Abstract
Colonization of the human small intestine by Vibrio cholerae is an essential step in pathogenesis that requires the type IV toxin-coregulated pilus (TCP). To date, three functions of TCP have been characterized: it serves as the CTXΦ receptor, secretes the colonization factor TcpF, and functions in microcolony formation by mediating bacterium-bacterium interactions. Although type IV pili in other pathogenic bacteria have been characterized as playing a major role in attachment to epithelial cells, there are very few studies to suggest that TCP acts as an attachment factor. Taking this into consideration, we investigated the function of TCP in attachment to Caco-2 cells and found that mutants lacking TCP were defective in attachment compared to the wild type. Overexpression of ToxT, the activator of TCP, significantly increased attachment of wild-type V. cholerae to Caco-2 cells. Using field-emission scanning electron microscopy (FESEM), we also observed TCP-mediated attachment to the small intestines of infected infant mice by using antibodies specific to TCP and V. cholerae. Remarkably, we also visualized matrices comprised of TCP appearing to engulf V. cholerae during infection, and we demonstrated that these matrices protected the bacteria from a component of bile, disclosing a possible new role of this pilus in protection of the bacterial cells from antimicrobial agents. This study provides new insights into TCP's function in V. cholerae colonization of the small intestine, describing additional roles in mediating attachment and protection of V. cholerae bacterial cells.
INTRODUCTION
Vibrio cholerae is the etiological agent of the acute diarrheal disease cholera and is characterized as a motile, Gram-negative, bacillus-shaped bacterium. Pathogenesis of V. cholerae O1 critically depends on production of the cholera toxin and the toxin-coregulated pilus (TCP), which are coordinately regulated by the toxR virulence regulon (24, 36). The cholera toxin is an ADP-ribosylating toxin that constitutively activates adenylate cyclase in host cells (reviewed in reference 17), causing copious amounts of diarrhea. Delivery of the cholera toxin requires successful colonization of the small intestine by V. cholerae, which is mediated by TCP, as demonstrated in the infant mouse cholera model (37), the new infant rabbit cholera model (29), and human volunteer studies (15).
Biogenesis of TCP is dependent on the tcp operon, which contains a large cluster of 12 genes (37). The first gene in the operon, tcpA, encodes the 20-kDa major pilin subunit of TCP. TcpA is expressed as a preprotein that is cleaved to its mature form by a prepilin peptidase, encoded by tcpJ (22). ToxT, an AraC family member, activates expression of tcp through signals from a transcriptional cascade called the toxR regulon. The remaining genes in the operon, with the exception of tcpF, encode components of the apparatus that are responsible for biogenesis of TCP. This apparatus is thought to span the inner membrane, periplasm, and outer membrane for the energy-dependent production of the pilus. The 10th gene in the operon, tcpF, encodes a 36-kDa protein that is secreted by the TCP apparatus and is also required for colonization of the infant mouse (18). In addition to the tcp operon, chromosomal dsbA, which encodes a periplasmic enzyme, is required for disulfide bond formation of the functional pilin subunit (28).
To date, three functions of TCP have been characterized. In addition to secretion of the colonization factor TcpF, TCP is also responsible for microcolony formation by mediating bacterium-bacterium interactions that are thought to promote in vivo colonization (20) and is the receptor for CTXΦ, the bacteriophage that carries the genes for cholera toxin (39). When cells are grown in vitro, TCP production causes the bacteria to form microcolonies or autoagglutinate in liquid culture. In the case of classical biotype strains, in which TCP production occurs maximally during growth under appropriate in vitro conditions, autoagglutination manifests itself as granular clumps of cells that fall out of suspension after overnight growth (20). TCP filaments protruding from the bacterial surface appear to align along their longitudinal axes to form bundles of filaments in vitro, comparable to the bundle-forming pilus (BFP) of enteropathogenic Escherichia coli (EPEC) (4). In addition, mutants lacking TCP show increased serum sensitivity, indicating a potential role for the pili in mediating protection against complement-like activity potentially associated with the intestinal environment (5).
TcpA belongs to the type IV family of pilins, which includes pilins from several pathogenic bacteria, including BFP of EPEC (14), gonococcal (GC) pilin from Neisseria gonorrhoeae (27), PAK pilin from Pseudomonas aeruginosa (9), and the CFA/III pilin of enterotoxigenic E. coli (ETEC) (34). All type IV pilin subunits share N-terminal sequence similarity, a C-terminal disulfide bond, and conserved cellular machinery for assembly into pilus filaments, suggesting a common subunit structure and filament architecture (9). Type IV pili share several cellular functions, including surface motility, microcolony and biofilm formation, adhesion, immune evasion, cell signaling, DNA transformation, and phage attachment. These structural similarities probably reflect certain functional similarities, although the biological roles of the pili seem to vary depending on the organism on which they are present (reviewed in reference 8). Although most type IV pili in pathogenic bacteria have been characterized to be involved in adhesion to the host, including pili from N. gonorrhoeae (40), P. aeruginosa (11), EPEC (7, 38), and ETEC (34), very few studies have characterized TCP as being an attachment factor. Early studies demonstrated that V. cholerae bacterial cells lacking tcpA were unable to agglutinate erythrocytes (37); however, other studies found that ΔtcpA and ΔtoxR mutants were only slightly defective in attachment to human intestinal HT-29 cells (3, 21). In spite of this, studies with EPEC have demonstrated that pilus-mediated attachment is cell type specific, since BFP mediates attachment to human intestinal Caco-2 cells but not to human intestinal CMT-93 cells (38). Since differentiated Caco-2 cultured epithelial cell assays have proved to be valuable in vitro assays for assessing EPEC and ETEC adhesion (7, 10, 34), we decided to use these cells to assess the function of TCP as a potential adhesin.
