Abstract
Expression of the high-risk human papillomavirus (HPV) E6 and E7 oncogenes is essential for the initiation and maintenance of cervical cancer. The repression of both was previously shown to result in activation of their respective tumor suppressor targets, p53 and pRb, and subsequent senescence induction in cervical cancer cells. Consequently, viral oncogene suppression is a promising approach for the treatment of HPV-positive tumors. One well-established method of E6/E7 repression involves the reexpression of the viral E2 protein which is usually deleted in HPV-positive cancer cells. Here, we show that, surprisingly, bovine papillomavirus type 1 (BPV1) E2 but not RNA interference-mediated E6/E7 repression in HPV-positive cervical cancer cells stimulates cellular motility and invasion. Migration correlated with the dynamic formation of cellular protrusions and was dependent upon cell-to-cell contact. While E2-expressing migratory cells were senescent, migration was not a general feature of cellular senescence or cell cycle arrest and was specifically observed in HPV-positive cervical cancer cells. Interestingly, E2-expressing cells not only were themselves motile but also conferred increased motility to admixed HeLa cervical cancer cells. Together, our data suggest that repression of the viral oncogenes by E2 stimulates the motility of E6/E7-targeted cells as well as adjacent nontargeted cancer cells, thus raising the possibility that E2 expression may unfavorably increase the local invasiveness of HPV-positive tumors.
INTRODUCTION
High-risk human papillomavirus (hrHPV)-positive cervical cancer cells harbor integrated HPV genomic DNA and are uniquely dependent upon the expression of two viral oncogenes, E6 and E7, for the maintenance of the transformed phenotype (40). hrHPV E6 is best known for its ability to target p53 for proteasomal degradation via its association with the E6-associated protein (E6-AP), a ubiquitin ligase (52, 53, 64). Additionally, hrHPV E6 activates telomerase to extend the life span of primary human keratinocytes (28, 33, 58) and binds to and deregulates several PDZ proteins that are known to regulate cell polarity, adhesion, and proliferation (27, 32, 41, 57). This results in deregulation of tumor-suppressive activity and, in so doing, contributes to carcinogenesis. hrHPV E7 binds to and degrades members of the retinoblastoma (Rb) family, resulting in the transcription of E2F target genes such as cyclin E and cyclin A which are responsible for S-phase progression (11, 37). Additional E7 activities include the inhibition of the cyclin-dependent kinase inhibitors p21CIP1 and p27KIP1 which activate Rb (22, 24). Together, both hrHPV E6 and E7 contribute to carcinogenesis by suppressing apoptosis and senescence and by stimulating cellular proliferation. Consequently, a number of reports have pioneered various approaches to inhibit E6 and E7 function in HPV-positive cancer cells for the specific induction of cellular growth arrest and death (1, 18, 48, 54). This includes the use of oncogene-specific peptide aptamers (3, 43), antisense technology (59), RNA interference (RNAi) (4, 30, 45), and the expression of viral E2 protein (7, 39, 44, 56, 63). Furthermore, in vivo experiments have provided proof of concept for the therapeutic targeting of E6/E7 in HPV-driven tumors in the presence and absence of conventional treatments (18, 25, 60).
Several laboratories, including ours, have previously described the consequences of virally delivered E2 expression in HPV-positive cervical cancer cells: E6/E7 transcription ceased, followed by the reactivation of Rb and p53 tumor suppressors, cell cycle arrest, and eventually the induction of a synchronized cellular senescence phenotype. This was observed following the expression of bovine papillomavirus type 1 (BPV1) E2 transactivator (E2-TA), but not the transcriptional repressor (E2-TR), in HPV-positive cancer cells (10, 17). Similar phenotypes were observed using a temperature-sensitive BPV1 E2 (E2ts) protein that is functional at the permissive temperature of 32°C but not at 37°C and above (8, 62). Senescence induction in cervical cancer cells was identified by the typical flat-cell morphology (34), upregulation of senescence markers including senescence-associated β-galactosidase (SA-β-Gal) activity (9), and a transcriptional profile that is reminiscent of replicatively senescent primary cells (23). Rescue of E2-expressing HeLa cells from either E6 or E7 repression further revealed that senescence was primarily dependent upon E7 repression and Rb-controlled transcription (6), whereas E6 repression resulted in a mixed phenotype that included apoptotic and senescent cells (6, 46).
Data from Goodwin et al. demonstrated that E2-TA expression in HeLa cell colonies results in gradual cellular dispersal over time (17). Here, we further characterize this phenotype and demonstrate that E6/E7 repression by E2, but not by short hairpin RNA (shRNA) or small interfering RNA (siRNA) knockdown, stimulates the rapid formation of cellular protrusions, together with increased migratory and invasive cell characteristics. This form of migration was specifically observed in HPV-positive cells but not in HPV-negative cells or in cells harboring HPV episomes. Furthermore, migration was nondirectional and was dependent upon cell-cell contact. The knockdown of an important E7 target, the human DEK oncogene, induced senescence, as previously published (23, 67), but did not stimulate motility, thus demonstrating that HeLa cell senescence per se was not sufficient for driving this phenotype. Finally, we show that E2-expressing cells could stimulate the migration of adjacent nontargeted HeLa cells, a finding which suggests that E2-mediated E6/E7 repression in cervical cancers might support local tumor cell dissemination and invasiveness.
MATERIALS AND METHODS
Cell culture.
The human cervical cancer cell lines utilized were HeLa (HPV18 positive), Caski (HPV16 positive), and C33A (HPV negative). These and the osteosarcoma cell line SAOS2 as well as IMR-90 human fibroblasts were maintained in Dulbecco's modified Eagle medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. Primary keratinocytes were prepared from human foreskins as described previously (66) and maintained in keratinocyte Epilife medium supplemented with human keratinocyte growth supplement (HKGS; Cascade Biologics, Portland, OR). Near-diploid immortalized keratinocytes that form skin (NIKS) as well as NIKS transfected with HPV16 (NIKS-HPV16) (12) (kind gifts from Paul Lambert, University of Wisconsin—Madison) were cultured on irradiated J2-3T3 feeder cells as previously described (42).
Viral and plasmid constructs.
Adenovirus (Ad) and Ad expressing E2ts (AdE2ts) have been described previously (62), as well as the E2-TA and E2-TR expression plasmids (63). Nontargeting and DEK-specific short hairpin (NTsh and DEKsh, respectively) lentiviral vectors were obtained through the Sigma Mission short hairpin RNA (shRNA) program (Sigma-Aldrich, St. Louis, MO). The relevant product numbers were SHC 002VC for the control nontargeting vector (NTsh) and TRCN0000013107 for the DEKsh targeting vector. The LXSN retroviral constructs were a generous gift from Denise Galloway (University of Washington, Seattle, WA). The cytomegalovirus (CMV)-driven DsRed lentiviral expression vector was a generous gift from the Malik laboratory (Cincinnati Children's Hospital, Cincinnati, OH). Specific lentiviral constructs targeting the HPV16 E6 open reading frame were custom designed through Sigma. The shRNA targeted the E6 coding region, nucleotides (nt) 196 to 216: 5′-GTACTGCAAGCAACAGTTACT-3′. Double-stranded RNAi oligonucleotides designed against the HPV18 E7 coding region (nt 694 to 712, 5′-GGAAGAAAACGATGAAATA-3′) as well as the siGenome nontargeting control were purchased from Dharmacon (Lafayette, CO).
Viral infections.
