Abstract
HIV-1 often evades cytotoxic T cell (CTL) responses by generating variants that are not recognized by CTLs. We used single-genome amplification and sequencing of complete HIV genomes to identify longitudinal changes in the transmitted/founder virus from the establishment of infection to the viral set point at 1 year after the infection. We found that the rate of viral escape from CTL responses in a given patient decreases dramatically from acute infection to the viral set point. Using a novel mathematical model that tracks the dynamics of viral escape at multiple epitopes, we show that a number of factors could potentially contribute to a slower escape in the chronic phase of infection, such as a decreased magnitude of epitope-specific CTL responses, an increased fitness cost of escape mutations, or an increased diversity of the CTL response. In the model, an increase in the number of epitope-specific CTL responses can reduce the rate of viral escape from a given epitope-specific CTL response, particularly if CD8+ T cells compete for killing of infected cells or control virus replication nonlytically. Our mathematical framework of viral escape from multiple CTL responses can be used to predict the breadth and magnitude of HIV-specific CTL responses that need to be induced by vaccination to reduce (or even prevent) viral escape following HIV infection.
INTRODUCTION
A hallmark of human immunodeficiency virus (HIV) infection of humans is the generation of viral variants that are not recognized by virus-specific cytotoxic T lymphocyte (CTL) responses (42, 51). Such variants result from point mutations in or around epitopes presented by host major histocompatibility complex (MHC) class I molecules (3, 28, 42, 86). The impact of escape from the CTL response on disease progression in HIV infection is not well established and may be epitope specific (for a critical overview, see reference 8). Several studies have documented an increase in the viral load and disease progression following escape from a CTL response (20, 40, 43, 54). However, CTL escape in other cases has no apparent effect on disease progression (46, 56). Escape from CTL responses has been considered one reason for the failure of T cell-based HIV vaccine trials in animals (14–16, 21, 26, 49). However, failure of a vaccine is not always due to viral escape (69) and may arise due to other factors, including induction of T cell responses of insufficient magnitude or with poor or inappropriate effector functions. Indeed, in the STEP trial, very few CTL responses were elicited, and these responses had limited ability to cross-react with circulating strains (17, 24). In a macaque simian immunodeficiency virus (SIV) protection model, the breadth of CTL responses was highly correlated with control of viremia and protection (59), and a profound CD8+ T cell- mediated benefit of a vaccine in a heterologous setting has been noted with live attenuated virus, which elicited multiple responses to different SIV proteins (90). Escape from CTL responses also occurs in nonpathogenic SIV infection of nonhuman primates, such as sooty mangabeys, which generally do not progress to develop disease (48). It has also been suggested that detrimental effects of some CTL escapes may be offset by benefits gained when escape leads to a reduction in the replicative fitness of the escape variant (25, 33); it is certainly plausible that escape from different epitopes may have different impacts on the viral load and that the same mutations may have different effects in the context of different host immune systems.
Mathematical models have been used previously to understand the importance, timing, and kinetics of CTL escape in HIV/SIV infection (6, 9, 30, 35, 62, 64, 65, 75, 77, 79, 83). Nowak et al. (79) proposed a model based on the escape of HIV from the immune response to explain disease progression of HIV-infected individuals. More recently, Fernandez et al. (30) derived a simple mathematical model for simian-human immunodeficiency virus (SHIV) escape from the CTL response and for the first time estimated the rate at which such CTL escapes accumulate in the virus population. Later studies have shown the importance of taking into account changes in the virus replication rate and CTL response in determining the rate of viral escape over the course of HIV/SIV infection (35, 64, 65, 83). In another important study, Asquith et al. (9), by analyzing a large number of CTL escapes in HIV-infected patients, concluded that the CTL response specific for a single epitope of HIV is not very efficient at killing virus-infected cells. They estimated that, on average, a single CTL response kills HIV-infected cells at the rate of 0.01 per day (9), which is about 1 to 2% of the death rate of cells productively infected with HIV (82). Interestingly, this study also found that the rate at which virus escaped from the CTL response was significantly higher during the early phase than in the chronic phase of HIV infection (9). A similar observation for several viral epitopes has also been made during SIV/SHIV infection of nonhuman primates (4, 10, 42, 64).
Many of these previous studies employed cross-sectional data, where escape in a single epitope was followed in a given patient or animal and comparisons of CTL escapes were done between different individuals. It is unclear whether the same conclusions about timing and kinetics of different CTL escapes hold within a given HIV-infected individual where the virus escapes from many CTL responses over the course of infection (70). Some earlier studies did involve analysis of viral escape from several CTL responses over the course of infection (47, 80), but the rates of viral escape were not quantified. In this work, we used data from our recently published study in which we followed several individuals from the very early stages of acute HIV infection to the viral set point (41). Using single-genome amplification and sequencing (SGA/S) (50, 52), we were able to predict the viral sequences that founded the infection and map the CD8+ T cell response to the founder virus. Analysis of the sequence data revealed that there is a rapid escape of HIV from several CTL responses occurring during the acute phase of the infection. In the chronic phase, however, the rate of viral escape was significantly reduced. Comparing the data on viral escape with the dynamics of CTL responses, we conclude that the low rate of escape of the virus from the CTL response in the chronic phase of the infection is unlikely to be determined by a single factor. Higher fitness costs of late escapes and the increasing breadth of the CD8+ T cell response over time are potential causes of the slow accumulation of late escapes.
MATERIALS AND METHODS
Basic mathematical model of viral escape from a single CTL response.
A model for the dynamics of escape of the virus from a single CTL response has been described in detail previously (9, 30, 35; see also the supplemental material). In brief, we assume that the founder (wild-type) virus replicates at rate r and cells infected with the virus are killed by the CTL response at rate k. The killing efficacy of the CTL response, k, is a parameter composed of two quantities: the per-cell killing efficacy of CTLs and the number of epitope-specific CTLs. Thus, k may change with time during the infection. The CTL escape mutant is assumed to have a lower replication rate, (1 − c)r (due to fitness cost c), and cells infected with the mutant virus are not killed by the epitope-specific CTL response. If the escape variant has a higher replication rate than the wild type, then c will be negative. When both viral variants (the wild type, w, and the escape mutant, m) are present in an infected host, their dynamics are described by the following equations (see also the supplemental material):
| (1) |
| (2) |
where w and m are the number of cells infected with the wild-type and CTL mutant viruses, respectively, c is the cost of the escape mutation, defined as a selection coefficient, and δ is the death rate of productively infected cells due to viral pathogenicity. Since both SIV and HIV virions are short-lived in vivo (82, 88, 99), densities of virus are likely to be proportional to the densities of cells productively infected with each virus variant given in equations 1 and 2. In this model, we assume that the CTL response controls growth of the virus by killing virus-infected cells (see equation 1). We also investigated the case where the CTL response controls viral growth by inhibiting viral replication through the secretion of soluble factors following MHC-dependent recognition of virus-infected cells (see below; see also the supplemental material).
