Abstract
This study is the first to demonstrate the activity of putative cellulosomal protease/peptidase inhibitors (named cyspins) of Clostridium cellulovorans, using the Saccharomyces cerevisiae display system. Cyspins exhibited inhibitory activities against several representative plant proteases. This suggests that these inhibitors protect their microbe and cellulosome from external attack by plant proteases.
TEXT
Some species of the Gram-positive anaerobic bacterium Clostridium can efficiently degrade polysaccharides in plant cell walls. Their efficient degradation is attributed to the cellulosome, which is the cellulolytic complex consisting of many cellulose-degrading enzymes. The cellulosome is composed of two major components: (i) a cell surface-attached nonenzymatic scaffolding protein with cohesion domains and (ii) a variety of cellulosomal enzymes with dockerin domains. Through an interaction between cohesin and dockerin, these cellulosomal proteins can assemble on cell surface-attached scaffolding protein. Assembly of enzymes in the cellulosome leads to effective and conjugative reactions (9). To understand the molecular mechanism underlying saccharification by the cellulosome, genomic analysis of several cellulosome-producing microorganisms was performed (Clostridium thermocellum and Clostridium cellulolyticum, analyzed by the Joint Genome Institute in 2007 and 2009, respectively). Whole-genome sequence analysis of Clostridium cellulovorans, which was suggested to have protoplast formation activity (1, 16), identified putative cellulosomal genes (14). Compared to genomes of other cellulosomal clostridia, C. cellulovorans has the smallest number of cellulosomal genes (15). The cellulosome of C. cellulovorans was indicated to be the most simple and suitable for the mechanistic analysis of the cellulosome. Genome analysis revealed 57 cellulosomal genes, including 4 scaffolding proteins and 53 cellulosomal enzymes. Most cellulosomal enzymes belong to the glycoside hydrolase family; there is, however, an interesting characteristic. Three protease/peptidase inhibitors are predicted in C. cellulovorans. Putative protease/peptidase inhibitors have been predicted in other cellulosome-producing microorganisms, such as C. thermocellum (20), C. cellulolyticum (3), and Piromyces sp. strain E2 (13). However, the role of protease inhibitors in cellulosomes has yet to be clarified. The prediction of gene function on the basis of genome analysis does not always correspond to protein profiling; therefore, gene function analysis at the level of protein production and activity is important to understand the true role of the gene. Because it is difficult to genetically manipulate C. cellulovorans, measurement of cellulosomal protein activity was examined with a Saccharomyces cerevisiae display system (17, 18), a heterologous gene expression system. This system is convenient to measure the activity of proteins because the displayed heterologous proteins can be quickly and directly analyzed using intact cells without the need for purification and concentration (17, 18). Furthermore, the proteins produced by the yeast display system are located on the yeast cell surface as well as cellulosomal enzymes that were naturally located on the cell surface of Clostridium species. In this study, using the yeast display system, we identified the function of the protease/peptidase inhibitors in C. cellulovorans cellulosomes and suggest their role in several cellulosome-producing microorganisms.