Adenoviral infections using empty vector (Ad) and temperature-sensitive E2-expressing Ad (AdE2ts) were carried out as described previously (62). Briefly, a total of 1 × 106 cells plated on 10-cm dishes were infected on two consecutive days with either 10 PFU or 10 infectious units (IU) of virus, as indicated in the figure legends, in 1 ml of phosphate-buffered saline (PBS) containing 4% fetal bovine serum (FBS) for 1 h. The virus was then aspirated, and cells were washed twice with PBS and then overlaid with medium. On the third day, cells were split and placed at 37°C for several hours to allow attachment and later shifted to the permissive temperature of 32°C for 3 days, after which they were shown to be irreversibly growth arrested (62). For lentiviral infections, cells were infected with 4 ml of shRNA-expressing nontargeting (NTsh), DEK-targeting (DEKsh), or HPV16 E6-targeting (HPV16 E6/E7sh) virus for 8 h. The cells were then washed twice with PBS and overlaid with fresh medium. At 1 day postinfection, the cells were overlaid with medium containing 650 ng/ml puromycin for selection of a pure, virally transduced population. For retroviral infections, cells were incubated with 4 ml of viral supernatant of empty vector (LXSN) or HPV16 E7- or HPV16 E6/E7-expressing vectors (LXSN E7 or LXSN 16E6/E7, respectively) or with 2 ml of viral supernatant of HPV16 E6 (LXSN E6)-expressing vector using 8 μg/ml Polybrene for 4 h and then overlaid with fresh medium. The same was done using a pBabe empty vector or vector expressing oncogenic H-ras. Cells were then selected and maintained in 500 μg/ml G418-containing medium.
Transient transfections and viral infections.
Transient transfections were conducted as previously described (66). Briefly, HeLa cells seeded on a 60-mm plate were transfected with a total of 4 μg of plasmid DNA using FuGENE transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. For cotransfections, cells were transfected with 400 ng of neomycin resistance plasmid and 3.6 μg of DNA. Cells were then selected in medium containing 900 μg/ml G418 for 8 days.
Migration and invasion assays.
Migration assays were conducted using 8.0-μm-ore-size polycarbonate transwell inserts (Corning Inc., Corning, NY) according to the manufacturer's instructions. Briefly, inserts were first rehydrated using 0.1 ml of medium in the top inserts and 0.6 ml in the wells of a 24-well dish for at least 1 h. A total of 1 × 105 cells were plated in the upper inserts and then allowed to migrate for at least 16 h. Nonmigrating cells were removed with a cotton swab while cells that had migrated were fixed in methanol for 10 min and stained with Giemsa dye (Sigma-Aldrich, St. Louis, MO) at a 1:20 dilution. Four quadrants of the membrane were then counted under a light microscope. For invasion assays, Matrigel-coated invasion inserts (BD Biosciences, Billerica, MA) were rehydrated with DMEM containing 10% FBS, for a final volume of 0.5 ml in the upper inserts and 0.75 ml in the wells of a 24-well dish. After incubation for a minimum of 2 h, the medium from the upper inserts was removed and replaced with 0.5 ml of serum-free medium containing 1 × 105 cells. Cells were allowed to migrate through the Matrigel for 20 to 22 h and then were stained and counted as described for migration assays.
Time-lapse video microscopy.
A total 100 μl of 1 × 105 cells were seeded as a colony in a four-chambered Lab-Tek coverglass slide (Nunc, Rochester, NY). The cells were allowed to attach for several hours and were gently washed with PBS and overlaid with Leibovitz's L-15 medium containing l-glutamine and 5% serum (Gibco, Invitrogen, Carlsbad, CA) in the absence of phenol red. Control and treated groups were imaged simultaneously using a Zeiss LSM510 confocal system attached to a Zeiss Axiovert 200 microscope and a heated stage. Images were captured at ×10 magnification every 5 min for at least 12 h using the LSM software with the MultiTime macro. Cell edges for at least 20 independent cells within the field of view were then tracked using ImageJ software with the MTrackJ plug-in, and average distance and/or velocity of each cell was determined. This was repeated for at least two different field views for each cell group.
Western blot analyses.
Cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (1% Triton, 1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.16 M NaCl, 10 mM Tris [pH 7.4], 5 mM EDTA) supplemented with protease inhibitor cocktail (Pharmingen, San Diego, CA). Protein concentrations were determined with Bradford reagent (Bio-Rad, Hercules, CA). Aliquots containing equal amounts of total protein were boiled in SDS sample buffer and resolved by SDS-polyacrylamide gel electrophoresis. Proteins were then transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) and probed overnight with the following antibodies used at the indicated dilutions: p53 monoclonal, 1:1,000; polyclonal cyclin A, 1:400; HPV18 E7, 1:200 (Santa Cruz Biotechnology Inc., Santa Cruz, CA); monoclonal DEK, 1:1,000 (BD Biosciences, San Diego, CA); monoclonal Rac1, 1:1,000 (Upstream Biosciences, Calgary, Alberta, Canada); monoclonal actin, 1:20,000 (Seven Hills Bioreagents, Cincinnati, OH), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Fitzgerald, Acton, MA). Membranes were exposed to enhanced chemiluminescence reagents (Perkin Elmer, Boston, MA), and protein bands were detected by autoradiography. For HPV16 E7 detection, a mixture of 1:50 HPV16 E7 8C9 monoclonal antibody (Invitrogen, Carlsbad, CA) and 1:200 HPV16E7 ED17 monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted in 3% milk was used. Signal was then detected using Super Signal West Femto substrate (Thermo Scientific, Rockford, IL).
Preparation of ECM.
Ad- or AdE2ts-infected cells plated at a confluent density were washed twice with PBS and then once with water. Cells were then treated with 20 mM NH4OH at room temperature for 5 min and aspirated, and the underlying endogenous extracellular matrix (ECM) was rinsed twice with PBS. Fresh Ad-infected cells were then seeded onto the AdE2ts ECM and vice versa, and time-lapse migration assays were conducted as described above.
Cell cycle analyses.
Cell cycle analysis was conducted as previously described (67). Briefly, HeLa cells were treated with medium containing the cell cycle inhibitors mimosine (0.5 mM) and thymidine (2.5 mM) for 16 h to induce cellular arrest in the G0/G1 and S phases of the cell cycle, respectively. Cells were then washed and pelleted by centrifugation, and 1 × 106 cells were resuspended in 800 μl of solution containing 1% bovine serum albumin (BSA) in PBS, 100 μl of 500 μg/ml propidium iodide in 10 mM sodium citrate, pH 7.0, and 100 μl of boiled RNase A (10 mg/ml in 10 mM Tris-HCl, pH 7.5). Cells were then incubated at 37°C for 30 min, and cell cycle profiles were obtained using a flow cytometer (BD Biosciences, San Jose, CA).
qRT-PCR.
Total RNA was harvested from cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription-PCR (RT-PCR) was performed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) or a QuantiTect reverse transcriptase kit (Qiagen, Valencia, CA) as described by the manufacturer. Quantitative RT-PCR (qRT-PCR) was carried out using an ABI 7500 system (Applied Biosystems, Foster City, CA) using SYBR green master mix (Applied Biosystems, Foster City, CA). The levels of mRNA assessed were all normalized to the c-abl, GAPDH, or EEFA1 reference gene. For verification of gene regulation identified from meta-analysis, RT-PCR was conducted using mRNA from three independent experiments, and all levels were normalized to the EEFA1 reference gene. A list of all primers used along with their respective sequences is given in Table 1.
Table 1.
