generating flat, swarming, and medusoid-shaped colonies while tan cells produce convex, round colonies that are more mucoid in appearance (4). One laboratory reported that a fresh yellow colony of the wild-type strain DK1622 contains 1 to 5% tan vegetative cells while a typical tan colony consists of 5 to 25% yellow vegetative cells (19). This suggests that the tan variant is less stable in a mixed population than the yellow phase variant and that tan-to-yellow conversion rates can vary widely. Environmental factors influence the proportions of yellow and tan variants in a population (28). Conditions that increase the production of yellow cells include the presence of high nutrient concentrations, UV radiation, low cell density, incubation at 36.5°C (the normal growth temperature is 32°C), and incubation in medium containing mitomycin C, nalidixic acid, or 2-phenylethanol (5, 29). These observations suggest that phase shifting to the yellow variant may be linked with M. xanthus stress responses. In contrast, the tan variant is more predominant in dense populations of M. xanthus (28). Growth rates may or may not differ between M. xanthus phase variants. At low cell densities or in low-nutrient media, the yellow variant grows faster but at higher cell densities or in media with a higher-nutrient content, the two variants grow equally well (26). Lastly, it has been reported that pure cultures of the tan M. xanthus variant are not capable of forming fruiting bodies or sporulating on starvation agar (19). Reduced phase variant stability, reduced motility, and reduced sporulation all raise the question of what evolutionary advantage the tan variant might provide that allows it to be maintained in a soil environment mixed with a population of yellow cells. This question was partly addressed by Laue and Gill, who showed that when a mixed culture of the two variants forms fruiting bodies, the resulting spores germinate into a population of tan vegetative cells that is disproportionately more numerous (35 to 37% of the total number of germinated cells) than the input pool (5% of vegetative cells) (19). This apparent enrichment of tan variants among fruiting body spores would help maintain numbers of tan cells, despite the yellow variant's being more motile and phenotypically stable in the vegetative state.
In addition to the challenges of finding nutrients (facilitated by the wolf pack feeding of vegetative cells) (2, 25) and surviving nutrient deprivation (resulting in fruiting body development and spore formation), M. xanthus may also serve as prey for bacteriophagous nematodes that coexist in the soil. It is not known if the phase variation of M. xanthus plays a role in its evasion of nematode predation. In the present study, we addressed in more detail the phenotypic differences between the tan and yellow phase variants of M. xanthus, specifically examining characteristics of phase variation that might play important roles in bacterial survival in the soil. We found that tan and yellow phase variants differ in the thickness of their biofilms, the stress resistance of their spores, and predation by the bacteriophagous nematode Caenorhabditis elegans.
MATERIALS AND METHODS
Bacteria and media.
M. xanthus strain DK1622 (14) was grown vegetatively in CTTYE broth (1% Casitone, 10 mM Tris-HCl [pH 7.6], 1 mM potassium phosphate [pH 7.6], 8 mM MgSO4, 0.5% yeast extract) at 32°C with shaking. Alternatively, cells were grown on CTTYE agar plates (containing 1.5% agar). Assays to measure S and A motilities on CTPM agar plates were performed as previously described (27). M. xanthus cultures were routinely started from fresh colonies to help maintain homogeneous populations of the phase variants. To determine numbers of CFU/ml, serial dilutions were spread onto CTTYE agar (1.5%) and colonies were scored after 4 to 5 days of incubation at 32°C. Gentamicin (3 μg/ml, final concentration) was included to reduce the chance of contamination. Comparisons of cultures on CTTYE agar with and without gentamicin showed that the antibiotic did not alter the proportions of tan and yellow CFU. To determine percentages of spontaneous phase variation from yellow to tan or from tan to yellow, isolated colonies were streaked onto fresh CTTYE agar, incubated for 3 days at 32°C, swabbed into CTTYE broth, incubated with aeration at 32°C for 2 days, and subjected to serial dilution onto CTTYE agar. After 5 days of incubation at 32°C, colonies were scored for color and the percentages of the total visible colonies that were tan or yellow were calculated.
M. xanthus sporulation and testing for stress resistance.
To generate starvation-induced spores, cultures were grown in CTTYE broth to 100 Klett units before being washed in TPM buffer (10 mM Tris-HCl [pH 7.6], 1 mM potassium phosphate [pH 7.6], 8 mM MgSO4), 100-fold concentration by centrifugation, and spotting of 20-μl aliquots of concentrated cells onto TPM agar (1.5%). Fruiting body development occurred for 5 days at 32°C before cells were scraped off with a razor blade and suspended in fresh TPM. Alternatively, fruiting body formation occurred on water agar plates (6.8 mM CaCl2, 1.5% agar) onto which a 2-day-old culture of Escherichia coli (strain DH5α grown on LB agar) had been swabbed. The E. coli swab pattern was in the shape of a cross with 20-μl aliquots of concentrated M. xanthus cells spotted onto the ends of the cross. M. xanthus bacteria swarmed over the E. coli prey and eventually formed fruiting bodies that were photographed 7 days after M. xanthus introduction. Fruiting bodies from TPM starvation agar were dispersed by three 10-s bursts of a sonicator (Fisher Scientific Sonic Dismembrator Model 100) set at 1.5 before any remaining vegetative cells were heat killed in a 57°C water bath for 15 min.