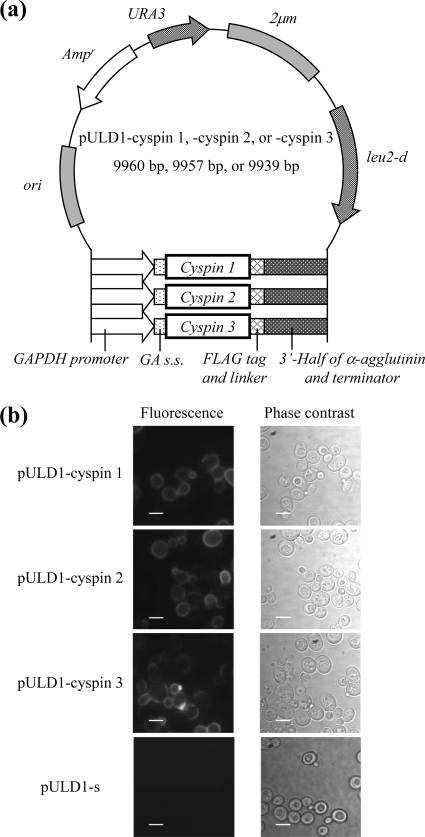
The regions encoding the protease/peptidase inhibitors cyspin 1 (putative cysteine peptidase inhibitor 1), cyspin 2, and cyspin 3 were amplified from the C. cellulovorans genome by PCR using one of three primer pairs, respectively: cyspin 1, 2-forward and cyspin 1-reverse; cyspin 1, 2-forward and cyspin 2-reverse; or cyspin 3-forward and cyspin 3-reverse (Table 1). By subcloning the resulting PCR product into the BglII-XhoI restriction site of pULD1 (8), three plasmids, pULD1-cyspin 1, pULD1-cyspin 2, and pULD1-cyspin 3, were constructed for display on the yeast cell surface by fusion with α-agglutinin and FLAG tag for detection (17, 18) (Fig. 1a). The constructed plasmids were introduced into Saccharomyces cerevisiae strain BY4741/sed1Δ (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YDR077w::KanMX4) by the lithium acetate method (5) using the EZ-Yeast transformation kit (BIO 101, CA). To detect displayed cyspins, cells were analyzed by immunofluorescence staining. After washing with phosphate-buffered saline (PBS) (pH 7.4), yeast cells were incubated in PBS (pH 7.4) containing 1% bovine serum albumin for 30 min. Mouse monoclonal anti-FLAG M2 antibody (Sigma-Aldrich, St. Louis, MO) was added as the primary antibody at a dilution factor of 1:300, and then the mixture of cells and antibody was incubated on a rotator at room temperature for 1.5 h. The cells were washed with PBS (pH 7.4) and incubated with the secondary antibody, Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, CA), diluted at 1:300 at room temperature for 1.5 h on a rotator. After washing with PBS (pH 7.4), cells were suspended in 20 μl PBS (pH 7.4) and then observed under a fluorescence microscope (IX71; Olympus, Tokyo, Japan) through a U-MNIBA2 mirror unit with a BP470-490 excitation filter, a DM505 dichroic mirror, and a BA510-550 emission filter (Olympus). Live images were obtained using AquaCosmos 2.0 software (Hamamatsu Photonics, Hamamatsu, Japan) controlling a digital charge-coupled device camera (C4742-95-12ER; Hamamatsu Photonics). Fluorescence was observed on the cell surface of yeast cells harboring pULD1-cyspin 1, pULD1-cyspin 2, and pULD1-cyspin 3, while pULD1-s-harboring cells (8), which displayed only a Strep-tag epitope, did not fluoresce (Fig. 1b).
Table 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| cyspin 1, 2-F | GAAGATCTATGTTAAAGAAAAAAAGAACATTTATTAGC (BglII) |
| cyspin 1-R | CCGCTCGAGCCTTAAAAGAGCCATTTTTAATAAGTTAAC (XhoI) |
| cyspin 2-R | CCGCTCGAGAGTTAAAAGAACCTTTCTTAATAATGCAAC (XhoI) |
| cyspin 3-F | GAAGATCTATGTTAAGGAAGAAAAAGAAATTTATCAG (BglII) |
| cyspin 3-R | CCGCTCGAGAGCTAAAAGAGCCTTTTTTAATAATGCAG (XhoI) |
Underlines indicate the restriction sites of the enzymes shown in parentheses.
Fig. 1.