Primers used for RT-qPCR
| Primer name | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
|---|---|---|
| c-abl | TCGCCAGAGAAGGTCTATGAACT | AACATTTCAAAGGCTTGGTG |
| hSnail1 | CCTCCCTGTCAGATGAGGAC | CAAGGAATACCTCAGCCTGG |
| hTwist1 | GGAGTCCGCAGTCTTACGAG | CCTCTACCAGGTCCTCCAGA |
| HPV16 E7 | CACGAGCAATTAAGCGACT | GCTCAATTCTGGCTTCAC |
| GAPDH | CCGCAGAGGTGTGGTGGCTG | CAGCCTGGCCTTTGGGGTCG |
| NME1 | GCGTTCCGTGCGTGCAAGTG | GCCATGGTTCCTTCCAGCTGCTT |
| Plat | GCTGCGCCCTGTGCTCAGAA | TCGTAGCACGTGGCCCTGGT |
| GSN | CTGCCGCTGTCGCCACCAT | GCCAGGCTCCTTCCCTGCCT |
| PTP4A1 | CGGGACCGGCTGTATGATTAGGC | GTCGTGCTGTGCCTGGCAGT |
| ADAM17 | TGAGTGCCCGCCTCCAGGAA | TAGGGCACACAGCGGCCAGA |
| CDH3 | CTGGGGGCTGTCCTGGCTCT | TGGTCCTCTTCGCCACCCCC |
| NOTCH1 | CTGCTGGGCCAGGTGAAGGC | GTTGAGGCTGCCCAGCGAGG |
| IGFBP3 | CAGCTCCAGGTGAGCCGCC | GGGATCAGACACCCGGTGCG |
| FGFR1 | ACAGCCACACTCTGCACCGC | TGGAGCTACGGGCATACGGT |
| PFN2 | GCCGGGGGCGTCTTTCAGAG | CCAATGCTCTACCAGCTCTGCCG |
| ACTN1 | TTGGCAACGACCCCCAGGGA | GGCGCAGCTCGTCCATGGTAA |
| FN1 | AGAAGTGGTCCCTCGGCCCC | ACCAGTTGGGGAAGCTCGTCTGT |
| S100A9 | CTGGGGCACCCAGACACCCT | CCAGGGCCCTCGTCACCCTC |
| PGF | TCCTCCTGTGCCAGGGGCTC | CTGCGGCCCCACACTTCCTG |
| JAG1 | AACGACCGCAACCGCATCGT | TTCAGCGTCTGCCACTGCCG |
| SEMA5A | AGCAACCCCACTCCCAGGCA | TGCGGCGAGCTTGAATGCCA |
| BMP4 | GCATCCGAGCTGAGGGACGC | AGGGAAGCTGCAGCAGTGCG |
| FGF2 | ACGGGGTCCGGGAGAAGAGC | TGCCCAGTTCGTTTCAGTGCCA |
| ITGB1 | ACGCCGCGCGGAAAAGATGA | GCACCACCCACAATTTGGCCC |
| PTGS2 | GCGAGGGCCAGCTTTCACCA | CCTGCCCCACAGCAAACCGT |
| SERPINE1 | TTTCAGCAGGTGGCGCAGGC | TACAGATGCCGGAGGGCGGG |
| SERPINE5 | TGAGCCACCGCTGCTTCTGC | TGCAGGGCATCCATTGCGGG |
| IL-6 | TTGCCTGCTGCCTTCCCTGC | TGCCAGTGCCTCTTTGCTGCT |
| EEFA1 | TGCGGTGGGTGTCATCAAA | AAGAGTGGGGTGGCAGGTATTG |
Immunofluorescence microscopy.
Ad- and AdE2ts-infected cells were plated onto poly-d-lysine hydrobromide (Sigma, St. Louis, MO)-coated coverslips and fixed with 2% paraformaldehyde in PBS. Coverslips were incubated in 0.2% Triton X-100 in PBS for 2 min, blocked with 2% BSA in PBS for 2 h, washed twice with PBS, and incubated with primary antibody at 4°C overnight. The following antibody dilutions were used: E-cadherin, 1:50 (BD Biosciences, San Diego, CA); vimentin, 1:300 (Santa Cruz Biotechnology Inc., Santa Cruz, CA); fibronectin, 1:300 (Sigma, St. Louis, MO); and Rac1, 1:200 (Upstream Biosciences, Calgary, Alberta, Canada). Cells were then washed and incubated in anti-mouse, anti-rabbit, or anti-goat Alexa-Fluor 568- or 555-conjugated secondary antibody (1:500; Invitrogen, Carlsbad, CA) at room temperature for 2 h. Coverslips were then mounted onto glass slides with DAPI (4′,6′-diamidino-2-phenylindole) Vector Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and images were taken using a Zeiss fluorescence microscope.
Rho GTPase pulldown assays.
HeLa cells infected with Ad or AdE2ts were washed with PBS and then lysed in buffer containing 20 mM Tris-HCl, pH 7.6, 100 mM NaCl, 10 mM MgCl, 1% Triton X-100, 0.2% SDS, and protease and phosphatase inhibitors. Cell lysates containing equal amounts of protein were then incubated with glutathione beads conjugated to glutathione S-transferase (GST)–p21-activated kinase 1 (PAK-1) for Rac1, and total levels were determined by immunoblotting using Rac1 (Upstream Biosciences, Calgary, Alberta, Canada) at a dilution of 1:500.
Senescence-associated β-galactosidase assay.
Cells were fixed in 2% formaldehyde and 0.2% glutaraldehyde for 15 min, washed with PBS, and incubated at 37°C for 16 to 36 h in SA-β-galactosidase staining solution as described previously (9). Cells were analyzed under a light microscope.
RESULTS
E2-mediated suppression of HPV E6/E7 stimulates migration in HPV-positive cancer cells.
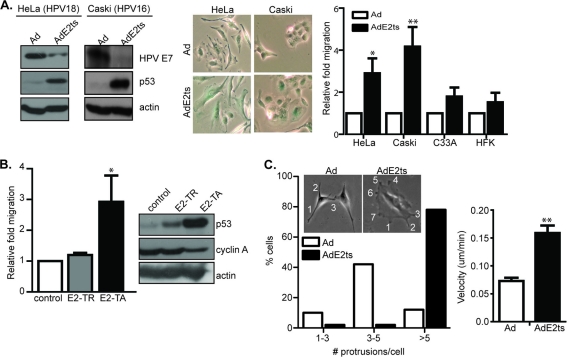
It is well established that the reexpression of either full-length BPV1 or hrHPV E2 protein in HPV-positive cervical cancer cell lines induces cell cycle arrest and cellular senescence through the repression of E6 and E7 (17, 63). E6/E7 repression by RNA interference also results in cellular senescence (20) and was recently shown to decrease motility (4). However, E2 expression was shown to increase the dispersal of tightly packed HeLa cells over time (17). To further characterize the effects of E2 expression on cervical cancer cell motility, both HPV18-positive HeLa cells and HPV16-positive Caski cells were infected with the empty adenoviral vector Ad or with AdE2ts virus as described previously for synchronous senescence induction (62). Immunoblot analysis of the respective protein lysates confirmed decreased E7 levels directly and decreased E6 levels indirectly based on p53 induction (Fig. 1A). E2 expression and subsequent E6/E7 suppression were also associated with cellular flattening and positive staining for SA-β-Gal activities (Fig. 1A). Transwell migration assays revealed that AdE2ts-infected HPV-positive HeLa and Caski cells, but not HPV-negative C33A cervical cancer cells and human foreskin keratinocytes (HFKs), showed elevated motility compared to the Ad-infected controls (Fig. 1A). Importantly, the observed motility was not dependent upon the presence of FBS in the top chamber, bottom chamber, or both chambers (data not shown), thus ruling out serum-dependent chemotaxis as the underlying mechanism. HeLa cells were also transiently transfected with an expression vector for full-length, E6/E7-repressive E2-TA protein or for the naturally occurring truncated E2-TR protein which lacks the N-terminal domain and is defective for oncogene repression and senescence induction (63). Transwell migration assays showed that E2-TA-expressing cells were significantly more motile than E2-TR-expressing and control cells (Fig. 1B). Western blot analysis confirmed that E2-TA but not E2-TR was effective in repressing E6, as demonstrated by upregulation of p53 protein levels, and E7, as shown by decreased levels of the upregulated E7 target, cyclin A (Fig. 1B).
Fig. 1.
E2-mediated suppression of HPV E6/E7 stimulates migration in HPV-positive cancer cells. (A) HeLa and Caski cells were infected with an adenovirus expressing temperature-sensitive E2 (AdE2ts) or Ad control. Western blot analyses showed decreased HPV E7 levels and increased p53 expression. Senescent AdE2ts-infected cells stained positive for SA-β-Gal while Ad controls did not. HeLa and Caski cells infected with empty Ad or AdE2ts were subjected to transwell migration, and both HeLa and Caski AdE2ts-infected cells showed increased migration compared to their proliferating controls. HPV-negative C33A cells and primary human foreskin keratinocytes did not show an increase in migration upon AdE2ts expression. (B) HeLa cells transfected with full-length E2-TA or mutant E2-TR as well as empty vector control were subjected to a transwell assay. E2-TA- but not E2-TR-transfected cells showed increased migration. Error bars represent standard deviation from a representative experiment. Western blot analysis indicated that only full-length E2-TA was effective in repressing E6, thus increasing p53 protein levels, while protein levels of cyclin A, a known target of E7, was decreased. (C) Ad- or AdE2ts-expressing HeLa cells were plated in a cloning ring and allowed to attach for at least 3 h. Time-lapse video microscopy was conducted as cells were allowed to migrate outwards over the course of 12 h. ImageJ software with the MTrackJ plug-in was then used to track cells. For every cell tracked by ImageJ, the final number of cellular protrusions formed (indicated by numbers) per cell within a given field was also counted. Total velocity over the course of the time lapse was also determined by tracking at least 20 cells per field, and three fields were tracked for each time lapse. Error bars represent standard errors of the means of cells tracked from three different time-lapse experiments.