Glycerol-induced spores were generated by growing M. xanthus cells as previously described (10). After 6 h of incubation with 0.5 M glycerol, cells were washed in TPM and sonicated at a setting of 1.5 as described above for the dispersion of fruiting body spores. Glycerol-induced spores were tested for resistance to sonication (setting 4.0 on the Sonic Dismembrator) and to lysozyme treatment (final concentration of 250 μg/ml at room temperature with agitation for 4 h) as previously described (9).
Microscopy.
A Nikon T1-SM inverted microscope was used to visualize intact fruiting bodies on the surface of agar plates. Alternatively, fruiting bodies were scraped off the agar and visualized with a Nikon Eclipse 50i phase-contrast microscope and methylene blue staining either before or after sonication to disperse cells. Scanning electron microscopy (SEM) was performed as previously described (9), using a JEOL model JSM-6490LV (JEOL, Tokyo, Japan). Transmission electron microscopy (TEM) of spores inside fruiting bodies was performed using a JEOL 1200 EX as previously described (9).
Biofilm assays.
Biofilms for M. xanthus were created and quantified using a modified protocol described by Peeters et al. (24). Briefly, overnight cultures of M. xanthus in CTTYE broth (∼100 Klett units) were added to 15-ml polystyrene tubes or to 12- or 96-well tissue culture plates, and the cultures remained stationary for 12 h at 28°C to allow cells to adhere. Supernatants were then removed, and plastic surfaces were washed with equal volumes of phosphate-buffered saline (PBS). Fresh CTTYE broth was then added, and cultures were allowed to incubate in stationary positions for 24 h at 32°C before the removal of supernatants, washing of plastic surfaces with PBS, and fixing of cells with 99% methanol for 15 min. Biofilms were stained for 20 min with a 1% (wt/vol) crystal violet solution before rinsing with H2O. To quantify the amounts of crystal violet retained by the biofilms, a 33% acetic acid solution was used to remove the stain. The acetic acid extraction liquid was centrifuged at 12,000 × g for 5 min to remove whole cells before measurement of the absorbance at 590 nm of the supernatant with a spectrophotometer.
Culturing of bacteria with worms.
C. elegans worms were cultured on E. coli strain OP50 (3) before the L4/young adult stage worms were purged of internal bacteria as previously described (17). Concentrations of worms were determined with a dissection microscope before aliquots of ∼100 worms were placed onto each agar plate containing M. xanthus cells. To assay worm feeding on starvation-induced or glycerol-induced spores, 20-μl aliquots of spores dispersed by sonication were spotted onto 6-cm-diameter petri plates containing nematode minimal medium agar (1.5%) (17) supplemented with gentamicin (3 μg/ml) to suppress E. coli growth and chloramphenicol (2 μg/ml) to inhibit M. xanthus spore germination and vegetative growth. To assay nematode feeding on vegetatively growing M. xanthus cells, bacteria were spotted onto CTTYE agar (1.5%) supplemented with 5 μg/ml cholesterol and 0.5 mM CaCl2 to support nematode reproduction and 3 μg/ml gentamicin to prevent E. coli growth. Plates containing nematodes and bacteria were incubated at room temperature (∼22°C).
Enumeration of nematodes and bacteria interacting together.
Three-milliliter aliquots of TPM were used to wash nematodes and bacteria off plates into sterile test tubes. These cell mixtures were used to enumerate both M. xanthus and C. elegans. To enumerate nematodes, the worms were pelleted by gentle centrifugation (3,000 × g) before the worms were resuspended in TPM containing 30 mM sodium azide to induce paralysis. Immobilized worms were then spotted onto agar plates, where individual worms were counted using a dissection microscope. To enumerate the viable M. xanthus cells that survived nematode predation, C. elegans worms were allowed to settle from the TPM liquid extracted from agar plates with bacteria. The bacteria still in suspension were removed, vortexed to disperse bacterial clumps, and subjected to serial dilution and plating onto CTTYE agar to allow CFU to form. CFU formation was scored after 5 days of incubation at 32°C.
Recovery of viable M. xanthus cells from inside worms.
Nematodes allowed to feed on yellow or tan variant spores of M. xanthus were examined for intestinal bacteria as previously described (17). Briefly, sodium azide was used to paralyze the pharynx muscles of the worms and close the anus to prevent entry of disinfectant into the worms. A 2% bleach solution was used for 20 min to decontaminate the worms of external bacteria, the bleach was washed away, and the nematodes were lysed with 1-mm zirconia beads using a FastPrep FP120 bead-beating device (ThermoSavant). Cell lysates were plated onto CTTYE agar plates, and CFU were enumerated after 6 days of growth at 32°C.
RESULTS
Phase variants are relatively stable and differ in bacterial motility.