Here we report that similar to other type IV pili, TCP plays a role in attachment to Caco-2 human intestinal epithelial cells. In addition to in vitro studies, we also visualized TCP-mediated attachment in vivo during infection of the infant mouse model by using high-resolution field-emission scanning electron microscopy (FESEM). Scanning electron microscopy studies in the past have observed V. cholerae pathogenesis in models such as the rabbit ileal loop model (25) and in vitro organ cultures (IVOC) (41, 42). These studies have been valuable in understanding some of the steps of pathogenesis; however, it is not clear that any of these surrogate models are completely suitable for evaluating the factors essential for colonization of the human gut. The infant mouse model is one of the more widely used models because it allows active peristalsis, digestion, and diarrhea, conditions which most likely resemble an actual human infection, and studies using the infant mouse model have demonstrated a correlation with human infection (15). Although the infant mouse model is used widely, to our knowledge there have not been any scanning microscopy studies of V. cholerae infection, probably due to the difficulty in locating the bacterial cells in a vast small bowel with active peristalsis. We have developed a technique to visualize V. cholerae pathogenesis during infection of the infant mouse model by using FESEM, revealing TCP-mediated attachment and microcolony formation. In addition to these roles, we report that TCP forms matrices that appear to engulf V. cholerae during infection, and these matrices can protect the V. cholerae cells against a component of the antibacterial compound bile. To our knowledge, this is the first study visualizing V. cholerae infection of an animal model most resembling human infection, revealing TCP's role in mediating attachment, microcolony formation, and protection of V. cholerae bacterial cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. Strains were stored at −80°C in Luria-Bertani (LB) broth containing 30% (vol/vol) glycerol. V. cholerae was grown in LB broth with a starting pH of 6.5 at 30°C for 16 h (overnight). When necessary, ampicillin was used in cultures at 100 μg/ml. Streptomycin (Sm) was used at a concentration of 100 μg/ml in LB agar for the attachment and protection assays. Arabinose was used at a final concentration of 0.01% when needed.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| V. cholerae strains | ||
| O395 | O1 classical Ogawa strain; Smr | 37 |
| RT4031 | O395 ΔtcpA11 | 20 |
| RT4045 | O395 with El Tor tcpA (allelic exchange at normal chromosomal location) | Laboratory collection |
| SJK190 | O395 pRH81 | This study |
| SJK290 | O395 ΔtcpA11 pRH81 | This study |
| JMM17 | O395 ΔtcpA11 pJMA1 | J. Marles |
| Plasmids | ||
| pBAD22 | Expression plasmid; araC Apr | Laboratory collection |
| pRH81 | pBAD22 with six-His toxT | 16 |
| pJMA1 | pBAD22 tcpA | J. Marles |
Attachment assays.
Attachment assays were performed as described previously (34), using Caco-2 cells, a human colonic carcinoma cell line. Briefly, Caco-2 cells were maintained in Dulbecco modified Eagle medium (Life Technologies, Inc., Rockville, MD) supplemented with 10% fetal calf serum (Life Technologies, Inc., Rockville, MD) at 37°C in 5% CO2. Caco-2 cells were seeded into 24-well tissue culture plates at a concentration of approximately 105 cells/cm2 and used at 15 days postconfluence, which is the condition for well-matured Caco-2 cells expressing microvilli, as previously described (10). Prior to adhesion assays, Caco-2 cells were washed once in warm phosphate-buffered saline (PBS) (pH 7.0). A bacterial suspension of approximately 106 cells/ml was prepared in culture medium, 1 ml of the suspension was added to the washed Caco-2 cells, and the mixture was incubated for 3 h at 37°C in 5% CO2. Samples were washed three times with PBS (pH 7.0) and then incubated for 30 min in a 1% Triton X-100 solution. The resulting suspensions were diluted appropriately and plated on LB medium plates containing streptomycin. The attachment percentage reported is the average for at least nine samples tested for each strain, and Student's t test was used to determine statistical significance compared to the O395 wild type.
To view bacterial attachment to Caco-2 cells by using FESEM, Caco-2 cells were seeded and grown on Formvar-coated glass coverslips in six-well tissue culture plates for 15 days until postconfluence was achieved as described above. After 3 h of incubation with V. cholerae, the Formvar-coated coverslips were washed 3 times in PBS, placed in fixative, critical point dried, and viewed using FESEM as described below.
Infection of infant mice.
Vibrio cholerae cell cultures were grown for 16 h at 30°C before being diluted 1:1,000 in LB. Four- to 5-day-old CD-1 mice were inoculated orally with 50 μl of diluted culture and kept in small plastic containers at 30°C in the absence of their mothers. At 3, 12, and 24 h postinoculation, mice were sacrificed; the small intestines were removed and placed directly into fixative for FESEM preparation (see below). Five mice were inoculated for each time point, and intestinal sections were viewed for each independent mouse.
For the bile protection assay, a total of 28 mice were inoculated with wild-type O395 bacteria. At 3 h postinoculation, 14 mice were sacrificed, and their small intestines were removed and sliced into six equal lengths. Of the 14 small intestines, intestines from seven mice were placed in LB and those from the remaining seven were placed in 20% sodium deoxycholate (Sigma, St. Louis, MO), a component of bile, for 1 h at 37°C with shaking. Bacteria were recovered by homogenizing harvested intestines in 3 ml of 10% glycerol with a Tissue Terror homogenizer (Fisher Scientific, Los Angeles, CA). The homogenate was diluted and plated on solid medium containing streptomycin. The survival index was calculated by dividing the number of bacteria that survived in 20% sodium deoxycholate by the number of bacterial cells that survived incubation in LB, normalized to the output number of bacteria in the intestine without further incubation. The same procedure was repeated for the remaining 14 mice at 24 h postinoculation. A survival index of 1 indicates that the bacteria associated with the intestines of the infected mice were completely protected against 20% sodium deoxycholate.