It can be shown that the ratio of mutant to wild-type density, z = m/w, in this model changes exponentially over time (e.g., see reference 35):
| (3) |
where z(t) and z0 are the ratio of the frequency of the escape mutant to the frequency of the wild-type virus in the population at some time, t, and at the time when the epitope-specific CTL response appears, which we arbitrarily call t = 0, respectively, while
where 〈k − cr〉 is the time average value of k − cr if these parameters vary in time, for example, due to a change in the magnitude of the epitope-specific CTL response, k, or due to deletion of target cells, which would affect the viral growth rate, r (35, 83). We call ε the escape rate since it determines how fast the escape mutant takes over the population. The frequency of the mutant virus in the viral population is given by f = z/(1 + z), and changes in the frequency over time are given by
| (4) |
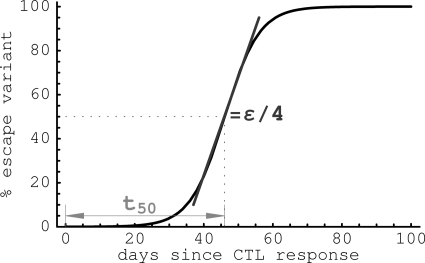
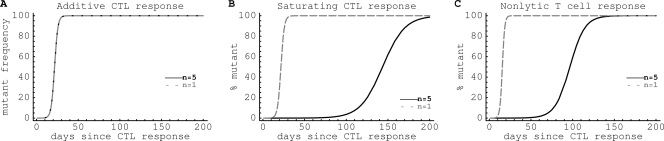
where f0 is the frequency of the escape mutant in the population at time t = 0. This equation is similar to one proposed in previous studies (9, 30, 35). Note that equations 3 and 4 also hold if CD8+ T cells do not kill infected cells but instead reduce the rate of virus replication. The time at which the escape variant reaches a frequency of 50% in the population is calculated as (Fig. 1). Similarly, is the time needed for the mutant to reach a frequency of 10%.
Fig. 1.
A cartoon of viral escape from a single CTL response and parameters calculated to characterize the escape. The slope of the change of the frequency of the escape variant at frequency f = 50% is proportional to the viral escape rate ε, and the time by which the variant reaches 50% in the viral population (t50) is determined by the initial frequency of the viral variant f0 and the rate of viral escape ε.
Mathematical model for multiple escapes.
The model given by equations 1 and 2 tracks changes in the density of the wild-type (founder) virus and a single variant that has escaped recognition from the epitope-specific CTL response. In acute HIV infection, the virus escapes from multiple CTL responses, specific to several viral epitopes (41, 93). To track the dynamics of viral escape from multiple responses, we extended equations 1 and 2. We assume that there are in total n CTL responses that control viral growth and, potentially, the virus can escape from all n responses. A CTL response that recognizes the ith epitope of the virus kills the virus-infected cells at rate ki, and escaping from the ith CTL response leads to viral replicative fitness cost ci. Assuming that all viral variants are present in the population initially, the dynamics of the wild type and the escape variants (see also the supplemental material) are given by
| (5) |
| (6) |
where w = m0, is the density of cells infected with the wild-type virus (which has not accumulated any escape mutations), mi is the density of cells infected with an escape variant denoted by a vector i = (i1, i2, …, in), with ij = 0 if there is no mutation in the jth CTL epitope and ij = 1 if there is a mutation leading to escape from the jth CTL response. The death rate of an escape variant due to remaining CTL responses is then simply . It should be noted that here we assume killing of infected cells by different CTL responses to be additive. Analysis of in vitro killing of targets suggests that the death rate of targets is a linearly declining function of the number of CTLs bound to a target, suggesting additive killing (81). Furthermore, recent analysis suggests that killing of peptide-pulsed target cells in vivo in mice may also be additive (37). Interestingly, most current models of virus and CTL dynamics assume additive killing, e.g., see references 6, 9, and 35). We also analyze models for viral escape when the death rate of infected cells is determined by the maximal CTL response or when CTLs control viral growth by reducing the rate of virus replication (see the supplemental material).
Escape from a given CTL response incurs a fitness cost to the virus. Assuming multiplicative fitness, the fitness cost of a variant i is as follows: .
Given that infection starts with the founder virus (41), we need to describe the generation of the escape variants from the wild type. To simplify the model, we assume that all viral variants are present at the start of the CTL response. The initial density of a variant that has escaped from j different CTL responses is μj, where μ = 2 × 10−5, the base substitution rate of HIV (66). Thus, the initial density of the founder virus is set to m0(0) = μ0 = 1. A variant of the model that includes the generation of escape mutants by mutation produces quantitatively similar dynamics (results not shown). The model equations 5 and 6 could also be modified to include limitation of viral growth by depletion/recovery of target cells or by explicitly modeling the dynamics of CTL responses (e.g., see equation S1 in the supplemental material).
To explicitly model the dynamics of the CTL response to HIV, we assume that prior to infection CD8+T cells are at the steady state determined by the ratio s/δE, where s is the rate of CD8+ T cell production from the thymus and δE is the CD8+ T cell death rate. Following HIV infection, CD8+ T cells differentiate into CTLs and start proliferating at a rate that depends on the density of cells infected with viral variants expressing CTL epitopes and die at a constant death rate, δE. Only viruses with unmutated epitopes support proliferation of a given CTL clone. The dynamics generated by this model are given by
| (7) |
where Ei denotes a CTL clone specific for epitope i, which proliferates with the maximum rate ρi, hEi is a constant, and αij is 1 if the ith epitope is not mutated in the variant j and 0 if it is mutated. CTLs, Ei, kill cells infected with the viral variant j at rate (see the supplemental material for derivation)
| (8) |
In this model, the death rate of infected cells expressing a particular set of viral epitopes is a saturating function of the density of CTLs recognizing these epitopes.
Nonlytic CD8+ T cell response.
CD8+ T cells can control viral growth by killing infected cells (19). Also, CD8+ T cells can affect virus replication by secreting antiviral factors, which in turn may reduce production of virus particles by infected cells (45, 57). Chemokines, produced by activated CD8+ T cells, can also prevent infection of uninfected cells by free virus (23, 87, 95). Clearly, production of antiviral factors, cytokines, and chemokines may lead to viral escape only if the production is MHC dependent. Since the relative contribution of killing of infected cells and nonlytic suppression of viral replication by CD8+ T cells during HIV infection is unknown (but see references 53, 73, and 98), we sought to determine whether our conclusions hold if we assume that CD8+ T cells control viral growth via nonlytic mechanisms.
Following previous work (35), we assume that the dynamics of the wild-type virus, w, and the single escape variant, m, is given by
| (9) |
| (10) |
where k and k′ represent the suppressive pressure exerted by the CD8+ T cell response from which the virus can escape and the pressure mediated by other CD8+ T cell responses, respectively. The model can be extended to include escape from multiple T cell responses. The average rate of escape of the virus from a single CD8+ T cell response is given by
| (11) |
and is now dependent on the immune pressure mediated by the total HIV-specific CD8+ T cell response, k + k′, and directly proportional to the rate of virus replication, r.