Unlike Laue and Gill (19), who reported that 5 to 25% of the cells within a tan colony typically would shift back to the yellow phase, we found both the tan and yellow variants of M. xanthus wild-type strain DK1622 to be relatively stable. Our observed rate of spontaneous conversion from yellow to tan was 1 to 3% of the cells in a single colony, and tan cells converted to yellow cells at a rate of about 2 to 5% of the cells in a colony. This observation is important for the interpretation of the experiments that follow, since it means that the two phase variants are stable enough to be tested for phenotypic differences. One possible reason why we do not see the high rate of tan-to-yellow conversion reported by Lau and Gill for strain DK1622 is that we may have isolated a spontaneous phase-locked variant of M. xanthus. The existence of such phase-locked mutants has been previously described (18). Although Laue and Gill reported a wide range of conversion rates (5 to 25%), our observed tan-to-yellow conversion rate (2 to 5%) did overlap that range. The phase-locked mutant reported by Laue and Gill no longer exists, making it impossible to directly compare that strain with our DK1622 strain.
Tan variants form smaller fruiting bodies with smaller spores.
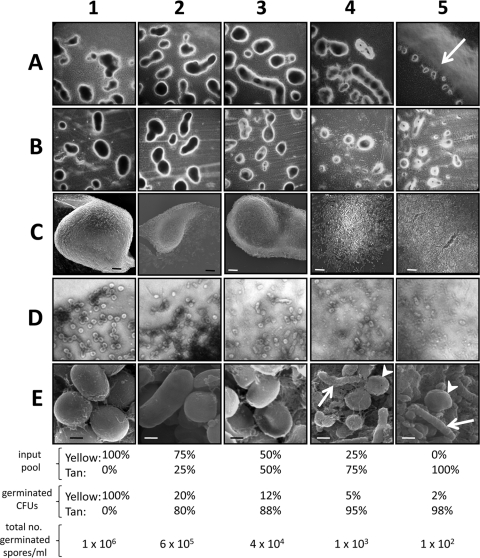
A previous investigation reported that tan variants of M. xanthus strain DK1622 could participate in the formation of fruiting bodies on TPM agar only if mixed with yellow variants (19). The tan variants, on their own, could not form fruiting bodies on the surface of agar but could form small, rudimentary fruiting bodies if development happened in submerged cultures (19). In contrast, we show here that an initial pool of tan variants is capable of forming fruiting bodies and spores independently of the addition of yellow cells to the mixture (Fig. 1, column 5). Cell aggregates and spores from tan cells are smaller than those that resulted when increasing amounts of yellow vegetative cells were included in the cell pool (Fig. 1, columns 1 to 4, rows A and B). When a population of 100% tan vegetative cells was used, fruiting body formation on TPM agar occurred only at the periphery of the 20-μl spot of cells (Fig. 1, panel A5, arrow). Fruiting body appearance varied slightly, depending upon whether TPM starvation or E. coli prey plates were used (Fig. 1, compare rows A and B). It has been shown that the inclusion of E. coli prey on starvation plates enhances myxospore formation 5-fold (33), and this may account for the larger fruiting bodies seen here (compare panels A5 and B5 in Fig. 1). Phase-bright, coccoid-shaped cells are visible throughout the range of fruiting bodies (Fig. 1, row D), but these cells are larger and more numerous with increasing percentages of yellow cells in the cell mixtures (Fig. 1, row E). SEM analysis of fruiting bodies with various mixtures shows that when yellow cells are >50% of the input pool, the fruiting body is tightly compacted with spores (Fig. 1, panels C1 to C3). However, lower starting percentages of yellow cells result in spores adhering less tightly in the fruiting body (Fig. 1, panels C4 and C5). Without a high number of input yellow cells, many cells in the aggregates remained in a rod shape even after 5 days of starvation on TPM (Fig. 1, row E, arrows). Yellow and tan variants possibly coexist in nature and jointly constitute fruiting bodies. However, here we show that it is possible for each phase variant to form multicellular structures largely independent of the other variant.
Fig. 1.
Tan variants are capable of fruiting body formation and sporulation on agar surfaces. Appearances of fruiting bodies and their spores from mixtures of vegetatively growing cells containing different ratios of yellow and tan phase variants (input pools) are shown. Percentages indicate relative numbers of cells mixed together before the initiation of starvation-induced sporulation. (A) Fruiting bodies on the perimeters of 20-μl spots on TPM starvation agar. Spots of cells containing 100% tan variants resulted in fruiting bodies forming only at the periphery of the 20-μl spots (panel A5, arrow). (B) Fruiting bodies allowed to form on water agar plates containing E. coli prey. (C) SEM (magnification, ×1,000) of fruiting bodies from TPM agar plates. Bars, 10 μm. (D) Phase-contrast microscopy (magnification, ×1,000) of the edges of methylene blue-stained fruiting bodies scraped from the surfaces of TPM starvation plates. (E) SEM (magnification, ×30,000) of sonicated fruiting bodies from TPM agar plates. Bars, 0.5 μm. Heat- and sonication-resistant spores were allowed to germinate, and the resulting colonies were enumerated and scored for color. Percentages of germinated spores are averages of three experiments.