FESEM.
To view mouse intestinal sections using FESEM, infant mice were sacrificed at various time points postinoculation, and the small intestines were removed and placed directly into a fixative solution of 4% paraformaldehyde–0.1 M sodium cacodylate for a minimum of 3 h. After being fixed, the small intestine was sliced into three segments of equal length, designated the proximal, middle, and distal intestinal sections, as described by Angelichio et al. (1). Since the majority of V. cholerae cells colonize the middle segment of the small bowel (1), this segment was cut laterally twice to expose the interior surface and then sliced into 7-mm-long pieces that were placed back into fresh fixative for 3 additional hours. After being washed with 0.1 M sodium cacodylate (pH 7.4), samples were dehydrated through an ethanol series and then critical point dried using a Samdri 795 critical point dryer (Tousimis Corp., Rockville, MD). Dried samples were coated with 3 nm of osmium by use of an SPI plasma coater (SPI Supplies, West Chester, PA) before examination. Images were generated at 15 kV by an FEI XL-30 (FEI Company, Salem, MA) field emission gun scanning electron microscope (FEG-SEM).
Immuno-field-emission scanning electron microscopy.
For immunolabeling, intestinal sections from infected and uninfected mice were extracted as described above and fixed for a minimum of 2 h in 4% paraformaldehyde–0.1 M sodium cacodylate at room temperature. Samples were washed twice in Tris-buffered saline with Tween 20 (TBST) before blocking in 3% bovine serum albumin (BSA)–TBST for 1 h. After blocking, samples were incubated for 3 h at room temperature with a 1:10 dilution of anti-TcpA 169.1 monoclonal antibody (32) or anti-O1 monoclonal antibody (cholera DFA; New Horizons Diagnostics Corporation, Columbia, MD) or with normal mouse serum (NMS) as a negative control. After several washes, the sections were incubated with goat anti-mouse secondary antibodies conjugated to 18-nm colloidal gold particles (Jackson ImmunoResearch Laboratories, Inc.) at a 1:50 dilution for 30 min. Following immunolabeling, intestinal sections were washed again several times before a final fixation in 2.5% glutaraldehyde–0.1 M sodium cacodylate for a minimum of 5 h. Samples were then dehydrated, critical point dried, and examined as described above.
Quantification of specific labeling was performed by counting the number of gold particles specifically labeling TCP or the bacterial cell surface in 10 randomly selected images for V. cholerae-infected or uninfected mice incubated with NMS, anti-TcpA, or anti-O1 monoclonal antibody in two independent experiments. Results are presented as the percentage of gold particles that specifically labeled TCP or the bacterial cell surface compared to the total number of gold particles in each image.
RESULTS
Attachment of V. cholerae to Caco-2 human intestinal cells mediated by TCP.
Caco-2 cells are a well-characterized human intestinal cell line used to demonstrate the requirement of type IV pili for attachment of other enteric pathogenic bacteria, such as BFP of EPEC and the CFA/III pilus of ETEC (7, 34). Because type IV pilus attachment can be cell type specific (38) and TCP is most similar to these other type IV pili that attach to Caco-2 cells, we also used this cell line to investigate if TCP plays a role in attachment. Caco-2 cells were differentiated for 15 days postconfluence and incubated for 3 h with cultures containing various V. cholerae strains (Table 1). V. cholerae lacking tcpA was significantly defective in attachment (10-fold) compared to the wild type (Fig. 1A). Overexpression of tcpA in a ΔtcpA mutant strain was unable to complement the attachment defect, probably due to the lack of balanced stoichiometry between protein levels of TcpA and the TCP apparatus. Supporting this hypothesis, strain JMM17 (O395 ΔtcpA pTcpA) produced large amounts of TcpA pilins but did not autoagglutinate as well as the wild type, suggesting that it did not produce as much functional TCP as the wild-type strain. Instead, tcpA from the El Tor biotype was exchanged into the native chromosomal location of the classical tcpA gene in the O395 ΔtcpA (RT4045) strain, and it complemented the ΔtcpA attachment defect (Fig. 1A). To provide further evidence that TCP functions as an attachment factor, toxT, encoding the activator of tcp gene expression, was overexpressed in wild-type bacteria to activate the entire tcp operon with stoichiometric levels of all proteins involved in TCP biogenesis. V. cholerae wild-type bacterial cell attachment to Caco-2 cells increased significantly, from 17% to approximately 88%, when toxT was overexpressed (Fig. 1A). However, attachment did not increase when toxT was overexpressed in a ΔtcpA strain (SJK290) (Fig. 1A). This strain was significantly defective in attachment compared to the wild type, indicating that the increased attachment in SJK190 (O395 pToxT) was due to increasing the production of TCP and not to overexpression of other attachment factors.
Fig. 1.

TCP mediates attachment of V. cholerae to Caco-2 cells. (A) Percentage of V. cholerae cells attaching to differentiated Caco-2 cells after 3 h of incubation at 37°C. Student's t test was used to determine that ΔtcpA strains were significantly defective in attachment compared to wild-type cells (P < 0.014), even when overexpressing ToxT. El Tor tcpA from strain N16961 was exchanged into the native chromosomal location of the classical tcpA gene and was able to complement the ΔtcpA attachment defect, and overexpression of toxT in the wild type significantly increased attachment to Caco-2 cells (P < 0.0001). (B) Field-emission scanning electron micrograph of wild-type V. cholerae TCP attaching to microvillus-expressing Caco-2 cells after 3 h of incubation at 37°C. Black arrowheads, bundled TCP; white arrowheads, microvilli; black arrows, TCP attaching to Caco-2 cells; white arrows, intact flagella. Bar, 1 μm.