Statistics.
Equation 4 was used to estimate the average rate of escape, ε, and the initial frequency of the escape variant in the population, f0, using methods of nonlinear least-squares regression (18). In several cases of viral escape, the frequency of the escape variant in the population changed from 0 to 1 between successive measurements, precluding precise estimation of the average escape rate. To estimate the minimal escape rate, we replaced the observed null frequency of the escape variant with 1/(N + 1), and when the frequency was 1, we replaced it with N/(N + 1), where N is the number of sequenced viral genomes at these time points. This methodology underestimates the true rate of viral escape (9, 35). To calculate 95% confidence intervals (CIs) for the estimated rates of escape, we recreated data on escape, assuming the number of variants observed was a sample from a binomial distribution (29). More specifically, for a given time point where m mutants out of a total of N sequences were detected, we generated a sample frequency of the mutant as BN(m/N)/N, where BN(p) is the binomial distribution for N trials with success rate per trial p = m/N. We recreated the data using this algorithm for each escape variant and all time points 1,000 times.
RESULTS
Rate of viral escape declines over the course of infection.
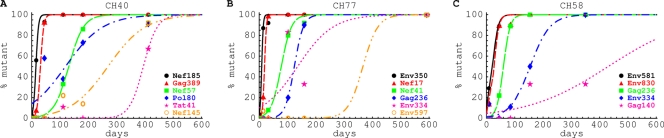
In our earlier study, three patients (CH40, CH77, and CH58) were diagnosed with acute HIV infection, and the dynamics of the HIV-specific CTL response and the virus evolution were followed longitudinally (41) (see Fig. S5 in the supplemental material). Infection in all three patients was established by a single virus. After the peak in viral load, the virus accumulated mutations that became fixed in the population. Many of these mutations were in CTL epitopes and thus appeared to be selected by CTL responses that arose around the time of peak viremia (see Fig. S5). Using a mathematical model (equation 4 in Materials and Methods), we estimated the rate of escape, ε, of HIV from CTL responses for every variant observed in these three patients, assuming that these escapes occur independently (Fig. 2; see also Fig. S6 to S8 in the supplemental material). This assumption of independent escapes is in part justified, because in our data the full genome is reconstructed from two independent halves, precluding linked analysis of escapes occurring on different parts of the genome. In addition, recombination, which can be frequent in HIV, will also destroy linkage relations (76). It should be noted that for some epitopes there is large variability in the percentage of mutant sequences in the viral population and this is poorly described by the mathematical model (e.g., Nef145 and Pol80 in Fig. 2A and Gag140 in Fig. 2C). Such variability can be explained by a limited sampling of viral genomes (around 5 to 10 at some time points), and this translates into relatively large confidence intervals in estimated escape rates for these epitopes (see Tables S1 to S3 in the supplemental material).
Fig. 2.
The dynamics of viral escape from epitope-specific CTL responses in the acute and chronic phases of HIV infection. Points represent the percentages of given escape variants at different times after the onset of symptoms and lines represent the best fit of the model given in equation 4 to these data. We show only a selected set of data and model fits for clarity; a complete analysis for these three patients is given in Fig. S6 to S8, and the estimates of escape rates with calculated confidence intervals are given in Tables S1 to S3, in the supplemental material. Each mutation is labeled according to the peptide corresponding to the sequence in which the mutation occurred (41).
According to the mathematical model, the estimated rate of escape, ε, in a particular epitope is given by the equation
| (12) |
and is determined by the average difference between the rate of killing mediated by the epitope-specific CD8+ T cell response, k, and the cost of escape, c, multiplied by the replication rate of the wild-type virus, r (9, 30, 35). Higher rates of escape imply stronger immune pressure (higher k) and a low fitness cost (low c), and slow escape implies low immune pressure (low k) or a high fitness cost (high c). For several escape variants, especially in cases when escape was very rapid, we can only estimate a minimal escape rate (i.e., minimal selective advantage) since there were no two consecutive data points where both the wild type and the escape mutant were present (see Fig. S6 to S8 and Tables S1 to S3 in the supplemental material). This implies that at these early time points, the actual rate at which escape occurred could be even higher than we estimate. (This indeed was found using 454 sequencing [32].) Within 10 to 50 days after the onset of symptoms (symptoms on average occur 2 to 3 weeks after the infection [38, 39]), we found that HIV escapes from a single CTL response with a mean rate of 0.24 day−1 (median = 0.22 day−1 [41]).
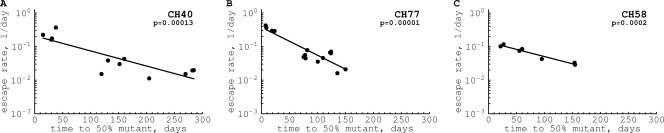
Our new observation, which is the focus of this article, is that the rate of viral escape declined with time after infection. At 1 year postinfection, the escape rate was >10-fold lower than that in the acute phase (Fig. 3). A similar observation was made earlier using cross-sectional data for humans and macaques (9, 10, 64).
Fig. 3.
Distribution of escape rates as a function of time since the onset of symptoms. For each escape variant, we calculate t50, the time at which the escape mutant is predicted to reach a frequency of 50% in the virus population (see Fig. 1 in Materials and Methods). The decline of the escape rate with time was highly significant in every patient (P values are indicated on the panels).
Contribution of CTL killing, fitness cost, and viral replication rate to determination of the rate of viral escape.
It is not clear why early escapes occur at higher rates than late escapes. In the model with a single founder virus and a single escape variant (see equations 1 and 2), the rate of viral escape from a single CTL response is determined by three parameters: k, the killing rate mediated by the CTL response against the wild-type epitope; c, the fitness cost of escape; and r, the rate of wild-type virus replication (35) (equation (12). Therefore, a lower escape rate later in infection could be because the killing mediated by the CTL response is lower, the cost of viral escape is higher, and/or the rate of virus replication is higher than that during the acute phase of the infection.
A decline in the magnitude of HIV-specific CTL responses over the course of acute HIV infection has been observed at least for some epitopes (41, 93) and is expected from the general pattern of T cell responses contracting after their peak during both acute and some chronic viral infections (2, 7, 16, 97). However, CTL responses specific to some viral epitopes increase in chronic HIV infection (or appear only in the chronic phase of the infection [5, 41, 93]), and therefore, at least for some epitopes, we might expect a higher rate of escape in the chronic phase of infection. For example, in patient CH77, response to the Nef17 epitope declined and the response to epitope Gag236 increased over the course of infection (see Fig. S5 in the supplemental material). To investigate this further, we developed a mathematical model that tracks the dynamics of different viral variants that escape from multiple CTL responses. The model predicts the dynamics of viral variants escaping from n different CTL responses depending on the killing efficacy of CTL responses, fitness costs associated with escape, and viral replication rate (see Materials and Methods).