Laue and Gill could not detect the formation of viable spores if yellow cells made up less than 50% of the input pool of cells in submerged cultures (19). However, we found that even with 100% tan cells present in the input pool, heat- and sonication-resistant cells could be recovered from the resulting fruiting bodies. We confirmed the previous observation of Laue and Gill that the number of tan germinated cells recovered from fruiting bodies greatly exceeded the number of tan cells that helped form the aggregate (Fig. 1, compare input pool with germinated CFU). These results suggest a greater efficiency of spore formation by tan cells than by yellow cells in the fruiting body. The greater number of tan spores might result from a preferential lysing of yellow cells in the developing fruiting body, as 90% of the viable yellow cells are lost during sporulation (33).
Glycerol induction of phase variants produces spores with phenotypic differences.
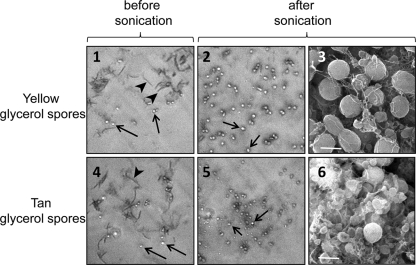
Glycerol can be added to cultures growing in liquid to produce spores independently of the motility and the cell-to-cell contact required for starvation-induced sporulation in fruiting bodies. Glycerol-induced spores have thinner spore coats, but they maintain many of the stress resistance phenotypes seen with starvation-induced myxospores (8). Both glycerol-induced yellow and tan phase variants produce coccoid cells that are phase bright (Fig. 2, arrows). For both phase variants, the glycerol-induced spores survived sonication while vegetative cells did not (Fig. 2, panels 2 and 5). Tan glycerol-induced spores are 50-fold more resistant to an increased intensity of sonication and are 500-fold more resistant to lysozyme than are yellow glycerol-induced spores (data not shown).
Fig. 2.
Glycerol can be used to induce spore formation by both phase variants. Both the yellow and the tan variants show the formation of phase-bright, coccoid-shaped cells (arrows) and vegetative, rod-shaped cells (arrowheads) after glycerol induction (panels 1 and 4). These phase-bright spores survive gentle sonication (setting 1.5, three pulses of 10 s each), whereas vegetative cells do not (panels 2 and 5). SEM analysis (magnification, ×14,000) shows coccoid cells of both glycerol-induced phase variants (panels 3 and 6). Bars, 1 μm.
Tan variants are less capable of forming biofilms.
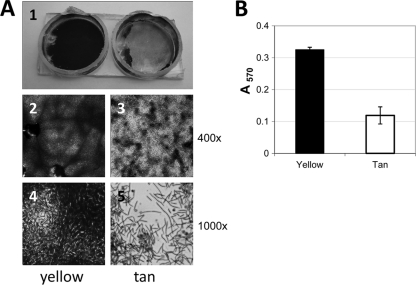
It was previously shown that in liquid-grown cultures the yellow variant will clump at a rate greater than that of the tan variant, showing that the yellow cells are more cohesive (19). Here we examined differences in the cell surfaces of the phase variants growing on solid surfaces (Fig. 3). Cells grown on CTTYE plates with 1.5% agar were swabbed into CTTYE broth and allowed to settle for 2 h before their appearances were recorded. Yellow cells were cohesive, and cell aggregates had a string-like quality and remained in suspension, while tan cells readily dispersed into the liquid and settled into a large pellet (data not shown). Analysis of these suspensions by light microscopy indicated much larger cell aggregates of the yellow variant than of the tan variant (data not shown). The two phase variants were compared for biofilm formation using 12-well polystyrene tissue culture plates (Fig. 3A) or 96-well polystyrene microtiter plates (Fig. 3B). Resulting biofilms were stained with crystal violet, and in all cases the yellow variant was more effective at forming a biofilm. This is documented by visual comparisons between yellow and tan biofilms (Fig. 3A), with multiple overlay layers of yellow cells visible compared to a dispersed monolayer of tan cells (compare panels 4 and 5 in Fig. 3A). Quantification of the amounts of extracted crystal violet shows that the tan biofilm is reduced almost 3-fold compared to the yellow biofilm (Fig. 3B).
Fig. 3.
Cells of the yellow variant of strain DK1622 can form thicker biofilms than cells of the tan variant. (A) Crystal violet-stained biofilms in two adjacent wells of a 12-well tissue culture plate (panel 1). Wells containing yellow variant cells (left) and tan variant cells (right) are shown. Wells were cut from the culture plate for ease of viewing by light microscopy. Magnifications of ×400 and ×1,000 were used to visualize biofilms of the yellow variant (panels 2 and 4, respectively) and those of the tan variant (panels 3 and 5, respectively). (B) Biofilms allowed to form in 96-well tissue culture plates were stained with crystal violet before extraction of the stain from cells and quantification with a spectrophotometer (absorbance at 570 nm). Error bars are standard deviations for 40 different wells for each phase variant.
Starvation-induced spores of the tan variant have a higher rate of ingestion by C. elegans than the yellow variant.