TCP-mediated attachment of wild-type V. cholerae to Caco-2 cells was also visualized using FESEM (Fig. 1B). After 3 h of incubation on differentiated Caco-2 cells, V. cholerae O395 cells were viewed proximal to TCP bundles appearing to attach to the Caco-2 cell surface (Fig. 1B). Similar images were observed for RT4045 (O395 with El Tor tcpA) and SJK190 (O395 pToxT) (data not shown). However, it was difficult to locate bacterial cells from strains RT4031 (O395 ΔtcpA) and SJK290 (O395 ΔtcpA/pToxT) (data not shown), suggesting that the V. cholerae cells were removed during the wash steps because TCP was not present for attachment. Taken together, these data suggest that TCP functions as a V. cholerae attachment factor for Caco-2 intestinal cells.
Visualization of V. cholerae infection of the infant mouse intestine after oral inoculation reveals that microcolony formation in vivo differs from microcolony formation in vitro.
It has previously been demonstrated that bacteria lacking tcpA are cleared rapidly from the intestine, in less than 24 h (20, 21). The kinetics of this rapid clearance has been suggested to be due to the lack of microcolony formation of these ΔtcpA bacterial cells. In order to determine if TCP could also participate in attachment in vivo, we decided to utilize the infant mouse model to visualize attachment of V. cholerae after oral inoculation. Besides the new infant rabbit model described by Ritchie et al., the infant mouse model is one of the only models that allows active peristalsis, digestion, and diarrhea, which most likely resemble an actual human infection (15, 29). In addition, results with V. cholerae colonization mutants have demonstrated that the infant mouse model reflects what is found in human infection (15). Four- to 5-day-old mice were inoculated orally with wild-type V. cholerae, and the infection was allowed to develop for 24 h prior to sacrifice. The small intestine was removed, and the midsection was sliced into 7-mm segments and prepared for visualization using FESEM. Visualization of the small bowel revealed the finger-like projections of villi lining the intestinal wall (see Fig. S1A in the supplemental material). To our knowledge, we are the first to visualize V. cholerae infection in the infant mouse model by using scanning electron microscopy.
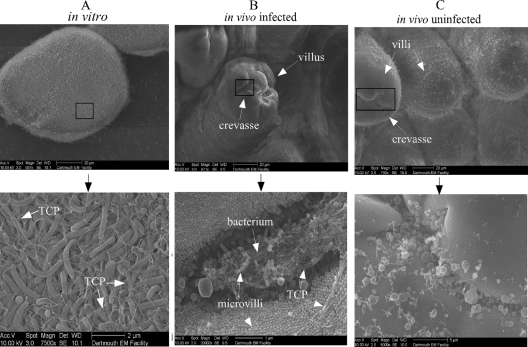
Initially, locating V. cholerae colonization in the vast intestinal sections was difficult; however, we were able to locate V. cholerae bacterial cells more easily when the villi displayed a bumpy appearance (see Fig. S1B in the supplemental material). This bumpy appearance was indicative of epithelial cells on the villi of the small intestine becoming denuded in the presence of V. cholerae infection. Although V. cholerae cells could still be located in sections of infected intestine that did not display this bumpy appearance (see Fig. S1C), colonization was extremely difficult to locate. Initially, we searched in vivo for microcolonies that resembled microcolonies formed under in vitro conditions of growth: 30°C for 16 h at a pH of 6.5 (Fig. 2A). The bacteria formed microcolonies under these conditions that fell out of solution, a characteristic attributed solely to TCP, called autoagglutination. In microcolonies in vitro, TCP was typically seen throughout the microcolony, mediating bacterium-bacterium interactions; however, the bacterial cells were visible mostly with TCP scattered throughout the microcolony-forming bundles (Fig. 2A). However, in viewing V. cholerae colonization in vivo, TCP appeared to engulf the bacterial cells, which were difficult to visualize, forming a matrix of TCP filaments around the bacterial cells (Fig. 2B).
Fig. 2.
Field-emission scanning electron micrographs of microcolony formation under in vitro conditions (A), V. cholerae colonization of a crevasse on top of a villus in the small intestine of an infected mouse (B), and a crevasse on top of a villus of an uninfected mouse (C). Boxes indicate the areas shown in the bottoms images (at a higher magnification). Scale bars are included for each image.
V. cholerae cells were found colonizing the crypts, as well as in the crevasses and folds along the sides of the villi. There were no visual qualitative differences between colonization of V. cholerae in the crypts versus the crevasses (data not shown). Intestinal crevasses of infected mice were drastically different from intestinal crevasses of uninfected mice (Fig. 2C), in which these bundles and matrices were not found in the crevasses or the folds along the villi. To confirm that the bacterial cells observed inside these matrices were V. cholerae, the intestines of infected mice were immunolabeled with anti-O1 monoclonal antibody, specific to the O antigen of classical V. cholerae O1 lipopolysaccharide (LPS) on the bacterial cell surface. Immunolabeling revealed that V. cholerae cells were located inside the filament matrices (Fig. 3A and B). Normal mouse serum inoculated with infected intestines was used as a negative control, where few gold particles were found to bind nonspecifically (Fig. 3C). Uninfected intestines inoculated with the anti-O1 monoclonal antibody were also used as a negative control (Fig. 3D). Quantification of specific immunolabeling of the bacterial cell surface in infected mice revealed that there was significant labeling on the V. cholerae bacterial cell surface when infected intestines were incubated with the anti-O1 monoclonal antibody compared to infected intestines incubated with normal mouse serum or uninfected mouse intestines incubated with anti-O1 monoclonal antibody (Fig. 3D).