To further analyze previous observations of E2-driven HeLa cell dispersal (17), we observed morphological changes over time by two-dimensional (2D) time-lapse video microscopy. Interestingly, E2-infected HeLa cells were characterized by increased numbers of cellular protrusions compared to their Ad-infected proliferating counterparts. Specifically, the majority of E2-expressing cells displayed greater than five protrusions per cell, while most control cells had 3 to 5 protrusions per cell (Fig. 1C). Protrusions on Ad control cells resembled filopodia while those associated with AdE2ts were reminiscent of lamellipodia. Given the role of the small Rho GTPases Rac1 and RhoA or the Cdc42 GTPase in the formation of cellular protrusions and migration (namely, Rac1 association with lamellipodia and Cdc42 with filopodia [47, 49]), we examined both Ad- and AdE2ts-infected cells for increased activity of GTP-bound Rac1. Pulldown assays from three independent experiments revealed not only that GTP-bound Rac1 was not upregulated but also that it was, in fact, downregulated in AdE2ts-infected compared to control cells. Interestingly, this decrease in active Rac1 was correlated with a decrease in total Rac1 levels as measured by Western blot analysis and immunofluorescence microscopy (see Fig. S1 in the supplemental material). Neither the Cdc42 nor RhoA member of the family of small GTPases was regulated by E2 expression at the level of activity or total protein (data not shown). Targeting Rac1 in Ad- or AdE2ts-infected cells through expression of either a dominant negative Rac1 molecule (Rac1 N17) (described in reference 19) or two separate shRNAs (61) did not change cellular migratory behavior compared to that of control transduced cells (data not shown). Taken together, these findings suggest that E2 expression causes Rac1 inhibition in HPV-positive cells but that Rac1 inhibition by itself is not sufficient to stimulate motility. Finally, we analyzed the motility of AdE2ts-infected versus Ad-infected HeLa cells by 2D time-lapse video microscopy. Cells were infected as above, densely seeded as a colony, and allowed to migrate outwards for at least 12 h. A comparison of total average velocity per cell revealed again that AdE2ts-infected cells were increasingly motile compared to control infected cells (Fig. 1C). Taken together, these data demonstrate that E2-mediated E6/E7 repression in HPV-positive cervical cancer cells stimulates dynamic protrusions and nondirectional cellular motility.
E2 expression stimulates invasion but not epithelial-mesenchymal transition (EMT).
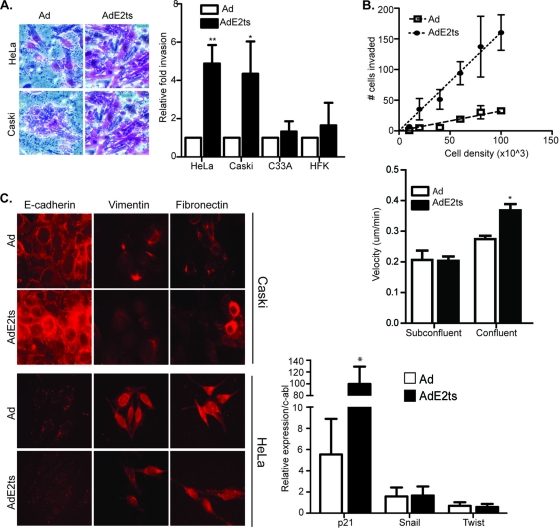
To determine whether E2-associated increased migration in HPV-positive cells translated into invasive potential, we measured the ability of E2-expressing cells to invade through extracellular matrix components in vitro. Matrigel invasion assays using AdE2ts-infected HeLa and Caski cells revealed increased invasion compared with Ad-infected control cells (Fig. 2A). Importantly, AdE2ts-infected HPV-negative C33A cervical cancer cells and primary human foreskin keratinocytes (HFKs) did not display increased invasion, demonstrating that this phenotype is specific to HPV-positive cells and thus likely associated with E6/E7 repression.
Fig. 2.
E2 expression stimulates invasion but not EMT. (A) HPV-positive HeLa and Caski cells as well as HPV-negative C33A and HFK cells infected with empty Ad or AdE2ts were subjected to Matrigel invasion assays. Images on the left show positive staining of invaded cells. Both HeLa and Caski AdE2ts-infected cells showed increased invasion compared to their proliferating controls, while HPV-negative cells did not respond to E2 expression. Error bars represent standard errors of the means taken from three or more independent experiments. (B) HeLa cells infected with AdE2ts or the Ad control were plated at increasing densities between 1 × 104 and 1 × 105 cells. Invaded cells were counted, and results are expressed as a regression graph. Increased invasion accompanied increased cell density in the senescent AdE2ts-infected cells with little effect in the Ad-infected control. Time-lapse video microscopy was carried out with using Ad- or AdE2ts-infected HeLa cells that were plated either at subconfluent or confluent densities. AdE2ts expression induced motility in confluent cells but not in subconfluent cells. (C) Both HeLa and Caski cells infected with Ad and AdE2ts were plated on coverslips at a confluent density and then analyzed for the expression of epithelial (E-cadherin) and mesenchymal markers (vimentin and fibronectin). HeLa cells infected with Ad or AdE2ts were analyzed for mRNA expression of the mesenchymal markers Twist and Snail; p21 was used as a positive marker of AdE2ts expression. Relative expression levels were normalized to expression of c-abl. Error bars represent standard errors of the means from three independent experiments.
Time-lapse video microscopy as well as a published report (17) suggested that E2-expressing cells tended to disengage from each other, indicating that cell-cell contact was important for the observed motility. Furthermore, cell-cell adhesion assays where control or E2-expressing cells were placed in single-cell suspension and allowed to attach to each other with gentle agitation showed that E2-expressing cells were deficient in forming adherent cell clusters (data not shown). To determine whether cell-cell contact was important for E2ts-associated invasion, Ad- and AdE2ts-infected cells were seeded in an invasion chamber at increasing densities (Fig. 2B). While increasing Ad control cell density stimulated migration in small increments, there was a significantly steeper increase in invasion with the corresponding E6/E7 repressed cells. Similarly, time-lapse video microscopy was conducted using Ad- and AdE2ts-infected cells at subconfluent and confluent densities. Migration was stimulated in E2-expressing cells only when the cells were confluent. These data suggest that E6/E7-repressed cells are particularly more motile and invasive when in contact with each other but do not rule out additional mediators that are unrelated to cell-cell contact.
Given a decrease in cell-cell attachment and increased invasion, both integral features of the EMT, we examined EMT induction in E2-expressing HeLa and Caski cells. EMT is an evolutionarily conserved process wherein epithelial cells break down contacts with neighboring cells and the extracellular matrix and acquire the motile and invasive phenotype of mesenchymal cells (50). EMT is closely linked to E-cadherin suppression (19), the upregulation of ECM components such as vimentin and fibronectin, and the transcriptional induction of mesenchymal markers including Twist and Snail. E2 expression did not decrease E-cadherin expression, which is already low in HeLa cells, or increase Snail and Twist transcription. Based on these markers, increased motility was therefore not associated with EMT (Fig. 2C).
HeLa cell migration is not a necessary consequence of cellular senescence but depends upon joint E6 and E7 repression.