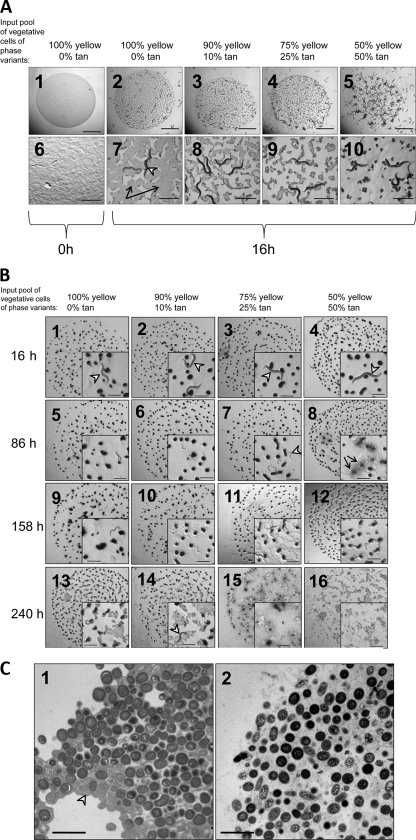
Nematodes were tested for the ability to ingest M. xanthus. We examined whether fruiting body spores could be ingested by C. elegans and whether M. xanthus phase variation affected the rates of predation by nematodes. Regardless of whether spores were dispersed by sonication (Fig. 4A) or remained intact in fruiting bodies (Fig. 4B), tan cells were more readily consumed by C. elegans. As early as 16 h after the exposure of dispersed spores to C. elegans, we observed a direct correlation between the initial ratio of tan cells to yellow cells and their removal by C. elegans (Fig. 4A, panels 7 to 10). Nematodes showed a similar preference for fruiting bodies containing more tan cells (Fig. 4B). However, the time required for nematode digestion of spores inside fruiting bodies was much longer than that required for the digestion of dispersed spores. After 16 h of exposure to nematode feeding, there was no visible change in the appearances of M. xanthus fruiting bodies, regardless of yellow and tan cell composition (Fig. 4B, panels 1 to 4). At this 16-h time point, the worms appeared to maintain the same L4 larval-adult stage seen when they were added to the agar plate (Fig. 4B, panel 1 to 4 insets, arrowheads). After 3 days of predator-prey interaction, the cells in the fruiting bodies were notably dispersed in those containing the highest percentage of tan cells (Fig. 4B, panel 8, arrows). Furthermore, at that time point, most of the worms appeared to be at a smaller L1-L2 stage (Fig. 4B, panel 7 inset, arrowhead), indicating that the original worms had produced progeny. After 10 days of nematode predation, fruiting bodies with a 50% input of yellow cells were completely dispersed (Fig. 4B, panel 16), while fruiting bodies with higher percentages of yellow input cells remained partially intact (Fig. 4B, panels 13 to 15). By 10 days, most of the nematodes visible were in the dauer stage (Fig. 4B, panel 14, arrowhead), presumably due to a lack of nutrition provided by the spores. The observation that spores of intact fruiting bodies took almost 15 times longer to be ingested/dispersed by nematodes than freely dispersed spores (compare Fig. 4A and B) suggests that the bacterial fruiting body structure may act as a defense against predation. Nematodes were observed bending around fruiting bodies but were not seen to move over or through fruiting bodies (examples of this are shown in the Fig. 4B panel 1 to 4 insets). This suggests that nematode predation of spores occurred on the fruiting body surface. It may be that an intact extracellular matrix (ECM) provides a steric hindrance to nematode digestion. TEM was used to analyze the ECM structures of fruiting bodies from a 100% yellow input pool and a 50% yellow-50% tan input pool (Fig. 4C, panels 1 and 2, respectively). A thicker electron-dense ECM correlated with more yellow cells in the input pool (Fig. 4C, panel 1, arrowhead).
Fig. 4.
C. elegans worms preferentially digest tan fruiting body spores. (A) Fruiting bodies from TPM starvation plates were harvested and then sonicated (setting 1.5) to dispersed spores into homogeneous solutions that were spotted onto agar plates in 20-μl aliquots (panels 1 and 6). Bars, 5 mm for panel 1 and 1 mm for panel 6. The same number of C. elegans worms was added to each mixture of fruiting body spores and allowed to feed for 16 h (panels 2 to 5 and 7 to 10). In panel 7, the arrows indicate the still existing lawn of spores and the arrowhead indicates a C. elegans L4 larva/adult feeding on bacteria. Bars, 5 mm for panels 2 to 5 and 1 mm for panels 7 to 10. (B) Fruiting bodies were allowed to form from various mixtures of phase variants on TPM starvation agar containing cholesterol and CaCl2 to support nematode replication. After 5 days of starvation-induced sporulation, intact and attached fruiting bodies were subjected to feeding by C. elegans. Appearances of fruiting bodies present in 20-μl aliquots were recorded after 16, 86, 158, and 240 h of nematode feeding. The more fruiting bodies were composed of tan variants, the more susceptible the fruiting bodies were to disruption by C. elegans (Fig. 4B, compare panel 16 inset with panel 13 to 15 insets). (C) TEM (magnification, ×30,000) analysis of a 5-day-old fruiting body from an input population of 100% yellow cells (panel 1) or 50% yellow and 50% tan cells (panel 2). The greater number of yellow cells corresponds to a greater electron-dense ECM packaging spores together (panel 1, arrowhead). Bars, 5 μm.