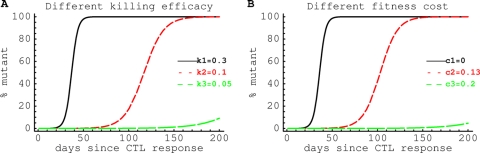
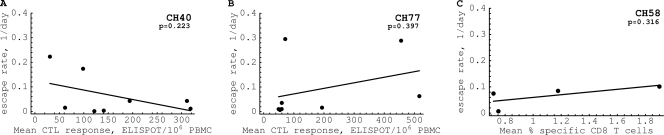
Simulations of viral escape in this model for a range of parameters suggest that even if there is no change in the magnitude of the CTL response over time, we still expect to see escapes that occur early during the infection accumulate at higher rates than those escapes that occur late in the infection (Fig. 4A). The reason for this is that if everything else is equal, the virus will first escape from the strongest CTL response (at the highest rate), and the last escape will be from the weakest CTL response (at the lowest rate). Interestingly, in our patient data we found no significant correlation between the magnitude of the epitope-specific CD8+ T cell response (which is expected to be correlated with killing rate k) and the rate of viral escape (Fig. 5). This suggests that the decreased rate of viral escape in the chronic phase of HIV infection is not due primarily to a lower magnitude of the HIV-specific CTL response.
Fig. 4.
Killing mediated by HIV-specific CTL responses and the cost of escape determine the sequence and the speed of CTL escapes during infection. We simulated the dynamics of HIV escape from multiple CTL responses using equations 5 and 6 (see Materials and Methods). On the plots, we show the changes in the percentages of variants that have escaped recognition of the first (solid line), second (small dashed line), and third (large dashed line) CTL responses over time. The model demonstrates that if there are three CTL responses from which the virus can escape, with everything else being equal, the first escape occurs from the strongest immune response (k1 = 0.3 day−1), and the last escape is from the weakest CTL response (k3 = 0.05 day−1) (A). Similarly, if the CTL-mediated pressure is similar for all epitopes, the first escape to occur is in the epitope that induces the lowest fitness cost (c1 = 0), and late escapes are those that incur the highest fitness cost (c3 = 0.20) (B). In both cases, early escapes occur at a higher rate than late escapes (A and B). In panel A, c1 = c2 = c3 = 0.005; k1 = 0.3 day−1, k2 = 0.1 day−1, and k3 = 0.05 day−1. In panel B, k1 = k2 = k3 = 0.3 day−1; c1 = 0, c2 = 0.13, and c3 = 0.20. Other parameters for both panels are as follows: r = 1.5 day−1; δ = 0.5 day−1. Mutants are assumed to be present when selection begins at frequencies μj, where μ = 2 × 10−5 and j is the number of mutated epitopes (e.g., m0,0,0 = μ0 = 1). As predicted by a simple model, the rate of escape from the ith CTL response is given by the difference ki − cir (see equation 3). Results were quantitatively similar if we explicitly included the dynamics of target cells in the model or modeled generation of escape variants from the founder virus by mutations (results not shown).
Fig. 5.
The absence of a significant correlation between the rate of HIV escape from a given epitope-specific CTL response and the average magnitude of the response in three patients, CH40 (A), CH77 (B), and CH58 (C). It is expected that CTL responses of a larger magnitude should select for more-rapid escapes. This is not observed for several measures of the CTL response, such as the mean response (shown in the figure), where the mean is calculated over the whole observation period, the maximal response (maximal value of the response over the whole observation period), or the total response (sum of all measurements of the epitope-specific response; results not shown). Furthermore, no significant positive correlation was observed between the rate of escape and the average CTL response prior to viral escape. The lack of a correlation could have arisen because only a limited number of responses and patients have been analyzed.
Several studies have established that escape from some CTL responses incurs a replication (fitness) cost to HIV when measured in vitro (28, 56, 68, 85, 86, 92), although recent analysis of in vivo data suggests only a minimal cost of escape during HIV infection (9). The model of the dynamics of multiple escape variants (equations 5 and 6 in Materials and Methods) suggests that when CTL pressure on several viral epitopes is similar, the virus will first escape at positions that confer the lowest fitness cost, and the last escapes will be those at positions incurring the highest possible fitness cost (Fig. 4B). Further, early escape will, according to equation 12, translate into a faster escape, leading to a negative correlation between the rate of escape and the time of escape.
Finally, since the escape rate in our model is given by ε = 〈k − cr〉, the rate of virus replication can also influence the rate of viral escape from the T cell response (35). Based on previous studies, it is expected that the rate of virus replication is high during the prepeak expansion phase (r ≈ 2 day−1) (91), is low during the postpeak virus decline (r < 1 day−1) (32, 58), and is average during the chronic phase (r ≈ 1 day−1) (67, 82). Two pieces of evidence suggest that during the decline of viremia after the peak, virus replication is limited. First, treatment of patients during the decline of viremia in acute infection does not speed up viral clearance (58). Second, the total density of viral variants, measured in HIV RNA copies per ml, escaping from the CTL response during viral decline accumulates at a relatively low rate (32). Because in our patients first escapes occur after peak viremia, the rate of virus replication is likely to be increasing after the peak of viremia into the chronic phase. An increase in the rate of virus replication is also expected due to potential recovery of target CD4+ T cells following viral control. As equation 12 indicates, if there is a fitness cost of viral escape (c > 0), higher rates of wild-type virus replication will lead to a lower rate of escape (35). The latter point is counterintuitive since more-rapid virus replication is expected to drive more viral mutations. Indeed, more rounds of virus replication will lead to the generation of higher numbers of escape variants. However, in order to be selected, the unmutated wild-type virus has to be killed at a sufficient rate so that escape variants rise in frequency. Due to the fitness cost of escape mutations, higher rates of virus replication will allow the wild type to preferentially reproduce and thus to offset the selective advantage of escape variants. The change in the rate of virus replication therefore is also expected to contribute to the slowing of CTL escape in the chronic phase of HIV infection.
Changes in the breadth and/or magnitude of the HIV-specific CTL response.
The dynamics of HIV-specific CTL responses over the time of infection are complex. In general, responses to only a few viral epitopes are detected in the acute phase of HIV infection (93). Over time, the breadth of the CTL response increases as more responses become detectable while some responses decline, possibly due to viral escape (41, 93). A recent study found a significant increase in the number of detectable CTL responses in a given patient over the course of HIV infection (93). Therefore, we investigated whether the increase in the number of HIV-specific CTL responses over the time of infection could be the principal cause of a lower rate of viral escape from CTLs in the late phase of the infection (Fig. 3).
An increase in the number of novel HIV-specific CTL responses can affect the existing CTL responses in two ways. First, a rise in novel responses may have no effect on already-existing HIV-specific CTL responses, as one may expect if there is no competition between CTL responses specific to different HIV epitopes. A recent study supports a lack of competition between CTL responses of different specificities (34). In the absence of competition, the model predicts that increasing the diversity of HIV-specific CTL responses (number of epitopes recognized) does not affect the rate of viral escape from a given CTL response (Fig. 6A). Having more responses does not affect the rate of escape from a given response, which is determined by the difference k − cr (see equation 12). Importantly, in this model there is a correlation between the magnitude of the CTL response when k is proportional to the CTL magnitude (see equations S1 to S5 in the supplemental material) and the rate of viral escape which is not observed in our data (Fig. 5).
