Abstract
In response to viral infection, reactive oxygen species (ROS) mediate innate immune signaling or generate danger signals to activate immune cells. The mechanisms of virally induced ROS are poorly defined, however. We demonstrate that ROS are produced within minutes of adenovirus type 5 (Ad5) infection of macrophages and that oxidative stress supports Ad5-induced cytokine secretion. We show that short hairpin RNA (shRNA) knockdown of TLR9 has no effect on ROS production despite observed decreases in Ad-induced cytokine secretion. A major source of ROS in macrophages is NADPH oxidase. However, shRNA knockdown of the NADPH oxidase subunit NOX2 does not attenuate Ad-induced ROS. Induction of ROS is not observed in cells infected with a temperature-sensitive mutant of Ad2, ts1, which is defective in endosomal membrane penetration during cell entry. Further, Ad5, but not ts1, induces the release of lysosomal cathepsin B into the cytoplasm of infected cells. In agreement with this finding, we observe a loss of mitochondrial membrane potential upon Ad infection which requires Ad endosomal membrane penetration and cathepsin B activity. Overexpression of Bcl-2 attenuates Ad5-induced ROS, further supporting the role for mitochondrial membrane destabilization as the source of ROS in response to Ad5 infection. Together, these data suggest that ROS produced in response to Ad5 infection depends on the virally induced endosomal membrane rupture to release lysosomal cathepsins. Furthermore, the release of cathepsins leads to mitochondrial membrane disruption and thus the release of ROS from the mitochondria.
INTRODUCTION
Adenovirus (Ad) interaction with macrophages or dendritic cells elicits a rapid proinflammatory response upon administration of Ad vectors for use in gene therapy and genetic vaccination (27, 37, 51). As sentinels of the immune system, resident macrophages such as alveolar macrophages or Kupffer cells respond to pathogens by secreting cytokines and chemokines to affect surrounding tissue and recruit additional immune cells to the site of infection. Additionally, these macrophages produce hydrolytic enzymes, membrane-disrupting peptides, and reactive oxygen and nitrogen species to destroy extracellular and phagocytosed cellular pathogens (51). These responses are less successful against viruses, which survive by gaining access to intracellular compartments to replicate. More recently, however, several macrophage effectors originally described for responses to extracellular bacteria have been shown to possess antiviral properties (46). In particular, the production of reactive oxygen species (ROS) has been shown to contribute to intracellular proinflammatory signaling in response to viral infection (15). Key questions remain regarding the contributions of ROS to antiviral and proinflammatory signaling cascades. Additionally, the source and stimuli leading to ROS production during viral infection have not been completely described.
As nonenveloped, double-stranded DNA (dsDNA) viruses, Ads trigger several innate signaling pathways during cell entry (27). Human adenovirus type 5 (Ad5) attaches to cells via the coxsackievirus and adenovirus receptor (CAR) (34). Secondary engagement of αv integrins by the Ad5 penton base triggers clathrin-mediated endocytosis (43). Within endosomes, partial uncoating of the Ad5 capsid leads to exposure of the viral dsDNA genome, which can be recognized by Toll-like receptor 9 (TLR9) (1, 8, 16, 45). Recognition of Ad5 DNA by TLR9 leads to the activation of NF-κB and production of several proinflammatory cytokines (1, 8, 45). Uncoating of the Ad capsid is required for the virus to penetrate endosomal membranes, a process facilitated by the internal membrane-lytic capsid protein VI (23, 26, 44). Within the cytoplasm, the viral DNA is recognized by an unidentified pattern recognition receptor leading to the activation of interferon (IFN) regulatory factor 3 (IRF-3) and production of beta interferon (IFN-β) (29, 30). More recent studies show that Ad5 activation of the NLRP3 inflammasome contributes to the proinflammatory response to incoming virions (4, 28). We have demonstrated that Ad5 rupture of endosomal membranes is a danger signal recognized by NLRP3 (3, 4). Furthermore, this danger signal depends on release of lysosomal cathepsin B into the cytoplasm during viral membrane rupture as well as the production of ROS. Additionally, activation of stress pathways such as the mitogen-activated protein kinase (MAPK) pathways during Ad cell entry has been reported, although the mechanisms that activate these pathways remain unknown (1, 5, 42).