Constructed plasmids and immunofluorescence staining of cyspin-displaying yeast cells. (a) Plasmid map of the pULD1-cyspin 1, 2, and 3 plasmids for display of cyspins on the yeast cell surface. Ampr, ampicillin resistance gene for bacterial selection; 2μm, replication origin of yeast; ori, replication origin of Escherichia coli; URA3, uracil marker gene; leu2-d, leucine marker gene; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GA s.s., glucoamylase secretion signal. (b) Immunofluorescence staining for cell surface display of cyspins with mouse monoclonal anti-FLAG M2 antibody as the primary antibody and Alexa Fluor 488 goat anti-mouse IgG as the secondary antibody. Scale bar, 5 μm.
To examine the property of each cyspin, their inhibitory activities were measured against several representative plant proteases, including papain, bromelain, and ficin. Cyspin-displaying yeasts were precultivated in SDC-HLM (0.67% [wt/vol] yeast nitrogen base without amino acids, 2% glucose, 2% Casamino Acids, 0.002% l-histidine-HCl, 0.003% l-methionine, 0.003% l-leucine) for 24 h, transferred into fresh medium to obtain an optical density at 600 nm (OD600) of 0.1, and cultivated for 24 h. After centrifugation at 3,000 × g, yeast cells were washed with PBS (pH 7.4) or 20 mM Tris-HCl (pH 7.8) and diluted to an OD600 of 40 with the same buffer. Similar display efficiencies were observed for cyspins 1, 2, and 3 by measuring the fluorescent intensity of cells after immunofluorescence labeling (data not shown). Fifty microliters of the yeast suspension was then added to a solution containing 130 μl of PBS (pH 7.4) or 20 mM Tris-HCl (pH 7.8) and 10 μl of protease (1 mg/ml papain [Wako Pure Chemical Industries, Ltd., Osaka, Japan] and ficin [Sigma-Aldrich] in PBS [pH 7.4] or bromelain [Wako Pure Chemical Industries] in Tris-HCl [pH 7.8]), and the solution was stirred gently for 10 min. Subsequently, 10 μl of benzyloxycarbonyl-l-phenylalanyl-l-arginine-7-4-methyl coumarylamide (Z-Phe-Arg-MCA) (100 μM; saturated condition) (Peptide Institute, Osaka, Japan) was added, and fluorescence was immediately measured with a Fluoroskan Ascent fluorometer (Labsystems OY, Helsinki, Finland) using a 96-well tissue culture plate (353072; Becton Dickinson Labware, NJ). A filter pair with excitation and emission at 355 nm and 460 nm, respectively, was used to detect the fluorescence of 7-amino-4-methyl-coumarin (AMC) liberated from Z-Phe-Arg-MCA. The relative protease activity was calculated by the following equation: relative protease activity = fluorescence intensity of the reaction solution by cyspin-displaying yeast per cell/fluorescence intensity by control yeast per cell.
Proteolytic enzymes in plants are involved in almost all aspects of growth and development, including germination, circadian rhythms, senescence, and programmed cell death (2). One function of a protease is to protect plant cells against outside attack. Proteases also appear to play key roles in regulating biological processes in plants, such as recognition of pathogens and pests and induction of effective defense systems. For example, papain, found in the latex of papaya trees, is excreted from wounds and contributes to defense response.
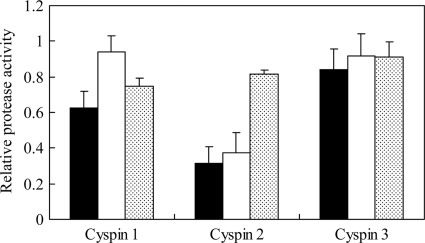
Cyspin 1 inhibited papain and bromelain, and cyspin 2 inhibited papain and ficin. In contrast, cyspin 3 inhibited all proteases, but its activity was lower than that of cyspins 1 and 2 (Fig. 2). These results indicate that these inhibitors exhibited cysteine-based activity against plant proteases, and of these, cyspins 1 and 2 were uniquely specific. This is the first report demonstrating the true inhibitory activity of cellulosomal protease/peptidase inhibitors against plant proteases. Several articles have proposed that cellulosomal protease/peptidase inhibitors could protect their respective microbes and cellulosomes from external proteases (3, 11). Our results with the inhibitory activity of cyspins actually demonstrated this hypothesis by measuring their inhibitory activity.