Fig. 6.
Stronger and more diverse CD8+ T cell responses can slow down the appearance of escape variants in the case of a saturating CTL or nonlytic CD8+ T cell response. We model escape of the virus from the T cell response using the model shown in Materials and Methods (equations 5 and 6), assuming that CTL responses are additive (A) [k = 0.5 day−1 and k = (0.5, 0.2, 0.1, 0.05, 0.01) day−1 for n = 1 and n = 5, respectively], CTL responses are saturating (B) k = 0.5 day−1 and k = (0.08, 0.12, 0.12, 0.12, 0.05) day−1 for n = 1 and n = 5, respectively; the death rate of infected cells due to CTL response is 0.5 day−1], or CD8+ T cells control viral replication nonlytically (C) [k = 0.3 day−1 and k = (0.3, 0.5, 0.5, 0.5, 0.19) day−1 for n = 1 and n = 5, respectively; see also equations 9 and 10]. Other parameters are as follows: c = (0, 1, 1, 1, 1) (to allow for escape only in the first epitope); r = 3 day−1; and δ = 0.5 day−1. In all panels, escape from the first CD8+ T cell response is shown.
When there is competition between different CTL responses, a rise in novel CTL responses can lead to a decrease in the contribution of already existing CTL responses. Competition could occur in many ways, for example, for costimulatory signals, for cytokines such as interleukin 2 (IL-2), or for binding to and killing of infected cells. In the model where only one CTL can bind to an infected cell at a time, as the number of CTL responses increases, the death rate of the infected cells due to killing by a given CTL response on average decreases (see the supplemental material for derivation, as well as reference 27). Under this assumption, mathematical modeling suggests that increasing the diversity of the HIV-specific CTL response reduces the contribution of a given CTL response to the killing of HIV-infected cells and as a consequence leads to a lower rate of viral escape (see equation 12) (Fig. 6B).
Finally, it is possible that CD8+ T cells control viral replication not by killing virus-infecting cells but by noncytolytic mechanisms, such as preventing infection of new uninfected targets (via production of CCR5-binding chemokines) or by reducing the rate of viral production by infected cells (via production of antiviral factors and cytokines) (19, 23, 45, 87, 95). If production of cytokines/chemokines and antiviral factors by epitope-specific CD8+ T cells is MHC restricted, occurs following recognition of virus-infected cells, and is localized, such nonlytic suppression will affect only cells infected with the wild-type virus, since these are the cells that would be able to trigger cytokine/chemokine production. Such a nonlytic T cell response should be able to select for viral escape. Recent studies suggest that nonlytic control of virus replication may be the major mechanism of suppression of SIV replication in monkeys (13, 53, 98).
Importantly, this model predicts a slower accumulation of the escape variant if the total magnitude of the CD8+ response is high (Fig. 6C) (see equation 11 in Materials and Methods, where a higher k′ value leads to a lower rate of escape, ε). Furthermore, because in this and in the CTL competition models pressure exerted on the viral population by a given CD8+ T cell response and by the total T cell response enters the expression for the rate of viral escape (see equation 11 and the supplemental material), we do not in general expect to see a correlation between the rate of viral escape and the absolute magnitude of the epitope-specific CD8+ T cell response. This lack of correlation is consistent with our findings for patients CH40, CH77, and CH58 (Fig. 5). The last two models also predict that the rate of viral escape should be lower at a higher magnitude of the total CD8+T cell response, which indeed is observed for 2 patients (see Fig. S10 in the supplemental material).
CTL response of sufficient breadth can prevent viral escape in acute HIV infection.
Results of the previous section suggest that CTL responses of a sufficient diversity should be able to slow down the appearance of viral escape at least in cases when CTLs of different specificities compete for access to infected cells or when the CD8+ T cell response controls viral replication nonlytically (Fig. 6). Assuming that CTLs compete for access to infected cells, the death rate of infected cells due to killing by a single CTL response when there is a total of n responses, all of a similar magnitude, is given by
| (13) |
where Ê is the average density of epitope-specific CTLs, Î is the density of infected cells, and kE and h are constants (see the supplemental material for more detail). Escape from a given CTL response will only occur if ki is >cir (see equation 12). Because ki decreases with n, escape should not occur when the number of HIV-specific CTL responses, n, is approximately equal to or greater than
| (14) |
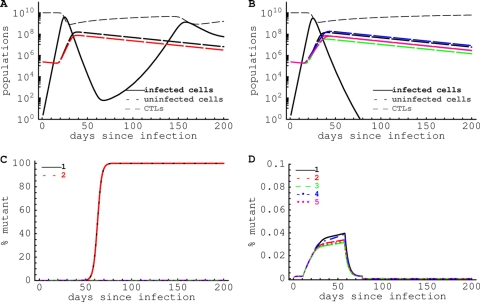
For the maximum estimated escape rate kE ≈ 0.5 day−1 (32), a possible cost of escape per epitope of 10% (ci = 0.10), ncritical ≈ 0.5/(0.1 × 1) = 5. Therefore, for these parameters, five CTL responses of a similar magnitude if they are present early in infection should be sufficient to prevent viral escape. Simulation of HIV dynamics during acute infection confirms this analytical prediction (Fig. 7). Indeed, if only two CTL responses are induced during the acute phase of infection despite initial control of viremia, escape from CTL responses occurs and virus rebounds (Fig. 7A and C). However, increasing the diversity of the CTL response to n = 5 prevents viral escape and leads to control of viral replication (Fig. 7B and D). Thus, increasing the diversity of the HIV-specific CTL response by vaccination should limit viral escape in acute HIV infection.
Fig. 7.
HIV-specific CD8+ T cell responses of a sufficient diversity can prevent viral escape in acute HIV infection. We model escape of the virus from the T cell response using the model given by equations 5 to 7, assuming that there are 2 (A and C) [ρ = (0.5, 0.45) day−1 and hE = (108, 108)] or 5 (B and D) [ρ = (0.5, 0.45, 0.4, 0.35, 0.3) day−1 and hE = (108, 108, 108, 107, 107)] HIV-specific CTL responses in the acute phase of the infection. Virus replication is limited by the availability of uninfected target cells, and the presence of the virus stimulates generation of the CTL response (A and B). Viral variants that escape recognition by a given CTL response are produced by mutation at the rate μ = 2 × 10−5 (C and D). Other parameters in simulations are as follows: s = 5 × 103 cells/day; δE = 0.02 day−1; kE = 0.5 day−1; h = 107; and c = 0.05. When 5 CTL responses control HIV replication, escape is prevented because the advantage of a variant escaping from a single response is low, and the likelihood of generating a variant with 5 escape mutations is very small. We assume that if the number of cells infected with a particular viral variant drops below 1 in the simulations, that variant does not replicate.