Fig. 3.
V. cholerae engulfed in filament matrices. (A) FESEM view of V. cholerae engulfed in a matrix of filaments alongside a villus in the infant mouse. The V. cholerae cell is slightly visible, as indicated by the white arrowhead. (B) Backscatter view of image A immunolabeled with anti-O1 V. cholerae specific monoclonal antibody, disclosing V. cholerae in the fibrous matrix. (C) Backscatter image of NMS incubated with a V. cholerae-infected mouse intestine to serve as a negative control. White arrowheads indicate V. cholerae, and black arrows indicate 18-nm gold particles. (D) Quantification of specific immunolabeling of the bacterial cell surface or TCP in infected mice, using anti-O1 and anti-TcpA monoclonal antibodies. Uninfected mouse intestines incubated with anti-O1 and anti-TcpA monoclonal antibodies also served as a negative control. The x axis indicates if the mice were infected or uninfected and labeled with either NMS or anti-O1 or anti-TcpA monoclonal antibody. Quantification of specific labeling was performed by counting the number of gold particles specifically labeling TCP or the bacterial cell surface in 10 randomly selected images for V. cholerae-infected or uninfected mice incubated with NMS or with anti-TcpA or anti-O1 monoclonal antibody in two independent experiments. Results are presented as the percentage of gold particles that specifically label TCP or the V. cholerae bacterial cell compared to the total number of gold particles in each image. Asterisks indicate significant specific binding to V. cholerae bacterial cells or TCP compared to the negative controls using NMS or uninfected intestines (P < 0.0001).
TCP participates in attachment to epithelial cells in vivo.
While colonization of the infant mouse intestine by V. cholerae was being visualized, it also appeared that TCP was bound to the epithelial cell microvilli (Fig. 4). Similar to other in vitro images of type IV pili appearing to attach to Caco-2 cells, these pili appeared to reach out toward the microvilli and form a mesh of TCP filaments that spread along the intestinal epithelial cells (Fig. 4). In order to confirm that these filaments were TCP, the intestines of infected mice were incubated with monoclonal antibody 169.1 (anti-TcpA). A scanning view of these filaments reaching out toward the microvilli on the epithelial cell is shown in Fig. 5A. These filaments were positively immunolabeled with monoclonal antibody 169.1 (Fig. 5B), demonstrating that these were TCP. The negative controls, incubating infected intestines with normal mouse serum and incubating uninfected mouse intestines with monoclonal antibody 169.1, did not reveal specific labeling (data not shown). Quantification of specific immunolabeling of TCP revealed that there was significant labeling on these filaments when infected intestines were incubated with monoclonal antibody 169.1 (anti-TcpA) compared to infected intestines incubated with normal mouse serum or uninfected mouse intestines incubated with monoclonal antibody 169.1 (Fig. 3D). These data suggest that TCP may play a role in attachment to epithelial cells of the small intestine during infection.
Fig. 4.
Field-emission scanning electron micrographs of TCP filaments that appear to be attaching to the microvilli of the epithelial cells in the small intestine. TCP filaments and microvilli are indicated with arrows. Bars, 500 nm.
Fig. 5.
Immunolabeling with 169.1 (anti-TcpA antibody) reveals TCP filaments in infected intestines. (A) FESEM image of V. cholerae surrounded by a matrix of TCP filaments that appear to be attaching to the microvilli of the epithelial cell in the crevasse of a villus. The box indicates the area shown in panel B (at a higher magnification). (B) Backscatter image of filaments positively immunolabeled with monoclonal antibody 169.1 (anti-TcpA). White arrowheads indicate a V. cholerae bacterial cell. Black arrows indicate some of the immunolabeling with 18-nm gold particles.
TCP matrices are formed in later stages of infection.
It has been demonstrated previously that V. cholerae lacking tcpA is cleared very rapidly from the intestine. By 8 h after inoculation of 107 bacterial cells, only 100 bacteria from intestines can be enumerated on solid media, and by 24 h, no bacteria are detected in the small bowel (21). However, wild-type bacteria are maintained at 105 to 106 bacterial cells in the small intestine throughout the course of infection (21). During infection, it has been demonstrated that the population of V. cholerae bacteria is dynamic: virulence genes are highly expressed early in the infection process, and during the later stages, the population of V. cholerae bifurcates into two fractions, namely, one subpopulation continues to express virulence genes, whereas these genes are silenced in the other subpopulation (26). Using FESEM, differences between initial infection and the later stages of infection of the infant mouse were visualized. Mice were sacrificed at 3, 12, and 24 h postinoculation before intestinal samples were prepared for FESEM. At 3 h postinoculation, intestines from mice infected with wild-type bacteria were visualized attaching to epithelial cells in what appeared to be a TCP-independent manner (Fig. 6). While bacterial cells had intact flagella, they were not enclosed in a matrix of TCP filaments compared to the later stages of infection (Fig. 6 to 8). Twelve hours after inoculation with wild-type V. cholerae, microcolonies were visualized in villus crevasses, with TCP-mediated bacterium-bacterium interactions (Fig. 7). Intestinal sections were immunolabeled with monoclonal antibody 169.1 (anti-TcpA), confirming the presence of TCP within these microcolonies (Fig. 7B and C). After 24 h, microcolonies were also visualized, but TCP appeared to form matrices that engulfed the bacteria within these microcolonies (Fig. 8A). Intestinal sections incubated with monoclonal antibody 169.1 revealed that these matrix filaments were positively labeled as TCP (Fig. 8B). Quantification of specific labeling and the negative controls are outlined in Fig. 3D. Similar observations were made with mice inoculated with RT4045, expressing the El Tor biotype tcpA gene (data not shown). These data suggest that TCP not only mediates bacterium-bacterium interactions to generate microcolonies but also forms a fibrous matrix around the bacterial cells.