Current studies demonstrate a role for ROS in the activation of the innate immune response to pathogens. Several of these studies implicate a role for NADPH oxidase in this ROS-dependent response. For instance, ROS activation of IRF-3 by TRIF-dependent signaling via TLR4 has been previously reported (10), In this case, TRIF-dependent activation of the NADPH oxidase subunit NOX4 leads to the production of ROS, which activates an ASK1/p38 MAPK pathway leading to IRF-3 activation (10). More recently, studies have shown that ROS produced by phagosomal NADPH oxidase contributes to augmented RIG-I-dependent IRF-3 activation (38). These data are in line with previous observations that many viruses that activate RIG-I also induce ROS production during infection (15, 38).
Additionally, RIG-I-dependent IRF-3 activation is thought to be enhanced by mitochondrial ROS. Current research demonstrates that defects in autophagy enhance IRF-3 activation via the RIG-I pathway due to elevated ROS associated with accumulation of dysfunctional mitochondria (40). Furthermore, pharmacological induction of mitochondrial oxidative stress can augment RIG-I-dependent IRF-3 activation (40). One recent study implicates a role for mitochondrial ROS in the activation of the NLRP3 inflammasome (49). These findings underscore the importance of ROS in activating the innate immune response. Understanding the mechanism by which ROS are produced during infection is key to understanding the interplay between this endogenous danger signal and the innate immune response.
We have found that Ad5 infection induces ROS production by human macrophage-like THP-1 cells. Furthermore, we have demonstrated that Ad5-induced ROS depends on viral penetration of endosomal membranes. Although recognition of Ad5 DNA by TLR9 contributes to the production of proinflammatory cytokines, stable short hairpin RNA (shRNA) knockdown of TLR9 does not affect Ad5-induced ROS (8). While NADPH oxidase is known to contribute to ROS production in the context of infection, stable knockdown of the NADPH oxidase subunit, gp91phox, does not attenuate Ad5-induced ROS. On the other hand, Ad5 infection leads to mitochondrial membrane disruption that depends on Ad5-mediated endosomal membrane rupture. Preventing this mitochondrial membrane instability by bcl-2 overexpression attenuates Ad5-induced ROS. Taken together, these data suggest that the mitochondria serve as a source for ROS generated during Ad5 infection. We are the first to demonstrate a role for mitochondrial ROS in activating the NLRP3 inflammasome upon viral infection.
Our studies also demonstrate that Ad5-induced membrane rupture leads to release of lysosomal cathepsins into the cytoplasm of infected cells. In concordance with previous data implicating cathepsin in mitochondrial membrane disruption, we have found that mitochondrial membrane disruption depends on the catalytic activity of lysosomal cathepsin B, released into the cytoplasm when Ad5 ruptures endosomal membranes. Furthermore, cathepsin activity is required for Ad5-induced ROS. Together, these data demonstrate that Ad5 rupture of endolysosomal membranes during cell entry triggers cathepsin B release that in turn facilitates mitochondrial membrane disruption that generates ROS during infection. Furthermore, these ROS are crucial to the inflammatory response to the virus. Our findings shed new light on the mechanisms of Ad5-induced inflammation and highlight potential mechanisms to attenuate inflammatory responses to Ad5 vectors for use in gene therapy.
MATERIALS AND METHODS
Cell lines and reagents.
The human monocyte cell line THP-1 was obtained from ATCC. Phorbol-12-myristate-13-acetate (PMA) was purchased from Sigma-Aldrich. RPMI 1640 was purchased from Mediatech. 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA; catalog no. D399) was purchased from Invitrogen. PAM3CSK4 was purchased from InvivoGen. The interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! kit was obtained from eBioscience. CA074me was purchased from EMD Biosciences. All horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Biomeda, Ltd. All other reagents were from Fisher.
Virus preparation.
An E1/E3-deleted adenovirus expressing enhanced green fluorescent protein (EGFP) under a cytomegalovirus (CMV) promoter, Ad5gfp, and a temperature-sensitive adenovirus, Ad2ts1, were propagated in HEK293 cells and subjected to cesium chloride gradient centrifugation twice to purify the virus. The ts1 virus was propagated at the nonpermissive temperature, 39.5°C, to generate mutant virus unable to penetrate the endosomal membrane. A Bradford assay determined the viral concentration as 1 μg of protein corresponding to 4 × 109 viral particles. To determine viral titer, the viruses were serially diluted on HeLa cells, and flow cytometry was used to quantify GFP expression. Of the viruses used in this study, specific infectivity ranged from 100 to 200 viral particles per GFP-transducing unit (GTU).