Fig. 2.
Inhibition activity of cyspin-displaying yeast cells against papain, ficin, and bromelain. Closed, open, and stippled bars indicate the remaining protease activity rates against papain, ficin, and bromelain, respectively. Z-Phe-Arg-MCA was used as the substrate. A value of 1 for relative protease activity (in relative fluorescence units [RFU]/min) means that there was no inhibitory activity. The initial rates of activity against papain, ficin, and bromelain were 1.80, 0.715, and 0.739 RFU/min. Each data set represents averages and standard deviations from three independent experiments.
Genome analysis of C. cellulovorans suggested that the cellulosome included three kinds of protease/peptidase inhibitors (15). The actual presence of protease/peptidase inhibitors in C. thermocellum and C. cellulolyticum, in which their presence was previously predicted, has been suggested only by transcriptional and proteomic analysis (3, 6). Our study first demonstrated that protease/peptidase inhibitors can exhibit their activity in cellulosomes from C. cellulovorans and may play a vital role.
Furthermore, cyspins 1 and 2 were regulated by the same promoter on the basis of genome sequencing results, suggesting the possibility of simultaneous expression. Analysis of amino acid homology indicated that three cyspins have two conserved chagasin family peptidase inhibitor I42 domains, initially found in Trypanosoma cruzi (10). The first chagasin domain showed a high degree of amino acid homology (76 to 77%) among the three cyspins. Despite little difference in the first chagasin domain among the three cyspins, cyspin 3 had wide substrate specificity and low activity, unlike cyspins 1 and 2. Therefore, the second chagasin domain could be important for the inhibitory activity specific to cyspins 1 and 2. In fact, the second chagasin domain of cyspin 3 had 40% and 46% amino acid homologies with that of cyspins 1 and 2, respectively, although there was 57% amino acid homology between cyspins 1 and 2, suggesting a difference in the activity between cyspins 1 and 2 and cyspin 3. There is the possibility that cyspin 3 might specifically inhibit other types of proteases and play a role similar to cyspins 1 and 2.
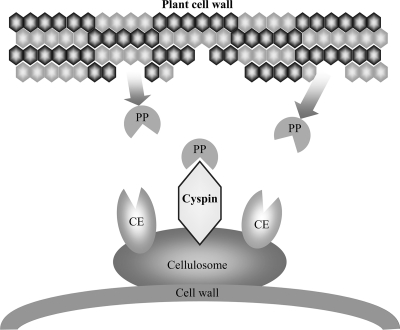
In latex, some plant cysteine proteases show strong toxicity and growth inhibition against insects (7). In addition, cysteine proteases are involved in the defense response in Arabidopsis thaliana (19). These studies indicate that plant cysteine proteases could play an important role in self-defense. C. cellulovorans was isolated from the wood chip of the poplar tree (12), whose protease gene family is similar to that of A. thaliana (4). Therefore, the poplar could have the same protection system, using proteases for self-defense. Considering the defense systems in plants and cyspin function, it is possible that cyspins in cellulosomes could contribute to the protection of C. cellulovorans against attacks by plant proteases (Fig. 3).
Fig. 3.
Putative role of cellulosomal cyspins in C. cellulovorans. Cellulosomal protease/peptidase inhibitors protect their microbe and cellulosome. PP, plant proteases; CE, various cellulosomal enzymes.
In conclusion, we demonstrated, for the first time, the true function of cellulosomal protease/peptidase inhibitors against plant cysteine proteases using three kinds of cyspin-displaying yeast cells. Our study suggests that cellulosomal inhibitors might protect their microbe and cellulosome from plant protease attack.