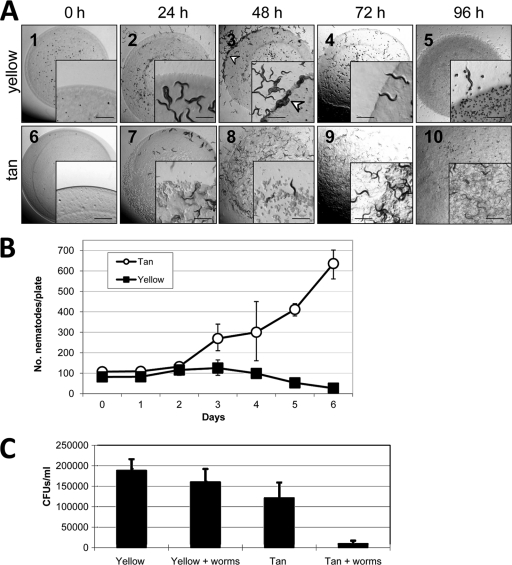
C. elegans prefers glycerol-induced tan variant spores to yellow spores.
As with dispersed tan fruiting body spores, C. elegans ingested all of the available glycerol-induced tan spores (Fig. 5A, panels 6 to 10). C. elegans appears to avoid even entering patches of yellow glycerol-induced spores and instead congregates at the perimeters of yellow patches (Fig. 5A, panel 3, arrowhead). Both predator nematodes and prey bacteria were enumerated after various lengths of cohabitation. Worms proliferated in the presence of tan glycerol-induced spores but showed no discernible increase in population size with yellow glycerol-induced spores as the food source (Fig. 5B). Following 3 days of cohabitation, cells were scraped off the plates and resuspended in liquid TPM and nematodes were allowed to settle before removal of the bacterial supernatant for cell enumeration by serial dilution and colony counting. In the presence of C. elegans on agar plates, the yellow cell population remained stable, while there was a 13-fold decrease in the number of recovered tan cells when worms were present (Fig. 5C). Collectively, Fig. 4 and 5 show that C. elegans has a strong predatory preference for tan cells over yellow cells. Furthermore, we found that C. elegans has a strong preference for tan vegetatively growing cells over yellow vegetative cells (see Fig. S1 in the supplemental material). This preference for tan vegetative cells was shown even when tan and yellow cells were present in adjacent patches on the same plate.
Fig. 5.
C. elegans preferentially digests tan glycerol-induced spores. (A) C. elegans digesting 20-μl spots of glycerol-induced spores. Insets are higher magnifications of the spot perimeters. Bars, 1 mm. (B) Enumeration of C. elegans worms recorded at several time points after a starter culture of 100 nematodes was added to plates containing spots of glycerol-induced spores. (C) Recovery of viable M. xanthus cells from agar surfaces after C. elegans was allowed to feed for 60 h. Error bars show standard deviations for three separate experiments.
DISCUSSION
We show here a predator-prey relationship between C. elegans and M. xanthus and the effect that bacterial phase variation may have on this relationship. Both of these organisms are popular model systems for studying multicellular development. However, no previous studies examined interactions between these two organisms. The myxobacterial fruiting body structure is well regarded as a survival mechanism in which Gram-negative cells form spores that are resistant to environmental stresses such as heat, toxic chemicals, UV light, and desiccation (29). We are among the first to show that, compared to individual vegetative cells, the myxobacterial fruiting body has enhanced resistance to the ubiquitous nematode predators that coexist with myxobacteria in soil. As both nematodes and myxobacteria are bacterial predators in the same environment, they have likely coevolved with one another.