Cell culture.
THP-1 cells were maintained in RPMI 1640 supplemented with 1 mg/ml streptomycin, 100 IU/ml penicillin, 0.25 μg/ml amphotericin B, nonessential amino acids, 2 mM glutamine, 10 mM HEPES buffer, and 1 mM sodium pyruvate. For all experiments, THP-1 cells were differentiated to macrophage-like cells by overnight stimulation with 100 nM phorbol-12-myristate-13-acetate (PMA).
Stable cell lines.
Lentiviral vectors were employed to generate TLR9 knockdown THP-1 cells (THP-1-TLR9KD), gp91phox knockdown THP-1 cells (THP-1-gp91phoxKD), or THP-1 cells expressing control shRNA (THP-1-control). Lentiviral vectors with an LKO.1 backbone expressing shRNA for TLR9 (Open Biosystems, catalog no. RHS3979-9624075, clone identification [ID] TRCN0000056891), shRNA for gp91phox (Open Biosystems, catalog no. RHS3979-97052978; clone ID TRCN0000064591), or control shRNA recommended by the RNAi (i.e., RNA interference) Consortium (AddGene no. 10879) were generated by cotransfection of 293T cells with the packaging plasmids pHEF-VSVG (catalog no. 4693; NIH AIDS Research and Reagent Program), pRSV-REV (Addgene no. 12253), and pMDLg/pRRE (Addgene no. 12251). Supernatants containing these recombinant lentiviral vectors were collected 48 h posttransfection and used to transduce THP-1 cells by spinoculation. Positive transductants were selected with puromycin. THP-1 cells stably overexpressing Bcl-2 were generated similiarly by generating transducing particles carrying either a pBMN-I-GFP vector (Addgene no. 1736) or pBMN-I-GFP containing the Bcl-2 gene.
ROS assay.
All THP-1 cells were plated at 200,000 cells per well in a black 96-well plate (Costar) in RPMI with 10% fetal bovine serum (FBS) containing 100 nM PMA to stimulate differentiation to macrophage-like cells. Medium was replaced the following morning, and cells were rested for 2 days. Following 3 h of serum starvation, cells were incubated with the ROS-sensitive fluorophore H2DCFDA (DCF; Invitrogen) at 10 μM for 30 min followed by two washes with 1× phosphate-buffered saline (PBS). If pretreated with drug, cells were incubated with the drug for 1 h following DCF incubation. Virus was added to wells when necessary, and fluorescence intensity was measured over time at an excitation wavelength of 485 nm and emission wavelength of 520 nm on a fluorescent plate reader.
Western blot analysis.
Western blotting confirmed TLR9 knockdown and Bcl-2 overexpression in THP-1-TLR9KD and THP-1-Bcl-2 cells, respectively. THP-1-TLR9KD and THP-1-Bcl-2 cells and their respective control cells were plated in a 6-well plate at 2 million cells per well in RPMI containing 100 nM PMA to stimulate macrophage differentiation. The next morning, cells were washed and lysed in a mixture of 25 mM Tris (pH 8), 25 mM NaCl, 0.1 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, and 15 mM β-mercaptoethanol (solution B) containing 1 mM serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF). Lysates were subjected to SDS-PAGE on 15% polyacrylamide gels, transferred to nitrocellulose, and immunoblotted for TLR9 (Santa Cruz, catalog no. sc-25468) or Bcl-2 (Santa Cruz, catalog no. sc-7382). As a loading control, both membranes were immunoblotted for actin (Sigma catalog no. A5441).
qPCR for gp91phox levels.
RNA was isolated from THP-1-gp91phoxKD and THP-1-control cells using the Qiagen RNeasy Plus minikit (catalog no. 74134) and the Qiagen QIAshredder (catalog no. 79654). Five hundred nanograms of RNA was subjected to DNase I treatment (Fermentas no. EN0521) and then reverse transcribed to single-strand cDNA (Fermentas RevertAid first strand cDNA synthesis kit, no. K1621). cDNA was then subjected to quantitative PCR (qPCR) on the Bio-Rad Opticon 2 using Bio-Rad IQ SYBR green supermix (catalog no. 170-8880). The primers for actin were forward primer ATGGGTCAGAAGGATTCCTATGTG and reverse primer CTTCATGAGGTAGTCAGTCAGGTC (20). The primers for gp91phox were forward primer CACAGGCCTGAAACAAAAGA and reverse primer GCTTCAGGTCCACAGAGGAA (QPPD [Quantitative PCR Primers Database], NCI, primer set 10932). Relative gp91phox expression was generated using the threshold cycle (2−ΔΔCT) method as described by Livak and Schmittgen, where the fold change in gp91phox expression was normalized to the housekeeping gene β-actin in each cell type and gp91phox expression in the THP-1-control cells is set to 1 (22).