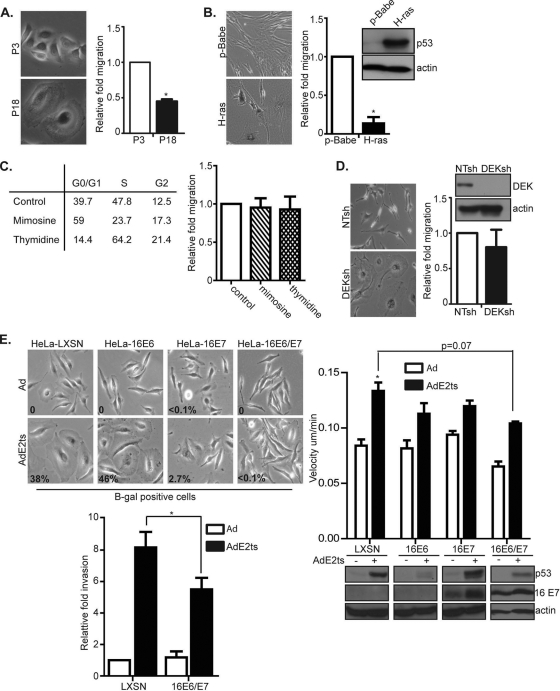
It is well established that E2 expression and E6/E7 repression in HPV-positive cancer cells result in cellular senescence. Since the above invasion phenotypes were equally specific to HPV-positive cells, we asked whether the two phenotypes might be linked. To determine whether senescence in general results in migration, human foreskin keratinocytes were passaged to replicative senescence and monitored for their motility. Similar to E6/E7-repressed HeLa cells, HFKs passaged to senescence showed positive SA-β-Gal activity as well as the flat-cell morphology which is a hallmark of senescence (Fig. 3A). Transwell migration assays, however, revealed that replicatively senescent cells were not increasingly motile compared to their proliferating controls (Fig. 3A). Furthermore, IMR-90 fibroblasts undergoing oncogene-induced senescence (OIS) by the expression of oncogenic H-ras showed a similar decrease in migration compared to proliferating controls (Fig. 3B). This indicated that migration was not generally associated with senescence (29).
Fig. 3.
HeLa cell migration is not a necessary consequence of cellular senescence but depends upon joint E6 and E7 repression. (A) HFKs passaged to replicative senescence and stained for SA-β-Gal activity showed that cells at later passage (passage 18 [P18]) were positively senescent and showed decreased migration compared to early-passage (P3) controls. Error bars indicate standard errors of the means taken from three independent experiments. (B) Similarly, decreased migration was observed with oncogene-induced senescent IMR-90 cells. Error bars indicate standard deviation of the mean from a representative experiment. Immunoblots from IMR-90 H-ras-expressing cells show increased p53 protein levels, indicative of senescence in this cell type. (C) HeLa cells were treated with mimosine or thymidine or left untreated and then subjected to cell cycle analyses. While the drugs were sufficient to arrest cells in their respective phases, no difference in migration patterns was observed. Error bars represent standard deviations. (D) HeLa cells were infected with lentiviral DEKsh RNA or a nontargeting (NTsh) control. DEKsh cells displayed the large, flattened morphology indicative of the senescence phenotype. However, DEKsh senescent cells did not show increased motility compared to proliferating NTsh controls. Error bars represent standard errors of the means from three independent experiments. Immunoblot analysis for DEK indicated that the knockdown vector significantly downregulated DEK protein levels. (E) HeLa cells were transduced with LXSN, LXSN 16E6, LXSN 16E7, or LXSN 16E6/E7 vector and then infected with Ad or AdE2ts. As expected, HeLa-LXSN and HeLa-16E6 cells were primarily senescent, as indicated by SA-β-Gal activity staining and quantitation. While all cells infected with AdE2ts were increasingly motile compared to control Ad-infected cells, only HeLa-LXSN cells coexpressing AdE2ts were significantly motile compared to controls. Invasion assays with LXSN- versus LXSN 16E6/E7-transduced cells further confirmed decreased motility upon E6/E7 reconstitution. Immunoblot analysis showed that each vector was effective in expressing its respective oncogene as HeLa-16E6 cells showed little or no expression of p53 protein levels, while HeLa-16E7-expressing and HeLa-16E6/E7-expressing cells showed increased 16E7 protein levels.
To next determine whether migration was a generic consequence of cell cycle arrest, which is intrinsic to the senescence phenotype, HeLa cells were chemically arrested at various stages of the cell cycle. G1 arrest was induced by mimosine, and S-phase arrest was induced by thymidine. Flow cytometry revealed that both chemicals caused cell cycle arrest in the expected phases of the cell cycle, but neither was sufficient to stimulate cell migration (Fig. 3C). Next, we determined whether migration was a generic consequence of HeLa cell senescence. The human DEK oncogene is reported to block cellular senescence in primary human foreskin keratinocytes and HeLa cells, and the knockdown of DEK causes senescence in HeLa cells (23, 67). HeLa cells were transduced with a lentivirus expressing DEK-specific shRNA to induce senescence and compared to cells transduced with nontargeting shRNA. Both cell populations were subsequently used for migration assays. Unlike their E2-expressing counterparts, senescent DEK knockdown cells did not migrate at increased rates (Fig. 3D). Taken together, these data suggest that neither cell cycle arrest nor cellular senescence in HeLa cells is sufficient for stimulating cellular motility.
Having established that E2 expression leads to increased motility in HPV-positive HeLa cells, we next determined whether E6 or E7 repression was required. An approach similar to that previously published by DeFilippis et al. was utilized (6). Briefly, HPV18 E6/E7 expression was inhibited by AdE2ts in HeLa cells while either HPV16 E6, E7, or E6/E7 was simultaneously expressed retrovirally. Since E2 does not regulate retroviral oncogene expression, the net result of this approach is the individual repression of E7 in HPV16 E6-expressing cells, the individual repression of E6 in HPV16 E7-expressing cells, or no repression in the presence HPV16 E6/E7-expressing cells. Western blot analysis verified the overexpression of the exogenous oncogenes; p53 protein levels were decreased—albeit not eliminated—in HPV16 E6- and HPV16 E6/E7-overexpressing cells while HPV16 E7 protein levels were upregulated in HPV16 E7- and HPV16 E6/E7-overexpressing cells (Fig. 3E). Of note, HPV16 E7 protein levels were further upregulated in cells that coexpressed E7 with E2 (Fig. 3E and 4C). These data may be explained by a published report wherein high-risk E2 protein bound and stabilized HPV16 E7 (14). As previously shown, the reexpression of HPV16 E7 or E6/E7 was sufficient to counteract E2 senescence in HPV-positive cells (6) (Fig. 3E), whereas HeLa cells expressing HPV16 E6 (HeLa-16E6) displayed little senescence and eventual apoptosis. Using time-lapse video microscopy, we found that the reexpression of either E6, E7, or E6/E7 inhibited E2 motility, thus indicating that cellular migration requires the repression of both E6 and E7 by E2. Furthermore, Matrigel invasion assays showed that E2-expressing, HeLa-16 E6/E7 reconstituted cells invaded to a significantly lesser extent than E2-expressing control cells (Fig. 3E). It is possible that remaining trends toward increased migration in E6- or E7-expressing cells are due to insufficient reconstitution of the oncogenes in a proportion of the polyclonal cell population. This experimental caveat is obvious in the E6/E7-reconstituted, E2-expressing cells which exhibit remaining p53 induction and thus likely insufficient E6 expression. Incomplete repression might also reflect independent E2-specific activities which contribute to the motility phenotype, as shown in Fig. 4.
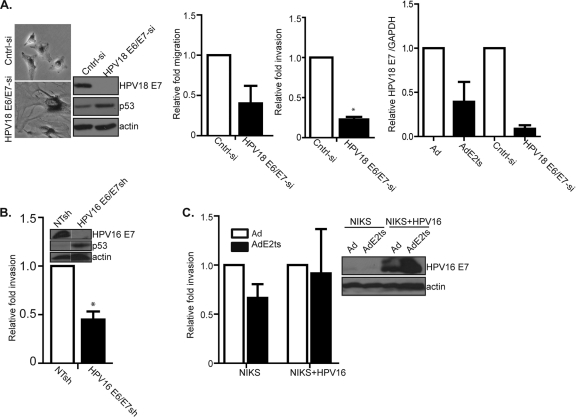
Fig. 4.