Footnotes
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Doi R. H., Tamaru Y. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24–32 [DOI] [PubMed] [Google Scholar]
- 2. Domsalla A., Melzig M. F. 2008. Occurrence and properties of proteases in plant latices. Planta Med. 74:699–711 [DOI] [PubMed] [Google Scholar]
- 3. Fendri I., et al. 2009. The cellulosomes from Clostridium cellulolyticum: identification of new components and synergies between complexes. FEBS J. 276:3076–3086 [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Lorenzo M., Sjodin A., Jansson S., Funk C. 2006. Protease gene families in Populus and Arabidopsis. BMC Plant Biol. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ito H., Fukuda Y., Murata K., Kimura A. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang S., Barak Y., Lamed R., Bayer E. A., Morrison M. 2006. The functional repertoire of prokaryote cellulosomes includes the serpin superfamily of serine proteinase inhibitors. Mol. Microbiol. 60:1344–1354 [DOI] [PubMed] [Google Scholar]
- 7. Konno K., et al. 2004. Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J. 37:370–378 [DOI] [PubMed] [Google Scholar]
- 8. Kuroda K., et al. 2009. Enhancement of display efficiency in yeast display system by vector engineering and gene disruption. Appl. Microbiol. Biotechnol. 82:713–719 [DOI] [PubMed] [Google Scholar]
- 9. Murashima K., Kosugi A., Doi R. H. 2002. Synergistic effects on crystalline cellulose degradation between cellulosomal cellulases from Clostridium cellulovorans. J. Bacteriol. 184:5088–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rigden D. J., Monteiro A. C., Grossi de Sa M. F. 2001. The protease inhibitor chagasin of Trypanosoma cruzi adopts an immunoglobulin-type fold and may have arisen by horizontal gene transfer. FEBS Lett. 504:41–44 [DOI] [PubMed] [Google Scholar]
- 11. Schwarz W. H., Zverlov V. V. 2006. Protease inhibitors in bacteria: an emerging concept for the regulation of bacterial protein complexes? Mol. Microbiol. 60:1323–1326 [DOI] [PubMed] [Google Scholar]
- 12. Sleat R., Mah R. A., Robinson R. 1984. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steenbakkers P. J., et al. 2008. A serpin in the cellulosome of the anaerobic fungus Piromyces sp. strain E2. Mycol. Res. 112:999–1006 [DOI] [PubMed] [Google Scholar]
- 14. Tamaru Y., et al. 2010. Genome sequence of the cellulosome-producing mesophilic organism Clostridium cellulovorans 743B. J. Bacteriol. 192:901–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamaru Y., Miyake H., Kuroda K., Ueda M., Doi R. H. 2010. Comparative genomics of the mesophilic cellulosome-producing Clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Environ. Technol. 31:889–903 [DOI] [PubMed] [Google Scholar]
- 16. Tamaru Y., et al. 2002. Formation of protoplasts from cultured tobacco cells and Arabidopsis thaliana by the action of cellulosomes and pectate lyase from Clostridium cellulovorans. Appl. Environ. Microbiol. 68:2614–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueda M., Tanaka A. 2000. Cell surface engineering of yeast: construction of arming yeast with biocatalyst. J. Biosci. Bioeng. 90:125–136 [PubMed] [Google Scholar]
- 18. Ueda M., Tanaka A. 2000. Genetic immobilization of proteins on the yeast cell surface. Biotechnol. Adv. 18:121–140 [DOI] [PubMed] [Google Scholar]
- 19. van der Hoorn R. A., Jones J. D. 2004. The plant proteolytic machinery and its role in defence. Curr. Opin. Plant Biol. 7:400–407 [DOI] [PubMed] [Google Scholar]
- 20. Zverlov V. V., Kellermann J., Schwarz W. H. 2005. Functional subgenomics of Clostridium thermocellum cellulosomal genes: identification of the major catalytic components in the extracellular complex and detection of three new enzymes. Proteomics 5:3646–3653 [DOI] [PubMed] [Google Scholar]