Measurement of cytosolic cathepsin B and cytochrome c.
THP-1 cells were plated in a 6-well plate at 2 million cells per well in RPMI containing 100 nM PMA to stimulate macrophage differentiation. The next morning, cells were serum starved for 3 h and left untreated (control), treated with adenovirus at 100,000 particles per cell (ppc), or treated with the ts1 mutant adenovirus at 100,000 ppc for 30 min. Cells were placed on ice, where they were washed and permeabilized with 50 μg/ml digitonin plus 1 mM PMSF in PBS (pH 7.4) for 15 min at 4°C to effectively lyse the plasma membrane while leaving the intracellular membranes intact. These cell lysates were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted for cathepsin B (Santa Cruz, catalog no. sc-13985) and cytochrome c (BioLegend, catalog no. 612302).
Assessment of mitochondrial membrane potential.
THP-1 cells were plated in a 96-well plate at 100,000 cells per well in RPMI plus 100 nM PMA to generate macrophage-like cells. The following day, the medium was replaced and cells were rested for 1 day in RPMI plus 10% FBS. Cells were serum starved for 3 h. If necessary, cells were pretreated with Ca074me (EMD Biosciences, catalog no. 205531) during the last 20 min of serum starvation. Cells were then left untreated, incubated with 50 μM uncoupling agent carbonyl cyanide m-chlorophenyl hydrazone (CCCP), infected with adenovirus at 50,000 ppc, or infected with the ts1 mutant adenovirus at 50,000 ppc for 30 min. Cells were then washed and incubated with the MitoPT JC-1 dye reagent per the manufacturer's recommendations (ImmunoChemistry Technologies, LLC, catalog no. 937). JC-1 oligomerized within the mitochondria, where it appears as red puncta throughout the cytoplasm. When mitochondrial membrane potential was lost, JC-1 appeared as diffuse green fluorescence throughout the cytoplasm, where it existed as a monomer. Briefly, cells were incubated with JC-1 for 15 min and washed twice carefully with wash buffer. Cells were analyzed by fluorescence microscopy, and then we quantified the percentage of cells containing red fluorescent puncta. The data were expressed as the percentage of cells with intact mitochondrial membrane potential (Ψm).
Quantification of IL-1β and TNF-α secretion by ELISA.
THP-1 cells stably expressing empty vector and THP-1-TLR9 knockdown cells were plated at 50,000 cells per well in a 96-well plate with 100 nM PMA to induce macrophage differentiation. The following morning, cells were serum starved for 2 h and left untreated or treated with adenovirus at 30,000 ppc for 6 h. Supernatants from each sample were collected, and an ELISA was performed using the Ready-SET-Go! IL-1β kit from eBioscience (catalog no. 88-7010-88). Similarly, THP-1 cells stably expressing empty vector and THP-1-Bcl-2 cells were plated at 50,000 cells per well in a 96-well plate with 100 nM PMA to induce macrophage differentiation. The following morning, cells were serum starved for 3 h, primed with 30 ng/ml of the TLR2 ligand PAM3CSK4, washed, and then either left untreated, treated with 3 mM ATP (positive control), or treated with adenovirus at 50,000 ppc for 2 h. Supernatants were used in the IL-1β ELISA. For tumor necrosis factor alpha (TNF-α) secretion, THP-1 cells were plated as done for the IL-1β ELISA and were serum starved for 3 h. They were then left untreated or were pretreated for 10 min with 30 mM N-acetyl-cysteine or 10 μM rotenone, and then they were infected with Ad5 at 30,000 ppc in the continued presence of the drug. After 4 h, supernatants were subjected to analysis by ELISA for TNF-α (eBioscience, catalog no. 88-7346-77).
RESULTS
ROS play a key role in the innate immune response to adenovirus type 5 infection.