E2 expression, but not E6/E7 knockdown, stimulates migration in HPV-positive cancer cells (A) HeLa cells were transfected with an siRNA targeting HPV18 E6/E7. This approach was effective in repressing the oncogenes and in inducing cellular senescence, as indicated by decreased HPV18 E7 and increased p53 protein levels, SA-β-Gal positivity, and the typical flattened cellular morphology. Repression of E6/E7 by siRNA resulted in decreased migration and invasion. Relative HPV18 E7 mRNA levels in E2 versus E6/E7 knockdown cells confirmed repression in each case. (B) Targeting HPV16 E6/E7 by shRNA expression in Caski cells also resulted in decreased invasion. (C) Near-diploid immortalized keratinocytes that form skin (NIKS) as well as NIKS-HPV16 were infected with AdE2ts. Neither E7 repression nor increased invasion was observed.
Given that joint E6 and E7 repression was required for significant E2-induced migration, we next sought to identify migration-associated genes that are controlled by E2 as well as E6/E7 and are thus potential mediators of this phenotype. We carried out a meta-analysis of migration-associated genes using microarray studies from several publications (23, 26, 50, 55, 62). Several candidate genes upregulated by HPV E2 and/or downregulated by HPV E6/E7 were selected for qRT-PCR analysis in the E2ts system. A list of genes along with their relative expression levels in these experiments is shown in Table 2. Individual knockdowns of Notch 1, semaphorin 3F, and plexin A1 did not repress migration (data not shown), thus indicating that other mediators are responsible or that migration requires the activities of multiple cellular E6/E7 targets.
Table 2.
E6/E7 repression stimulates the expression of migration-associated genes
| Gene symbol | Description | Relative fold inductiona |
|---|---|---|
| NME1 | Nonmetastatic cells 1 | + |
| Plat | Plasminogen activator tissue | + |
| GSN | Gelsolin | + |
| PTP4A1 | Protein tyrosine phosphate 4A | + |
| ADAM17 | ADAM metallopeptidase 17 | + |
| CDH3 | Cadherin 3 | + |
| NOTCH1 | Notch1 | + |
| IGFBP 3 | Insulin GF binding protein 3 | + |
| FGFR1 | Fibroblast GF receptor1 | + |
| PFN2 | Profilin 2 | + |
| ACTN1 | Actinin alpha-1 | + |
| FN1 | Fibronectin 1 | + |
| S100A9 | S100 calcium binding protein 1 | + |
| PGF | Placental growth factor | ++ |
| JAG1 | Jagged 1 | ++ |
| SEMA5A | Semaphorin 5A | ++ |
| BMP4 | Bone morphogenic protein 4 | ++ |
| FGF2 | Fibroblast growth factor2 | ++ |
| ITGB1 | Integrin-beta-1 | ++ |
| PTGS2 | Prostaglandin endoperoxide synthase 2 | +++ |
| SERPINE 1 | Serpin peptidase inhibitor clade 1 | +++ |
| SERPINE 5 | Serpin peptidase inhibitor clade 5 | +++ |
| IL-6 | Interleukin-6 | +++ |
Gene expression levels relative to EEFA1 using Ad and AdE2ts mRNAs from three independent experiments were measured by real-time PCR using the primer sequences listed in Table 1. +, fold change greater than 0 but less than 2; ++, fold change equal to or greater than 2 but less than 4; +++, fold change equal to or greater than 4.
E2 expression, but not E6/E7 knockdown, stimulates migration in HPV-positive cancer cells.
Our data showed that E2 expression stimulated cellular motility and that E6/E7 repression contributed to this phenotype. In contrast, E6/E7 repression by RNA interference was recently shown to repress motility (4). In order to determine whether E2-specific activities in addition to E6/E7 suppression are important, we knocked down the HPV18 oncogenes in HeLa cells via siRNA transfection (Fig. 4A). As expected, this approach resulted in E7 repression and p53 upregulation, together with cellular flattening and positive staining for SA-β-Gal activity. In line with recently published data, migration and invasion were repressed by E6/E7 knockdown. Direct detection of E7 mRNA levels further verified that oncogene repression by siRNA was at least as efficient as, if not more efficient than, that induced by E2 expression. Similar data were obtained in Caski cells transduced with a lentiviral vector delivering E6/E7-specific shRNA compared to nontargeting shRNA (Fig. 4B). Taken together, we concluded that oncogene repression by E2 is not sufficient for motility and that other E2 activities must be involved. Since BPV1 E2 protein does not appear to transcriptionally regulate cellular targets (23), such activities are likely to involve posttranscriptional mechanisms.
HeLa and Caski cells harbor integrated high-risk HPV DNA. In order to determine whether E2 was capable of stimulating migration in cells which maintain viral genomes episomally, we used near-diploid immortalized keratinocytes that form skin (NIKS), either positive or negative for HPV16 episomes. The cells were infected with either Ad or AdE2ts virus, followed by invasion assays. E2 expression did not decrease E7 expression in HPV16-positive NIKS (Fig. 4C), in line with published data from Bechtold et al. (2). Furthermore, E2 expression did not stimulate cellular invasion. Together, these data support the notion that E2-driven motility in HPV-positive cancer cells requires E6/E7 suppression in conjunction with specific E2 activities.
Senescence induction in AdE2ts-infected HeLa cells is reversible during the first 2 days of continuous E2 expression and becomes irreversible by day 3 (62). To determine whether E2-driven invasion is a reversible phenotype, we carried out timed E2 inactivation experiments similar to previous senescence experiments (62). Cells were infected with Ad or AdE2ts at the permissive temperature of 32°C and shifted to the nonpermissive temperature on either day 2 or on day 3, followed by invasion assays on day 4. Control cells were kept at the permissive temperature throughout. The results show that E2-associated invasion is a reversible phenotype at least up until day 3 post-E2 activation (see Fig. S2 in the supplemental material).
E2-expressing cells stimulate the migration of neighboring cancer cells independent of secreted factors.
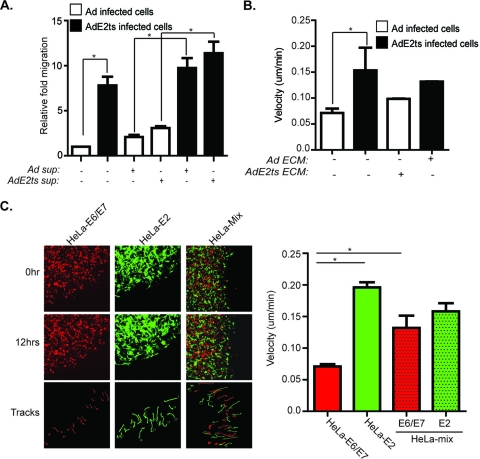
Based on our findings that E2-expressing cells stimulate motility, we explored the functional contribution of soluble and matrix factors in E2-associated migration. To test the role of soluble secreted factors, conditioned medium was transferred from the AdE2ts-expressing cells onto the empty Ad control HeLa cells and vice versa, and then transwell migration assays were conducted (Fig. 5A). The addition of AdE2ts medium to Ad-infected HeLa cells did not significantly stimulate their migration above baseline levels. Conversely, the addition of control Ad supernatant onto the E6/E7-repressed cells did not decrease their migration. While these data do not rule out contributions of soluble factors, they implicate cell-intrinsic properties as the primary determinant of the observed E6/E7-repressed cellular motility. To examine possible contributions of the underlying extracellular matrix (ECM), E2-expressing HeLa cells were treated with ammonium hydroxide which removes the cells while leaving the native endogenous ECM intact (31). Control and AdE2ts-infected cells were then reciprocally transferred onto ECM, and 2D time-lapse video microscopy was conducted. ECM components of E2ts-infected cells did not stimulate the migration of control infected cells, and ECM of Ad-infected cells did not decrease E2ts-induced motility (Fig. 5B). Finally, the concomitant exposure of control infected cells to E6/E7-repressed ECM plus supernatant and exposure of E6/E7-repressed cells to control ECM plus supernatant did not change cellular migration rates (data not shown), thus further emphasizing the importance of cell-intrinsic mechanisms (Fig. 2B).