DISCUSSION
There are only limited quantitative data suggesting an important role for the CTL response in control of HIV replication in the acute phase of infection. We have recently shown for three patients that within 2 months after the onset of symptoms, HIV rapidly escapes from several CTL responses with a median escape rate of 0.22 day−1 (41). Here we extend our analysis to the data for the first 2 years following HIV infection in the same three patients and show that the rate of viral escape decreases dramatically as infection progresses, reaching 10- to 100-fold-lower levels. Based on a model of viral escape from several CTL responses, changes in the rate of escape with time since infection could occur due to differences in the immune pressure exerted by early and late CTL responses, a higher fitness cost associated with late escapes, and an increase in the rate of viral replication after the peak of infection as target cells replenish. All of these factors combined will lead to a synergistic situation where early escapes occur rapidly and late escapes occur slowly (equation 12 and Fig. 4).
Indeed, modeling suggests that with everything else being equal, escapes that occur early in infection are aimed at avoiding the strongest CTL responses. It is expected that the quality of the HIV-specific CD8+ T cell response will decrease over time, for example, due to a loss of virus-specific CD4+ T cells. However, a recent study has challenged this conclusion by showing that the frequency of polyfunctional HIV-specific CD8+ T cells increases over time in a given patient (31).
Previous studies have also suggested that changes in the magnitude of individual CTL responses in chronic HIV infection could be responsible for driving sequential viral escapes (6, 77, 78). However, we found no significant positive correlation between the rate of escape in a particular epitope and the magnitude of the epitope-specific CTL response (Fig. 5). This contrasts with a conclusion reached in a recent study of SHIV infection of macaques, where a strong (although nonlinear) correlation between the magnitude of the KP9-specific CTL response as measured by tetramer staining and the rate of viral escape in the KP9 epitope was observed (64). Also, our data are inconsistent with another analysis of SIV infection of monkeys that predicted a faster escape with a larger CTL response (65). The difference in conclusions could arise because these previous studies analyzed escape in a single epitope in several animals while we looked at the escape from multiple CTL responses in a given patient. Also, a difference in infection type (HIV versus SHIV/SIV) or different methods of measuring the CTL response (enzyme-linked immunosorbent spot [ELISPOT] assay versus tetramers) may have played a role. Importantly, tetramer staining quantitates epitope-specific cells accurately but provides no information about their functional capacity, and gamma interferon (IFN-γ) ELISPOT assays enumerate only epitope-specific cells producing this cytokine, which may not be very predictive of the efficiency of CTL responses in vivo (22, 63, 94). It should be noted that in one of our patients (CH58), the CTL response was measured using multicolor flow cytometry and expression of a number of markers (e.g., IFN-γ, tumor necrosis factor alpha [TNF-α], and IL-2) was used to identify HIV-specific CD8+ T cells (41). Changes occurring in the per-cell efficacy of epitope-specific CTL over time following infection may have obscured a relationship between the response magnitude and the escape rate in our study. It is also possible that the lack of correlation between the CTL response and the rate of viral escape is due to only a few responses having been analyzed or because the per-cell killing efficacy of HIV-specific CTLs depends strongly on specificity. Indeed, in several recent studies, it was found that the per-cell killing efficacy of mouse CTLs depends on TCR specificity (37, 89). Infrequent sampling of viral sequences at later time points may also have contributed to the lack of a correlation between the magnitude of the CTL response and the rate of viral escape. It should also be noted that a number of studies failed to find a significant negative correlation between the magnitude of the total HIV-specific CD8+ T cell response (as measured by IFN-γ ELISPOT assay) and the viral set point (1, 44).
Our model also predicts that early escapes may occur at a higher rate because they bear minimal fitness cost while late escapes are costly. Indeed, several studies suggest that late escapes often arise in conserved regions of the viral genome, such as that for Gag (42, 74, 96). In our recent paper, late escapes indeed arose in Gag and Pol regions of the genome (41). Furthermore, the model in which CD8+ T cells control virus replication nonlytically or compete for access to target cells (see Materials and Methods) predicts a lower rate of viral escape as the diversity or the magnitude of the HIV-specific T cell response increases (Fig. 6C). Although we observed only a moderate change in the diversity of the CTL response in our patients (see Fig. S10 in the supplemental material), a strong negative correlation between the total magnitude of the CTL response and the rate of viral escape supports the nonlytic mechanism of virus control by CD8+ T cells (53, 98). A recent study also found that early escapes occur in epitopes with the highest nucleotide variability as estimated from the HIV sequence database, suggesting that first escapes occur in epitopes with minimal fitness cost (31).
The decline of the rate of HIV escape from CTL responses in a given patient over time since infection is a novel observation made in this study. This observation is consistent with conclusions of several other studies that looked at the kinetics of a single CTL escape in a patient but studied many different patients (9, 11, 84). Using 454 deep sequencing, we further found that the rate of viral escape may also change during rapid early escapes in both HIV and SIV infection (32; V. V. Ganusov et al., unpublished), suggesting that the decline in the rate of CTL escape over the course of infection is a robust observation. It is also clear that because of the limited sampling during the chronic phase of the infection, detecting fast escapes can be difficult (6). Our current data suggest that even early in infection (the first 100 days) where sampling is relatively similar, there is a strong correlation between the rate of escape and the timing of escape (Fig. 3). Using 454 deep sequencing of the whole viral genome over the course of HIV infection, we found that late escapes (e.g., after 1 year of infection) indeed occur at a low rate since the frequency of different escape variants changes little over the period of several months (B. T. Korber et al., unpublished data), which is in line with the results in this article.
In our analysis, we used deterministic models of virus escape from the CD8+ T cell response and assumed that escape mutants preexist. In the context of a deterministic model, assuming escape mutants are generated by mutation rather than preexisting hardly affects the dynamics, since mutation will generate the escape variants, albeit in low numbers, during the first time step of a simulation. However, in a stochastic model, the appearance of escape mutants generated by mutation will be delayed. In part, the use of a deterministic model is justified by the large population size of virus-infected cells generated during acute HIV infection before any escape variants are detected. Rough estimates suggest that about 1010 CD4+ T cells reside in the gut lamina propria in humans (36), and 50% of these are infected and/or depleted in the acute phase of infection (71, 72). If such a large population of infected cells exists prior to observed viral escape, the generation of escape mutants and their selection occur almost deterministically. However, in the chronic phase of infection, the number of infected cells and more importantly the effective population size of HIV (Ne) are unknown, and current estimates vary drastically (from 103 to >105, [12, 55, 60]). Previous work suggested that escape from T cell responses can be greatly delayed and variable at low effective population sizes (61). We have confirmed this result by performing stochastic simulations of virus escape using the mathematical model given by equations 5 and 6 (see also Fig. S11 in the supplemental material). Importantly, however, while the timing of viral escape (estimated by the time to a 50% mutant population, t50) was greatly influenced by the effective population size, the rate of escape was on average independent of the effective population size (see Fig. S11). Furthermore, the rate of escape in these stochastic simulations on average followed the predictions of the deterministic model, with the first escape occurring from the strongest immune response and occurring at the highest rate (e.g., Fig. 4). Thus, our observation that the rate of virus escape from the CD8+ T cell response declines with time since infection also holds when escape is modeled stochastically for a small effective population size of HIV.