Fig. 6.

Field-emission scanning electron micrograph of V. cholerae appearing to initially attach in a TCP-independent manner to a crevasse on top of a villus in the small intestine of an infected mouse at 3 h postinoculation. Bar, 1 μm.
Fig. 8.
Specific immunolabeling with anti-TcpA reveals TCP engulfing V. cholerae bacterial cells. (A) FESEM backscatter view of TCP forming a matrix of filaments around the V. cholerae bacterial cells in the small intestine of an infected infant mouse at 24 h postinoculation. The box indicates the area shown in panel B (at a higher magnification). (B) Immunolabeled filaments with monoclonal antibody 169.1 (anti-TcpA). Arrows point toward some of the specific immunolabeling with 18-nm gold particles. Bars, 1 μm.
Fig. 7.
Positive immunolabeling with anti-TcpA reveals TCP mediating bacterium-bacterium interactions. (A) FESEM of TCP-mediated microcolony formation by V. cholerae on top of a villus in the small intestine of an infected mouse at 12 h postinoculation. The box indicates the area shown in panels B and C (at a higher magnification). (B) Scanning view of TCP filaments interacting with V. cholerae bacterial cells. (C) Backscatter view of image B revealing immunolabeling of TCP filaments with monoclonal antibody 169.1 (anti-TcpA). Arrows point toward the mesh of TCP filaments. Bars, 1 μm.
TCP matrices protect V. cholerae from bile.
Because TCP matrices appeared to form in a time-dependent manner, we wanted to determine if these TCP matrices could protect V. cholerae from antibacterial compounds such as bile. Infant mice were inoculated with wild-type bacteria and divided into two groups. The first group was sacrificed at 3 h postinoculation (when no TCP matrices are found), and their intestines were removed and placed into either LB or a solution containing 20% sodium deoxycholate for 1 h at 37°C. After 1 h, the samples were thoroughly homogenized, plated onto solid medium, and enumerated the following day. The second group was sacrificed at 24 h postinoculation (when V. cholerae is enclosed in a matrix of TCP), and the same procedure was followed. The survival index was calculated and averaged for 7 mice at each time point. A survival index of 1 is indicative of 100% survival of exposure to an antibacterial compound. Bacterial cells at 24 h postinoculation survived the bile treatment significantly better (100-fold) than did bacterial cells at 3 h postinoculation (Fig. 9), suggesting that the TCP-mediated matrices formed after 24 h could play a role in protecting the bacteria from antimicrobial agents such as bile.
Fig. 9.

V. cholerae cells in TCP matrices are protected from sodium deoxycholate compared to V. cholerae cells not enclosed in pilus matrices. The small bowels of infected infant mice were removed at either 3 h (when no TCP matrices are found) or 24 h (when V. cholerae is enclosed in a matrix of TCP) postinoculation and exposed to 20% bile for 1 h at 37°C. Black circles represent the survival index for infected intestines from each mouse exposed to bile, and lines represent the average survival index for 7 mice tested at each postinoculation time point. Bacterial cells within the infected intestine at 24 h postinoculation survived the bile treatment significantly better than did bacterial cells within the infected intestines at 3 h postinoculation, by 2 log (P < 0.013), suggesting that TCP matrices aid in the protection of V. cholerae cells.
DISCUSSION
Microbial attachment and host colonization are key events in V. cholerae infection and result in the ability of the infecting bacterial cells to persist and multiply. In this study, we sought to determine if V. cholerae utilizes TCP to attach to Caco-2 cells, similar to other type IV pili, and to visualize the steps of V. cholerae infection in the infant mouse model. We found that TCP may play additional roles in colonization beyond microcolony formation alone. Here we report that TCP is involved in epithelial cell attachment, similar to other type IV pili, and in protection of V. cholerae, by forming matrices that appear to engulf the bacterial cells at later stages of infection.
Using a well-documented intestinal epithelial cell line to which other type IV pili attach, we found that TCP mediates attachment to Caco-2 cells in vitro. It is also possible that another, unidentified V. cholerae protein may interact with TCP to directly mediate epithelial cell adhesion, and not the pilus itself. In a previous study, purified TCP did not adhere to the surface of human intestinal epithelium (33), likely due to either the conformational changes that occurred in TCP during the purification process or the possibility that TCP alone cannot mediate attachment without a potential unknown minor subunit. It has been documented that in some cases the major subunit directly mediates adhesion, while in other instances this role is mediated directly by one of the minor subunits, with the polymerized major subunits providing a stalk on which the minor subunits are spaced throughout or appear at the tip (35). For example, the Neisseria pilus consists of at least two distinct proteins that are involved in adhesion: the highly variable major subunit PilE, forming the pilus filament, and PilC, the adhesin localized to the tip of the filaments (30, 31). TcpF is secreted by the TCP apparatus and may be a candidate protein for this interaction. However, ΔtcpF mutants are only slightly defective (not significantly) in Caco-2 cells, and TcpF does not localize to the pilus, based on immunolabeling electron microscopy studies in vitro and in vivo (data not shown). In addition, passive immunization of infant mice using polyclonal and monoclonal antibodies directed against TCP suggests that the adhesive moiety lies within the major pilin subunit itself rather than being a minor pilus adhesion protein (35). Also, because ΔtcpA mutants are defective in attachment to Caco-2 cells, these data suggest that TCP is able to mediate attachment either directly or indirectly. Since the El Tor pilus was able to adhere just as well as the classical pilus in vitro and in vivo, examining the conserved surface residues between the pilin subunits could provide some clues to identify critical amino acids of the pilus required for attachment. Studies are under way to use a library of tcpA point mutants to map out the possible TCP binding domain.