Fig. 5.
E2-expressing cells stimulate the migration of neighboring cancer cells independent of secreted factors. (A) Cell-extrinsic components are not sufficient to stimulate migration in E2-expressing cells. Supernatant taken from AdE2ts-infected cells was transferred onto Ad-infected cells in the top chamber of the transwell insert. The transfer of supernatant from these AdE2ts cells was not sufficient to stimulate the migration of control cells significantly. Conversely, supernatant from control Ad-infected cells transferred onto AdE2ts-infected cells was not sufficient to significantly reduce cell migration of AdE2ts cells. (B) A total of 1 × 105 Ad control cells were plated on the ECM of AdE2ts-infected cells (see Materials and Methods). Conversely, AdE2ts-infected cells were plated onto the ECM of control cells. Quantitation after time lapse showed that there was no significant difference in migration patterns when Ad cells were transferred onto the ECM of AdE2ts cells and vice versa. (C) E2 expression confers migration to nontargeted adjacent HeLa cells. A total of 1 × 105 parental HeLa cells (HeLa-E6/E7) labeled with DsRed or those infected with AdE2ts (HeLa-E2) which are GFP positive as well as 50:50 mix of both were subjected to time-lapse video microscopy. Images show cells used for time lapse at the beginning (0 h) and end (12 h) as well outlines of cellular tracks made throughout the course of the time lapse. ImageJ quantitation of velocity showed that HeLa-E2 cells migrated as expected. Additionally, HeLa-E6/E7-positive cancer cells became increasingly motile when mixed with HeLa-E2 cells. Error bars represent standard deviations of the mean from at least 20 cells tracked per field. Three or more fields of cells were tracked per group.
Targeting the HPV oncogenes is a promising approach to cancer treatment, given that senescence is a barrier to tumor development once it is irreversibly established. The observed increased motility of E2-expressing cells would therefore appear irrelevant to cancer spread. However, senescence irreversibility depends upon prolonged E6/E7 repression (63), and, additionally, not all cells in a given tumor are expected to be targeted even under ideal treatment circumstances. To determine the effect of E2-expressing HeLa cells on the motility of neighboring nontargeted cells, E2-expressing green fluorescent protein (GFP)-positive cells were mixed with DsRed-marked HeLa cells to observe the motility of each within a 50:50 mix (Fig. 5C). Time-lapse video microscopy revealed, as expected, that E2-expressing cells were more motile than their DsRed-marked, E6/E7-proficient counterparts (Fig. 5C). Interestingly, E2-expressing HeLa cells not only were themselves motile but also stimulated the motility of adjacent nontargeted HeLa cells (Fig. 5C). This suggests that oncogene repression via E2 in a cervical cancer environment stimulates the migration of both targeted and adjacent nontargeted cells, thus potentially contributing to a locally invasive environment.
DISCUSSION
The advent of new preventative HPV vaccines promises to reduce cervical cancer incidence significantly in the future (36, 51). However, these vaccines are ineffective for the treatment of existing infections and will therefore not impact the HPV-related disease burden for years to come. Alternative treatments are needed, which might include therapeutic vaccines or gene therapy approaches. It is well established that the HPV oncogenes E6 and E7 are a necessary requirement for disease development and progression (38, 40). As such, proof of concept for oncogene repression and resulting cell death has been published by multiple laboratories in vitro and in vivo (13, 18, 20, 45, 54) and has led to ongoing clinical trials wherein E6 and E7 are targeted by several vaccine strategies in patients affected with HPV-related malignancies, including recurrent cervical cancer (35). With regard to E2, its effectiveness in targeting E6 and E7 has been demonstrated in vitro (17, 63), and clinical trials carried out in Mexico have shown promising results in the treatment of high- and low-grade cervical intraepithelial neoplasia (5, 15).
We report here that targeting E6/E7 by E2, but not by RNA interference, stimulated motility and invasion. Our data confirm a recent report wherein E6/E7 knockdown repressed migration (4), while further extending a previous published report wherein E2-expressing, densely packed HeLa cells dispersed from each other (17). Surprisingly, however, E2-expressing cells not only were themselves motile but also conferred motility to neighboring cells. The underlying mechanism of E2-driven motility was independent of chemotaxis, dependent upon cell-cell contact, and neither mediated by EMT nor accompanied by a reduction in EMT. In fact, published data suggested a repression of EMT and invasion should be expected in response to E2 expression and subsequent E6/E7 repression. First, early studies have linked E-cadherin suppression to the invasive properties of HPV16 E6/E7 immortalized keratinocytes (65). Second, a recent publication has implicated E6 and E7 independently in causing EMT-like phenotypes in human keratinocytes (21). This was in line with earlier findings of EMT features in a cellular model of the HPV-positive precancerous stage (16). The marked discrepancy in phenotypes between E6/E7 repression and E2 expression is suggestive of E2-specific molecular activities that work in conjunction with E6/E7 repression. Regardless of the underlying mechanism, however, our data indicate possible risks associated with E2-based treatments of cervical cancer and a need for long term follow-up of tumor regression and recurrence in such patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R01 CA102357 and CA116316.
We thank Christopher Wylie and Marie-Dominique Filippi for insightful comments on the manuscript. We also thank Tomoyasu Higashimuto and James Lessard for reagents.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Au W. W., Abdou-Salama S., Al-Hendy A. 2007. Inhibition of growth of cervical cancer cells using a dominant negative estrogen receptor gene. Gynecol. Oncol. 104:276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bechtold V., Beard P., Raj K. 2003. Human papillomavirus type 16 E2 protein has no effect on transcription from episomal viral DNA. J. Virol. 77:2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butz K., Denk C., Ullmann A., Scheffner M., Hoppe-Seyler F. 2000. Induction of apoptosis in human papillomavirus positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc. Natl. Acad. Sci. U. S. A. 97:6693–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L., et al. 11 January 2011, posting date Down-regulation of HPV18 E6, E7, or VEGF expression attenuates malignant biological behavior of human cervical cancer cells. Med. Oncol. [Epub ahead of print.] doi:10.1007/s12032-010-9690-1 [DOI] [PubMed] [Google Scholar]
- 5. Corona Gutierrez C. M., et al. 2004. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum. Gene Ther. 15:421–431 [DOI] [PubMed] [Google Scholar]
- 6. DeFilippis R. A., Goodwin E. C., Wu L., DiMaio D. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 77:1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desaintes C., Demeret C., Goyat S., Yaniv M., Thierry F. 1997. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 16:504–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiMaio D., Settleman J. 1988. Bovine papillomavirus mutant temperature sensitive for transformation, replication and transactivation. EMBO J. 7:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dimri G. P., et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U. S. A. 92:9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowhanick J. J., McBride A. A., Howley P. M. 1995. Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol. 69:7791–7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dyson N., Howley P. M., Munger K., Harlow E. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934–937 [DOI] [PubMed] [Google Scholar]
- 12. Flores E. R., Allen-Hoffmann B. L., Lee D., Sattler C. A., Lambert P. F. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344–354 [DOI] [PubMed] [Google Scholar]
- 13. Fujii T., et al. 2006. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. Int. J. Oncol. 29:541–548 [PubMed] [Google Scholar]
- 14. Gammoh N., Grm H. S., Massimi P., Banks L. 2006. Regulation of human papillomavirus type 16 E7 activity through direct protein interaction with the E2 transcriptional activator. J. Virol. 80:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Hernandez E., et al. 2006. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 13:592–597 [DOI] [PubMed] [Google Scholar]