Overall, our analysis suggests that the most likely explanation for the low rate of accumulation of late escapes is a consequence of two factors: a high fitness cost of the escape mutations and an increase in the diversity of the T cell response over time in most patients. Our model thus predicts that a sufficiently diverse response should limit viral escape from the CTL response, supporting future development of T cell-based HIV vaccines as long as they elicit cross-reactive responses to a broad range of viral epitopes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Center for HIV/AIDS Vaccine Immunology, A1067854-03. Additional support came from the MRC Human Immunology Unit, the NIHR Oxford Biomedical Research Centre, grant 37874 from the Bill and Melinda Gates Foundation, NSF grant PHY05-51164, and NIH grants RR006555 and AI028433. Parts of this work were done under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396 , and V.V.G. was supported by the LDRD program of the Los Alamos National Laboratory, start-up funds from the University of Tennessee, and in part by a grant from the Russian Ministry of Education (NK-550P/2). P.B. and A.J.M. are Jenner Institute Investigators.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Addo M., et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed R. 1996. Tickling memory T cells. Science 272:1904. [DOI] [PubMed] [Google Scholar]
- 3. Allen T., et al. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen T. M., et al. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386–390 [DOI] [PubMed] [Google Scholar]
- 5. Allen T. M., et al. 2005. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J. Virol. 79:12952–12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Althaus C., De Boer R. 2008. Dynamics of immune escape during HIV/SIV infection. PLoS Comput. Biol. 4:e1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Althaus C., Ganusov V., De Boer R. 2007. Dynamics of CD8+ T cell responses during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 179:2944–2951 [DOI] [PubMed] [Google Scholar]
- 8. Asquith B. 2008. The evolutionary selective advantage of HIV-1 escape variants and the contribution of escape to the HLA-associated risk of AIDS progression. PLoS One 3:e3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asquith B., Edwards C., Lipsitch M., McLean A. 2006. Inefficient cytotoxic T lymphocyte-mediated killing of HIV-1-infected cells in vivo. PLoS Biol. 4:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asquith B., McLean A. 2007. In vivo CD8+ T cell control of immunodeficiency virus infection in humans and macaques. Proc. Natl. Acad. Sci U. S. A. 104:6365–6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asquith B., et al. 2007. In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc. Natl. Acad. Sci. U. S. A. 104:8035–8040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balagam R., Singh V., Sagi A. R., Dixit N. M. 2011. Taking multiple infections of cells and recombination into account leads to small within-host effective-population-size estimates of HIV-1. PLoS One 6:e14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balamurali M., et al. 2010. Does cytolysis by CD8+ T cells drive immune escape in HIV infection? J. Immunol. 185:5093–5101 [DOI] [PubMed] [Google Scholar]
- 14. Barouch D., Kunstman J., Glowczwskie J., Kunstman K., Egan M., et al. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barouch D., et al. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335–339 [DOI] [PubMed] [Google Scholar]
- 16. Barouch D., et al. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486–492 [DOI] [PubMed] [Google Scholar]
- 17. Barouch D. H., Korber B. 2010. HIV-1 vaccine development after STEP. Annu. Rev. Med. 61:153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bates D. M., Watts D. G. 1988. Nonlinear regression analysis and its applications. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 19. Bolitho P., Voskoboinik I., Trapani J. A., Smyth M. J. 2007. Apoptosis induced by the lymphocyte effector molecule perforin. Curr. Opin. Immunol. 19:339–347 [DOI] [PubMed] [Google Scholar]
- 20. Borrow P., et al. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205–211 [DOI] [PubMed] [Google Scholar]
- 21. Casimiro D. R., et al. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung C., et al. 2007. Not all cytokine-producing CD8+ T cells suppress simian immunodeficiency virus replication. J. Virol. 81:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cocchi F., et al. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811–1815 [DOI] [PubMed] [Google Scholar]
- 24. Corey L., McElrath M. J., Kublin J. G. 2009. Post-step modifications for research on HIV vaccines. AIDS 23:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crawford H., et al. 2009. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J. Exp. Med. 206:909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davenport M. P., Loh L., Petravic J., Kent S. J. 2008. Rates of HIV immune escape and reversion: implications for vaccination. Trends Microbiol. 16:561–566 [DOI] [PubMed] [Google Scholar]
- 27. De Boer R. J., Perelson A. S. 1995. Towards a general function describing T cell proliferation. J. Theor. Biol. 175:567–576 [DOI] [PubMed] [Google Scholar]
- 28. Draenert R., et al. 2004. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J. Exp. Med. 199:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Efron B., Tibshirani R. 1993. Introduction to the bootstrap. Chapman & Hall, New York, NY: [Google Scholar]
- 30. Fernandez C., et al. 2005. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J. Virol. 79:5721–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari G., et al. 2011. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog. 7:e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischer W., Ganusov V. V., Giorgi E. E., Hraber P. T., Keele B. F., et al. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frater A. J., et al. 2007. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J. Virol. 81:6742–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fryer H. R., Scherer A., Oxenius A., Phillips R., McLean A. R. 2009. No evidence for competition between cytotoxic T-lymphocyte responses in HIV-1 infection. Proc. Biol. Sci. 276:4389–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ganusov V., De Boer R. 2006. Estimating costs and benefits of CTL escape mutations in SIV/HIV infection. PLoS Comput. Biol. 2:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ganusov V., De Boer R. 2007. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 28:514–518 [DOI] [PubMed] [Google Scholar]
- 37. Ganusov V. V., Barber D. L., De Boer R. J. 2011. Killing of targets by CD8 T cells in the mouse spleen follows the law of mass action. PLoS One 6:e15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gasper-Smith N., et al. 2008. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J. Virol. 82:7700–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gay C., et al. 2011. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS One 6:e19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geels M., et al. 2003. Identification of sequential viral escape mutants associated with altered T-cell responses in a human immunodeficiency virus type 1-infected individual. J. Virol. 77:12430–12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goonetilleke N., et al. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206:1253–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goulder P., Watkins D. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630–640 [DOI] [PubMed] [Google Scholar]
- 43. Goulder P. J., et al. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212–217 [DOI] [PubMed] [Google Scholar]
- 44. Gray C. M., et al. 2009. Human immunodeficiency virus-specific gamma interferon enzyme-linked immunospot assay responses targeting specific regions of the proteome during primary subtype C infection are poor predictors of the course of viremia and set point. J. Virol. 83:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guidotti L. G., Chisari F. V. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65–91 [DOI] [PubMed] [Google Scholar]