Parallels can be drawn between the ecologies of myxobacteria and soil-dwelling cellular slime molds, which interact with nematodes in a single-cell or multicellular fashion (15). The slime mold Dictyostelium purpureum can exist as a single-cell amoeba that preys upon bacteria, or it can aggregate into a multicellular structure called a slug that is composed of up to 100,000 individual cells. This number is comparable to the number of M. xanthus spores in a typical fruiting body. Dictyostelium slugs eventually undergo metamorphosis to form fruiting body structures composed of balls of spores projecting up over a surface by slender stalks. Typically, up to 20% of the cells in the slug die during the formation of the stalk-like fruiting body, compared with upwards of 90% of the cells aggregating into the M. xanthus fruiting body lysing during spore formation. While individual amoeba cells are rapidly digested by C. elegans, cells within the Dictyostelium slug secrete a mucopolysaccharide coating that makes them resistant to predation by adult C. elegans (15). While adult C. elegans worms appear unable to disperse the slug or stalk-like fruiting body masses, the starvation-induced dauer form of the nematodes can climb up into the spore mass of the slime mold fruiting body and disperse the spores from the fruiting body (15). In this dauer state, the nematodes are incapable of ingesting spores because the buccal cavity is sealed (1) but they disperse spores that can then be eaten by neighboring adult worms on the substrate below the fruiting body stalk. Dictyostelium spores survive passage through the adult C. elegans digestive tract. In this way, the dauer and adult forms of C. elegans collectively distribute Dictyostelium spores to new locations, where the spores germinate and grow. Similarities between M. xanthus and D. purpureum are the following. (i) Both are predators of bacteria capable of responding to nutrient deprivation by multicellular development that leads to sporulation. (ii) While in a single-cell vegetative state, both are readily ingested and destroyed by C. elegans. (iii) While in a multicellular state, both secrete an ECM that collectively prevents predation. (iv) Both appear to have their multicellular structures disrupted by the dauer state of C. elegans (Fig. 4B) (15). Whereas mature Dictyostelium spores survive passage through the C. elegans intestinal tract, this is less clear for myxospores from fruiting bodies. We recovered viable tan variants from the intestines of C. elegans that had been feeding on lawns of dispersed fruiting body spores consisting of mixed pools of tan and yellow variants (data not shown). However, we do not know if ingested tan spores can survive eventual passage through the entire intestinal tract, as Dictyostelium spores are known to do (15). Likewise, we do not know if tan fruiting body spores have a selective advantage over yellow spores inside the worm since its preference for tan spores makes it difficult to determine if C. elegans ingests the yellow variant.
Just like the protective advantage of the mucopolysaccharide coating of the Dictyostelium slug, the ECM packaging of M. xanthus fruiting body spores may be important. The more pronounced ECM of fruiting bodies rich in yellow variants (Fig. 4C) correlates with a greater resistance to disruption by C. elegans (Fig. 4B). Furthermore, Wireman and Dworkin have reported that tan variants initially undergo myxospore formation within aggregation centers but later this tan cell sporulation can occur outside the aggregation center (32). It is likely these individual tan spores would be much more vulnerable than spores packaged inside the fruiting body. We show that M. xanthus yellow cells form thicker biofilms (Fig. 3), and this may account for the greater resistance of yellow vegetative cells to nematode predation (see Fig. S1 in the supplemental material). Palsdottir et al. have recently shown that M. xanthus biofilms are filled with vesicles bound to the vegetative cell surface (23). It is not known if yellow or tan biofilm cells differ in the number or appearance of these extracellular vesicles. It is possible that there is more than a physical obstruction of greater ECM or biofilm structures preventing C. elegans from disrupting fruiting bodies with many yellow cells. The yellow variants may actually secrete a substance that repels worms. The existence of such a “chemorepellant” is suggested by the avoidance of C. elegans from entering patches of dispersed yellow glycerol-induced spores (Fig. 5A, panel 3). The apparent greater sensitivity of yellow glycerol-induced spores to lysozyme treatment than the tan equivalent suggests that yellow spores might make a more digestible meal in the lysozyme-rich interior of nematodes (12, 20, 21), although this does not appear to be the case (Fig. 5). Despite the presence of chloramphenicol in the agar, after a 48-h incubation, the yellow glycerol-induced spores may have germinated into vegetative cells that established biofilms, making them more resistant to C. elegans than the tan variant. When C. elegans is allowed to choose between adjacent spots containing either vegetatively growing yellow or tan M. xanthus cells, the nematodes strongly prefer to feed upon the tan variants (see Fig. S1 in the supplemental material). Whether this preference is due to positive or negative chemotaxis or to differences in biofilm structure remains to be determined.
Whereas the yellow variant appears to be a superior bacterial predator because it has greater A and S motility (see Fig. S2 in the supplemental material), the tan variant appears more efficient at sporulating inside the fruiting body (Fig. 1). Because the tan variant is dependent upon the yellow variant for the production of large numbers of spores inside fruiting bodies (Fig. 1), the tan variant may act as a social cheater, a phenomenon previously reported for myxobacteria (30). Numerous pure cultures of antisocial mutants of M. xanthus show defects in spore formation, but mixing with an altruistic wild-type strain leads to disproportionately large numbers of cheater spores (11). A correlation between the amounts of the M. xanthus pigment DKxanthene and the numbers of spores resulting in fruiting bodies was previously reported (22), and results shown here confirm this direct correlation (Fig. 1). Phase variation plays a role in two manifestations of M. xanthus multicellular development, as the yellow variant contributes more to biofilm formation (Fig. 3) and to the overall size of the fruiting bodies than does the tan variant (Fig. 1). It remains to be determined if tan fruiting body spores can survive passage through the C. elegans intestine. However, there is precedence for Gram-positive spores surviving ingestion by C. elegans (17), and if M. xanthus spores survive this process, then nematodes may play a role in the dissemination of viable spores, as they do in that of Dictyostelium spores (15).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dan Fordice for helping to review the manuscript.