- 16. Geiger T., Sabanay H., Kravchenko-Balasha N., Geiger B., Levitzki A. 2008. Anomalous features of EMT during keratinocyte transformation. PLoS One 3:e1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodwin E. C., et al. 2000. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 97:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gu W., et al. 2006. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 13:1023–1032 [DOI] [PubMed] [Google Scholar]
- 19. Guo F., Zheng Y. 2004. Involvement of Rho family GTPases in p19Arf- and p53-mediated proliferation of primary mouse embryonic fibroblasts. Mol. Cell Biol. 24:1426–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall A. H., Alexander K. A. 2003. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J. Virol. 77:6066–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hellner K., Mar J., Fang F., Quackenbush J., Munger K. 2009. HPV16 E7 oncogene expression in normal human epithelial cells causes molecular changes indicative of an epithelial to mesenchymal transition. Virology 391:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helt A. M., Galloway D. A. 2003. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis 24:159–169 [DOI] [PubMed] [Google Scholar]
- 23. Johung K., Goodwin E. C., DiMaio D. 2007. Human papillomavirus E7 repression in cervical carcinoma cells initiates a transcriptional cascade driven by the retinoblastoma family, resulting in senescence. J. Virol. 81:2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones D. L., Alani R. M., Munger K. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jonson A. L., Rogers L. M., Ramakrishnan S., Downs L. S., Jr 2008. Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention in a mouse model of cervical cancer. Gynecol. Oncol. 111:356–364 [DOI] [PubMed] [Google Scholar]
- 26. Karstensen B., et al. 2006. Gene expression profiles reveal an upregulation of E2F and downregulation of interferon targets by HPV18 but no changes between keratinocytes with integrated or episomal viral genomes. Virology 353:200–209 [DOI] [PubMed] [Google Scholar]
- 27. Kiyono T., et al. 1997. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. U. S. A. 94:11612–11616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klingelhutz A. J., Foster S. A., McDougall J. K. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79–82 [DOI] [PubMed] [Google Scholar]
- 29. Kondo H., Yonezawa Y. 1992. Changes in the migratory ability of human lung and skin fibroblasts during in vitro aging and in vivo cellular senescence. Mech. Ageing Dev. 63:223–233 [DOI] [PubMed] [Google Scholar]
- 30. Lea J. S., et al. 2007. Silencing of HPV 18 oncoproteins With RNA interference causes growth inhibition of cervical cancer cells. Reprod. Sci. 14:20–28 [DOI] [PubMed] [Google Scholar]
- 31. Le Beyec J., et al. 1997. A complete epithelial organization of Caco-2 cells induces I-FABP and potentializes apolipoprotein gene expression. Exp. Cell Res. 236:311–320 [DOI] [PubMed] [Google Scholar]
- 32. Lee S. S., Weiss R. S., Javier R. T. 1997. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. U. S. A. 94:6670–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X., et al. 2009. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc. Natl. Acad. Sci. U. S. A. 106:18780–18785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lleonart M. E., Artero-Castro A., Kondoh H. 2009. Senescence induction; a possible cancer therapy. Mol. Cancer 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma B., Yijie Xu, Chien-Fu Hu ng, Wu T.-C. 2010. HPV and therapeutic vaccines: where are we in 2010? Curr. Cancer Ther. Rev. 6:81–103 [Google Scholar]
- 36. McCormack P. L., Joura E. A. 2010. Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (Gardasil): a review of its use in the prevention of premalignant genital lesions, genital cancer and genital warts in women. Drugs 70:2449–2474 [DOI] [PubMed] [Google Scholar]
- 37. McLaughlin-Drubin M. E., Munger K. 2009. The human papillomavirus E7 oncoprotein. Virology 384:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moody C. A., Laimins L. A. 2010. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer 10:550–560 [DOI] [PubMed] [Google Scholar]
- 39. Moon M. S., et al. 2001. Effect of BPV1 E2-mediated inhibition of E6/E7 expression in HPV16-positive cervical carcinoma cells. Gynecol. Oncol. 80:168–175 [DOI] [PubMed] [Google Scholar]
- 40. Munger K. 2002. The role of human papillomaviruses in human cancers. Front. Biosci. 7:d641–d649 [DOI] [PubMed] [Google Scholar]
- 41. Nakagawa S., Huibregtse J. M. 2000. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 20:8244–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakahara T., Peh W. L., Doorbar J., Lee D., Lambert P. F. 2005. Human papillomavirus type 16 E1^E4 contributes to multiple facets of the papillomavirus life cycle. J. Virol. 79:13150–13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nauenburg S., Zwerschke W., Jansen-Durr P. 2001. Induction of apoptosis in cervical carcinoma cells by peptide aptamers that bind to the HPV-16 E7 oncoprotein. FASEB J. 15:592–594 [DOI] [PubMed] [Google Scholar]
- 44. Nishimura A., et al. 2000. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol. 74:3752–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niu X. Y., Peng Z. L., Duan W. Q., Wang H., Wang P. 2006. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int. J. Gynecol. Cancer. 16:743–751 [DOI] [PubMed] [Google Scholar]
- 46. Psyrri A., et al. 2004. Role of the retinoblastoma pathway in senescence triggered by repression of the human papillomavirus E7 protein in cervical carcinoma cells. Cancer Res. 64:3079–3086 [DOI] [PubMed] [Google Scholar]
- 47. Raftopoulou M., Hall A. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23–32 [DOI] [PubMed] [Google Scholar]
- 48. Rampias T., Sasaki C., Weinberger P., Psyrri A. 2009. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J. Natl. Cancer Inst. 101:412–423 [DOI] [PubMed] [Google Scholar]
- 49. Ridley A. J. 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11:471–477 [DOI] [PubMed] [Google Scholar]
- 50. Ruutu M., Peitsaro P., Johansson B., Syrjanen S. 2002. Transcriptional profiling of a human papillomavirus 33-positive squamous epithelial cell line which acquired a selective growth advantage after viral integration. Int. J. Cancer 100:318–326 [DOI] [PubMed] [Google Scholar]
- 51. Schauner S., Lyon C. 2010. Bivalent HPV recombinant vaccine (Cervarix) for the prevention of cervical cancer. Am. Fam. Physician 82:1541–1542 [PubMed] [Google Scholar]
- 52. Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495–505 [DOI] [PubMed] [Google Scholar]
- 53. Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129–1136 [DOI] [PubMed] [Google Scholar]
- 54. Tan T. M., Ting R. C. 1995. In vitro and in vivo inhibition of human papillomavirus type 16 E6 and E7 genes. Cancer Res. 55:4599–4605 [PubMed] [Google Scholar]
- 55. Thierry F., et al. 2004. A genomic approach reveals a novel mitotic pathway in papillomavirus carcinogenesis. Cancer Res. 64:895–903 [DOI] [PubMed] [Google Scholar]
- 56. Thierry F., Yaniv M. 1987. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 6:3391–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thomas M., et al. 2008. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 27:7018–7030 [DOI] [PubMed] [Google Scholar]
- 58. Veldman T., Horikawa I., Barrett J. C., Schlegel R. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Knebel Doeberitz M., Rittmuller C., zur Hausen H., Durst M. 1992. Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6–E7 anti-sense RNA. Int. J. Cancer 51:831–834 [DOI] [PubMed] [Google Scholar]
- 60. Wang W., et al. 2010. Selective targeting of HPV-16 E6/E7 in cervical cancer cells with a potent oncolytic adenovirus and its enhanced effect with radiotherapy in vitro and vivo. Cancer Lett. 291:67–75 [DOI] [PubMed] [Google Scholar]
- 61. Wei J., et al. 2008. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell 13:483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wells S. I., et al. 2003. Transcriptome signature of irreversible senescence in human papillomavirus-positive cervical cancer cells. Proc. Natl. Acad. Sci. U. S. A. 100:7093–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wells S. I., et al. 2000. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21(CIP)-dependent pathways. EMBO J. 19:5762–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Werness B. A., Levine A. J., Howley P. M. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76–79 [DOI] [PubMed] [Google Scholar]
- 65. Wilding J., et al. 1996. E-cadherin transfection down-regulates the epidermal growth factor receptor and reverses the invasive phenotype of human papilloma virus-transfected keratinocytes. Cancer Res. 56:5285–5292 [PubMed] [Google Scholar]
- 66. Wise-Draper T. M., et al. 2006. Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol. Cell. Biol. 26:7506–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wise-Draper T. M., et al. 2005. The human DEK proto-oncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7. J. Virol. 79:14309–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.