- 46. Hay C., et al. 1999. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J. Virol. 73:5509–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones N. A., et al. 2004. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 200:1243–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaur A., et al. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur. J. Immunol. 31:3207–3217 [DOI] [PubMed] [Google Scholar]
- 49. Kawada M., et al. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keele B. F., et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kent S., Fernandez C., Dale C., Davenport M. 2005. Reversion of immune escape HIV variants upon transmission: insights into effective viral immunity. Trends Microbiol. 13:243–246 [DOI] [PubMed] [Google Scholar]
- 52. Kirchherr J. L., et al. 2007. High throughput functional analysis of HIV-1 env genes without cloning. J. Virol. Methods 143:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klatt N. R., et al. 2010. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 6:e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koenig S., et al. 1995. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1:330–336 [DOI] [PubMed] [Google Scholar]
- 55. Kouyos R., Althaus C., Bonhoeffer S. 2006. Stochastic or deterministic: what is the effective population size of HIV-1? Trends Microbiol. 14:507–511 [DOI] [PubMed] [Google Scholar]
- 56. Leslie A., et al. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282–289 [DOI] [PubMed] [Google Scholar]
- 57. Levy J. 2003. The search for the CD8+ cell anti-HIV factor (CAF). Trends Immunol. 24:628–632 [DOI] [PubMed] [Google Scholar]
- 58. Little S. J., McLean A. R., Spina C. A., Richman D. D., Havlir D. V. 1999. Viral dynamics of acute HIV-1 infection. J. Exp. Med. 190:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu J., et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y., Mittler J. E. 2008. Selection dramatically reduces effective population size in HIV-1 infection. BMC Evol. Biol. 8:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Y., Mullins J. I., Mittler J. E. 2006. Waiting times for the appearance of cytotoxic T-lymphocyte escape mutants in chronic HIV-1 infection. Virology 347:140–146 [DOI] [PubMed] [Google Scholar]
- 62. Liu Y., et al. 2004. Molecular clock-like evolution of human immunodeficiency virus type 1. Virology 329:101–108 [DOI] [PubMed] [Google Scholar]
- 63. Loffredo J. T., et al. 2007. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J. Virol. 81:2624–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Loh L., Petravic J., Batten C., Davenport M., Kent S. 2008. Vaccination and timing influence SIV immune escape viral dynamics in vivo. PLoS Pathog. 4:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mandl J. N., Regoes R. R., Garber D. A., Feinberg M. B. 2007. Estimating the effectiveness of simian immunodeficiency virus-specific CD8+ T cells from the dynamics of viral immune escape. J. Virol. 81:11982–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mansky L. M. 1996. Forward mutation rate of human immunodeficiency virus type 1 in a T lymphoid cell line. AIDS Res. Hum. Retroviruses 12:307–314 [DOI] [PubMed] [Google Scholar]
- 67. Markowitz M., et al. 2003. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J. Virol. 77:5037–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martinez-Picado J., et al. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McDermott A. B., et al. 2005. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J. Virol. 79:15556–15566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McMichael A. J., Borrow P., Tomaras G. D., Goonetilleke N., Haynes B. F. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehandru S., et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mehandru S., et al. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81:599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Migueles S. A., et al. 2008. Lytic granule loading of CD8(+) T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miura T., et al. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare Gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J. Virol. 83:2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Monteiro L., Goncalves C., Piqueira J. 2000. A condition for successful escape of a mutant after primary HIV infection. J. Theor. Biol. 203:399–406 [DOI] [PubMed] [Google Scholar]
- 76. Neher R. A., Leitner T. 2010. Recombination rate and selection strength in HIV intra-patient evolution. PLoS Comput. Biol. 6:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nowak M. 1996. Immune responses against multiple epitopes: a theory for immunodominance and antigenic variation. Semin. Virol. 7:83–92 [Google Scholar]
- 78. Nowak M., et al. 1995. HIV results in the frame: results confirmed. Nature 375:193. [DOI] [PubMed] [Google Scholar]
- 79. Nowak M. A., et al. 1991. Antigenic diversity thresholds and the development of AIDS. Science 254:963–969 [DOI] [PubMed] [Google Scholar]
- 80. O'Connor D. H., et al. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493–499 [DOI] [PubMed] [Google Scholar]
- 81. Perelson A. S., Macken C., Grimm E., Roos L., Bonavida B. 1984. Mechanism of cell-mediated cytotoxicity at the single cell level. VIII. Kinetics of lysis of target cells bound by more than one cytotoxic T lymphocyte. J. Immunol. 132:2190–2198 [PubMed] [Google Scholar]
- 82. Perelson A. S., Neumann A. U., Markowitz M., Leonard J. M., Ho D. D. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell lifespan, and viral generation time. Science 271:1582–1586 [DOI] [PubMed] [Google Scholar]
- 83. Petravic J., Loh L., Kent S., Davenport M. 2008. CD4+ target cell availability determines the dynamics of immune escape and reversion in vivo. J. Virol. 82:4091–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Petravic J., et al. 2008. Estimating the impact of vaccination on acute simian-human immunodeficiency virus/simian immunodeficiency virus infections. J. Virol. 82:11589–11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peyerl F. W., et al. 2004. Fitness costs limit viral escape from cytotoxic T lymphocytes at a structurally constrained epitope. J. Virol. 78:13901–13910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Prado J. G., et al. 2009. Functional consequences of human immunodeficiency virus escape from an HLA-B*13-restricted CD8+ T-cell epitope in p1 Gag protein. J. Virol. 83:1018–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Price D. A., et al. 1998. Antigen-specific release of beta-chemokines by anti-HIV-1 cytotoxic T lymphocytes. Curr. Biol. 8:355–358 [DOI] [PubMed] [Google Scholar]
- 88. Ramratnam B., et al. 1999. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 354:1782–1785 [DOI] [PubMed] [Google Scholar]
- 89. Regoes R., Barber D., Ahmed R., Antia R. 2007. Estimation of the rate of killing by cytotoxic T lymphocytes in vivo. Proc. Natl. Acad. Sci. U. S. A. 104:1599–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reynolds M. R., et al. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 205:2537–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ribeiro R. M., et al. 2010. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J. Virol. 84:6096–6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Troyer R. M., et al. 2009. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 5:e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Turnbull E. L., et al. 2009. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J. Immunol. 182:7131–7145 [DOI] [PubMed] [Google Scholar]
- 94. Valentine L. E., et al. 2008. Recognition of escape variants in ELISPOT does not always predict CD8+ T-cell recognition of simian immunodeficiency virus-infected cells expressing the same variant sequences. J. Virol. 82:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wagner L., et al. 1998. Beta-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature 391:908–911 [DOI] [PubMed] [Google Scholar]
- 96. Wang Y. E., et al. 2009. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J. Virol. 83:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wherry E., Blattman J., Murali-Krishna K., Van Der Most R., Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wong J. K., et al. 2010. In vivo CD8+ T-cell suppression of SIV viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 6:e1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang L., et al. 1999. Rapid clearance of simian immunodeficiency virus particles from plasma of rhesus macaques. J. Virol. 73:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.