Utilizing FESEM on intestinal sections of mice infected with V. cholerae, we visualized V. cholerae colonizing crevasses on the tips of the villi, folds along the length of the villi, and crypts between the villi within the infant mouse small intestine, similar to previous studies (25). We did not visualize any qualitative differences between colonization of V. cholerae in the crypts versus the crevasses. V. cholerae colonization was easier to locate when the epithelial cells were undergoing dramatic denudation, giving a bumpy appearance of the villi. This finding of denudation is consistent with histological and microscopic studies of intestinal biopsy specimens from cholera patients (2, 12). At 3 h postinoculation, V. cholerae was found attaching to the intestine in a TCP-independent manner. Although we found that TCP is necessary for attachment to Caco-2 cells in vitro, TCP is not the only characterized V. cholerae attachment factor. GlcNAc binding protein (GbpA) also mediates attachment by binding to GlcNAc sugar residues on epithelial cells (19). Also, previous studies found that tcp is expressed as early as 1 h postinfection, as measured by resolution of a Tet resistance cassette (23), suggesting that TCP may not play a role in initial attachment but, rather, at later stages of infection. Expression was strongly increased by 4 h and reached maximal TetR resolution by 6 h (23).
At 12 h postinoculation, when tcp is expressed, V. cholerae can be visualized forming TCP-mediated microcolonies in the small intestine, and as the disease progresses to 24 h, the bacterial cells appear to be engulfed by pilus matrices. TCP undergoes a dramatic transformation in structural organization from being in bundled filaments that align longitudinally to forming a matrix of filaments that appear to surround the bacteria in a cocoon. Some matrices were visualized in as little as 12 h; however, the formation of these matrices was probably continuous upon tcpA expression over the course of 24 h. The pili observed in vivo appeared smaller in diameter than pili observed in vitro, and there is a possibility that TcpA could be secreted to aid in formation of the matrix as part of an in vivo-formed biofilm. Experiments to determine if there are other components of the matrix are under way.
When intestines from infected mice containing these pilus matrices were exposed to a high concentration of bile, V. cholerae bacterial cells were able to survive significantly better than bacterial cells in intestines of infected mice that did not form these matrices, although both infected intestines had equal numbers of bacteria. These data suggest that a role of these pilus matrices is to protect the bacterial cells from antibacterial compounds. It is possible that the protection against bile at 24 h postinoculation was due to other factors involved in establishing infection and protection of V. cholerae and not because of the TCP-mediated matrices physically engulfing V. cholerae. However, since ΔtcpA bacterial cells never establish an infection (21), the definitive genetic experiment of exposing intestines infected with ΔtcpA bacterial cells to bile and comparing them with intestines infected with the wild type cannot be performed. Also, it is not known whether these protective matrices are found in other model systems for testing additional putative factors in persistence. These matrices may be formed only in a system that has active peristalsis, resembling a true infection. In spite of this, V. cholerae cells expressing TCP in vitro are more resistant to serum (5), bile, and antibiotics (S. J. Krebs, unpublished data), suggesting a role for the pili in mediating protection against antibacterial compounds associated with the intestinal environment during infection. A previous study by Kirn et al. (20) demonstrated that tcpA point mutants capable of autoagglutination exhibited levels of complement resistance similar to wild-type levels, whereas those mutants incapable of autoagglutination were invariably sensitive to cytolysis. These data suggest that autoagglutination itself protects bacteria from complement-mediated cytolysis by sequestering bacteria inside microcolonies (20). We propose that TCP matrices physically protect bacteria from the harsh intestinal environment and allow V. cholerae to persist and survive within this environment in the presence of defensins, bile acids, pancreatic secretions, and secretory IgA antibodies. Longus pilus-expressing ETEC strains have also been associated with improved survival (10-fold) when exposed to antibacterial factors, including lysozyme and antibiotics. This suggests that longus-mediated bacterial self-aggregates also protect bacteria against antimicrobial environmental agents and may promote gut colonization (6).
The population of bacteria in the intestine is dynamic. Although a total cell count of 105 to 106 V. cholerae bacterial cells is maintained in the gut throughout infection, some of the bacteria are multiplying in the gut while others are disseminating out into the environment. It is most likely that both the molecular mechanisms of detachment of V. cholerae and the host response are concomitantly responsible for the dissemination of V. cholerae into the environment. It is possible that the shedding of epithelial cells of the intestinal villi may be a host response to clear the V. cholerae-containing pilus matrices. Aggregates of bacterial cells visualized in the fecal matter of cholera-infected patients have been termed “in vivo-formed biofilms,” and it has been hypothesized that their presence is related to infectivity and persistence of V. cholerae in the environment (13). It has been suggested that enhanced infectivity of V. cholerae shed in human stools is due largely to the presence of these aggregates of bacterial cells that disperse in vivo, providing a higher dose of the pathogen (13). These in vivo-formed biofilms/aggregates may be the same TCP-mediated protective cocoons described here, which we visualized in V. cholerae-infected infant mice.
V. cholerae requires TCP to mediate efficient intestinal colonization. Without TCP, V. cholerae cells cannot colonize and are cleared from the small intestine very rapidly. In this study, we sought to determine the role of TCP in colonization in vivo and found that in addition to microcolony formation, TCP functions in attachment and protection of V. cholerae bacterial cells by forming TCP matrices. Using FESEM and the infant mouse model, we also illustrated the importance of examining infection in a model system that resembles actual human infection. These results indicate that TCP plays an important role in the progression of disease at later stages of V. cholerae infection and potentially provide us with steps associated with successful colonization.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Louisa Howard and the Ripple Imaging Facility at Dartmouth for help in the generation of the FESEM images.