This research was supported by internal funds from the University of Minnesota Duluth.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Albert P. S., Riddle D. L. 1983. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J. Comp. Neurol. 219:461–481 [DOI] [PubMed] [Google Scholar]
- 2. Berleman J. E., Kirby J. R. 2009. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol. Rev. 33:942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burchard R. P. 1974. Growth of surface colonies of the gliding bacterium Myxococcus xanthus. Arch. Microbiol. 96:247–254 [DOI] [PubMed] [Google Scholar]
- 5. Burchard R. P., Burchard A. C., Parish J. H. 1977. Pigmentation phenotype instability in Myxococcus xanthus. Can. J. Microbiol. 23:1657–1662 [DOI] [PubMed] [Google Scholar]
- 6. Burchard R. P., Dworkin M. 1966. Light-induced lysis and carotinogenesis in Myxococcus xanthus. J. Bacteriol. 91:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Citti C., Nouvel L. X., Baranowski E. 2010. Phase and antigenic variation in mycoplasmas. Future Microbiol. 5:1073–1085 [DOI] [PubMed] [Google Scholar]
- 8. Dahl J. L., Fordice D. 2011. Small acid-soluble proteins with intrinsic disorder are required for UV resistance in Myxococcus xanthus spores. J. Bacteriol. 193:3042–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dahl J. L., et al. 2007. Identification of major sporulation proteins of Myxococcus xanthus using a proteomic approach. J. Bacteriol. 189:3187–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dworkin M., Gibson S. M. 1964. A system for studying microbial morphogenesis: rapid formation of microcyst in Myxococcus xanthus. Science 146:243–244 [DOI] [PubMed] [Google Scholar]
- 11. Fiegna F., Velicer G. J. 2003. Competitive fates of bacterial social parasites: persistence and self-induced extinction of Myxococcus xanthus cheaters. Proc. Biol. Sci. 270:1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jolles P. (ed.). 1996. Lysozymes: model enzymes in biochemistry and biology, p. 9–87 Birkhäuser-Verlag, Basel, Switzerland [Google Scholar]
- 13. Kaiser D. 2004. Signaling in myxobacteria. Annu. Rev. Microbiol. 58:75–98 [DOI] [PubMed] [Google Scholar]
- 14. Kaiser D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. U. S. A. 76:5952–5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kessin R. H., Gundersen G. G., Zaydfudim V., Grimson M., Blanton R. L. 1996. How cellular slime mould evade nematodes. Proc. Natl. Acad. Sci. U. S. A. 93:4857–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S. K. 1991. Intercellular signaling in Myxococcus development: the role of C factor. Trends Genet. 7:361–365 [DOI] [PubMed] [Google Scholar]
- 17. Laaberki M.-H., Dworkin J. 2008. Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J. Bacteriol. 190:6197–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laue B. E., Gill R. E. 1994. Use of a phase variation-specific promoter of Myxococcus xanthus in a strategy for isolating a phase-locked mutant. J. Bacteriol. 176:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laue B. E., Gill R. E. 1995. Using a phase-locked mutant of Myxococcus xanthus to study the role of phase variation in development. J. Bacteriol. 177:4089–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGhee J. D. 27 March 2007, posting date The C. elegans intestine. In The C. elegans Research Community (ed.), WormBook: the online review of C. elegans biology. doi/10.1895/wormbook.1.133.1. http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- 21. McGhee J. D., et al. 2007. The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev. Biol. 302:627–645 [DOI] [PubMed] [Google Scholar]
- 22. Meiser P., Bode H. B., Muller R. 2006. The unique DKxanthene secondary metabolite family from the myxobacterium Myxococcus xanthus is required for developmental sporulation. Proc. Natl. Acad. Sci. U. S. A. 103:19128–19133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palsdottir H., et al. 2009. Three dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography. J. Bacteriol. 191:2077–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peeters E., Nelis J. J., Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157–165 [DOI] [PubMed] [Google Scholar]
- 25. Reichenbach H. 1999. The ecology of the myxobacteria. Environ. Microbiol. 1:15–21 [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg E., Keller K. H., Dworkin M. 1977. Cell density-dependent growth of Myxococcus xanthus on casein. J. Bacteriol. 129:770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi W., Zusman D. R. 1993. The two motility systems of Myxococcus xanthus show different selective advantage on various surfaces. Proc. Natl. Acad. Sci. U. S. A. 90:3378–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimkets L. J. 1990. Social and developmental biology of the myxobacteria. Microbiol. Rev. 54:473–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sudo S. Z., Dworkin M. 1969. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J. Bacteriol. 98:883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Velicer G. J., Kroos L., Lenski R. E. 2000. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404:598–601 [DOI] [PubMed] [Google Scholar]
- 31. Whitworth D. E. (ed.). 2008. Myxobacteria: multicellularity and differentiation. ASM Press, Washington, DC [Google Scholar]
- 32. Wireman J. W., Dworkin M. 1975. Morphogenesis and developmental interactions in Myxococcus xanthus. Science 189:516–522 [DOI] [PubMed] [Google Scholar]
- 33. Wireman J. W., Dworkin M. 1977. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J. Bacteriol. 129:798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zieg J., Silverman M., Hilmen M., Simon M. 1977. Recombinational switch for gene expression. Science 196:170–172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.