This work was supported by NIH grant AI025096 to R.K.T. S.J.K. was also supported by NIH training grant T32GM08704.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Angelichio M. J., Spector J., Waldor M. K., Camilli A. 1999. Vibrio cholerae intestinal population dynamics in the suckling mouse model of infection. Infect. Immun. 67:3733–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asakura H., Tsuchiya M., Watanabe Y., Enomoto Y., Morita A. 1974. Electron microscopic study on the jejunal mucosa in human cholera. Gut 15:531–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benitez J. A., et al. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 65:3474–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bieber D., et al. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114–2118 [DOI] [PubMed] [Google Scholar]
- 5. Chiang S. L., Taylor R. K., Koomey M., Mekalanos J. J. 1995. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol. Microbiol. 17:1133–1142 [DOI] [PubMed] [Google Scholar]
- 6. Clavijo A. P., Bai J., Gomez-Duarte O. G. 2010. The longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microb. Pathog. 48:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cleary J., et al. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527–538 [DOI] [PubMed] [Google Scholar]
- 8. Craig L., Pique M. E., Tainer J. A. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363–378 [DOI] [PubMed] [Google Scholar]
- 9. Craig L., et al. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11:1139–1150 [DOI] [PubMed] [Google Scholar]
- 10. Darfeuille-Michaud A., et al. 1990. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect. Immun. 58:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doig P., et al. 1988. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun. 56:1641–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dutt A. R., Sen S. K. 1969. Some observations on cholera epidemics in Calcutta. J. Indian Med. Assoc. 52:326–329 [PubMed] [Google Scholar]
- 13. Faruque S. M., et al. 2006. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. U. S. A. 103:6350–6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giron J. A., Ho A. S., Schoolnik G. K. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710–713 [DOI] [PubMed] [Google Scholar]
- 15. Herrington D. A., et al. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hulbert R. R., Taylor R. K. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J. Bacteriol. 184:5533–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaper J. B., Morris J. G., Jr., Levine M. M. 1995. Cholera. Clin. Microbiol. Rev. 8:48–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirn T. J., Bose N., Taylor R. K. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49:81–92 [DOI] [PubMed] [Google Scholar]
- 19. Kirn T. J., Jude B. A., Taylor R. K. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438:863–866 [DOI] [PubMed] [Google Scholar]
- 20. Kirn T. J., Lafferty M. J., Sandoe C. M., Taylor R. K. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35:896–910 [DOI] [PubMed] [Google Scholar]
- 21. Kirn T. J., Taylor R. K. 2005. TcpF is a soluble colonization factor and protective antigen secreted by El Tor and classical O1 and O139 Vibrio cholerae serogroups. Infect. Immun. 73:4461–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaPointe C. F., Taylor R. K. 2000. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J. Biol. Chem. 275:1502–1510 [DOI] [PubMed] [Google Scholar]
- 23. Lee S. H., Hava D. L., Waldor M. K., Camilli A. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625–634 [DOI] [PubMed] [Google Scholar]
- 24. Miller V. L., Taylor R. K., Mekalanos J. J. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271–279 [DOI] [PubMed] [Google Scholar]
- 25. Nelson E. T., Clements J. D., Finkelstein R. A. 1976. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect. Immun. 14:527–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen A. T., et al. 2010. A bistable switch and anatomical site control Vibrio cholerae virulence gene expression in the intestine. PLoS Pathog. 6:e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parge H. E., et al. 1995. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378:32–38 [DOI] [PubMed] [Google Scholar]
- 28. Peek J. A., Taylor R. K. 1992. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 89:6210–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ritchie J. M., Rui H., Bronson R. T., Waldor M. K. 2010. Back to the future: studying cholera pathogenesis using infant rabbits. MBio 1:e00047–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudel T., Scheurerpflug I., Meyer T. F. 1995. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 373:357–359 [DOI] [PubMed] [Google Scholar]
- 31. Scheuerpflug I., Rudel T., Ryll R., Pandit J., Meyer T. F. 1999. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect. Immun. 67:834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun D. X., Seyer J. M., Kovari I., Sumrada R. A., Taylor R. K. 1991. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect. Immun. 59:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamamoto T., et al. 1998. Adhesive property of toxin-coregulated pilus of Vibrio cholerae O1. Microbiol. Immunol. 42:41–45 [DOI] [PubMed] [Google Scholar]
- 34. Taniguchi T., et al. 2001. Gene cluster for assembly of pilus colonization factor antigen III of enterotoxigenic Escherichia coli. Infect. Immun. 69:5864–5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor R. K. 1991. Bacterial adhesion to mucosal surfaces. J. Chemother. 3(Suppl. 1):190–195 [PubMed] [Google Scholar]
- 36. Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. 1986. Identification of a pilus colonization factor that is coordinately regulated with cholera toxin. Ann. Sclavo Collana Monogr. 3:51–61 [PubMed] [Google Scholar]
- 37. Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tobe T., Sasakawa C. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 4:29–42 [DOI] [PubMed] [Google Scholar]
- 39. Waldor M. K., Mekalanos J. J. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910–1914 [DOI] [PubMed] [Google Scholar]
- 40. Winther-Larsen H. C., et al. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. U. S. A. 98:15276–15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto T., Kamano T., Uchimura M., Iwanaga M., Yokota T. 1988. Vibrio cholerae O1 adherence to villi and lymphoid follicle epithelium: in vitro model using formalin-treated human small intestine and correlation between adherence and cell-associated hemagglutinin levels. Infect. Immun. 56:3241–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto T., Yokota T. 1988. Electron microscopic study of Vibrio cholerae O1 adherence to the mucus coat and villus surface in the human small intestine. Infect. Immun. 56:2753–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.