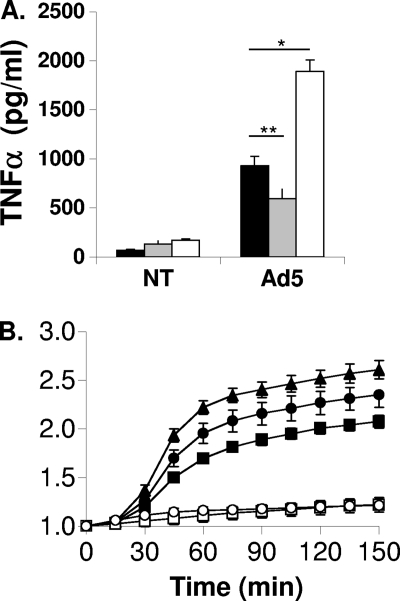
We have demonstrated previously that reactive oxygen species contribute to NLRP3 inflammasome activation in response to adenovirus infection. Specifically, we have shown that the ROS scavenger N-acetyl-cysteine significantly attenuates Ad5-induced IL-1β production by PMA-differentiated THP-1 cells. It is not yet known how ROS are produced in response to Ad5 infection or how they contribute to the activation of the innate immune response. In this study, we have found that the enhancement of mitochondrial ROS by treatment with rotenone, a complex I inhibitor, significantly augments Ad5-induced TNF-α production in PMA-differentiated THP-1 cells (Fig. 1 A). In contrast, N-acetyl-cysteine, which neutralizes ROS, significantly attenuates TNF-α production in response to Ad5 infection (Fig. 1A). Neither treatment influences Ad5 transduction (not shown). These data suggest that ROS also contribute more broadly to the innate immune response to Ad5 by inducing secretion of NLRP3-independent proinflammatory cytokines such as TNF-α.
Fig. 1.
Ad5-induced ROS production and the role of ROS in Ad5-induced TNF-α secretion. (A) PMA-differentiated THP-1 cells were serum starved for 3 h and either left untreated (black bars) or pretreated for 10 min with 30 mM N-acetyl-cysteine (gray bars) or with 10 μM rotenone (white bars), and then cells were left uninfected (NT) or infected with Ad5 at 30,000 ppc in the continued presence of drug for 4 h. Secreted TNF-α was measured by ELISA. (B) PMA-differentiated THP-1 cells were serum starved for 3 h and then incubated with 10 μM DCFDA for 30 min, washed, and left untreated (open squares), infected with ts1 mutant adenovirus (Ad2ts1) at 500,000 ppc (open circles), or infected with Ad5 at 50,000 ppc (closed squares), 100,000 ppc (closed circles), or 500,000 ppc (closed triangles). DCF fluorescence was measured on a fluorescent plate reader at an excitation wavelength of 485 nm and emission wavelength of 520 nm. The graph depicts DCF relative fluorescence intensity (normalized to time zero) over time.
Adenovirus type 5 rapidly induces ROS upon infection.
We have previously shown that ROS are produced rapidly after Ad5 infection and contribute to the innate immune response (4). However, the mechanism of ROS production during Ad5 infection has not yet been described. To more precisely define the steps in Ad5 cell entry required to elicit ROS from infected cells, we infected the macrophage-like PMA-differentiated THP-1 cells with an E1/E3-deleted Ad5 strain or the temperature-sensitive mutant virus Ad2ts1 (ts1) and monitored ROS production by measuring increases in the fluorescence intensity of the fluorophore DCF, which only fluoresces after becoming oxidized by ROS. When produced at the nonpermissive temperature, the ts1 virions are hyperstable virions that can bind receptors and become endocytosed but fail to rupture endosomal membranes because the capsid does not properly uncoat in endosomes (17). While infection with increasing doses of Ad5 induces a dose-dependent increase in ROS-dependent fluorescence, maximal doses of ts1 do not elicit ROS above background levels (Fig. 1B). Although virus multiplicities of infection (MOI) necessary to observe ROS using this fluorescence assay are much higher than those necessary to elicit secretion of proinflammatory cytokines, more rapid cell association of virus at these MOI allows us to determine that ROS production occurs at times corresponding to early events in Ad5 cell entry. Additionally, by comparison with ts1, we conclude that virus uncoating within endosomes is required to elicit ROS.
Adenovirus-induced ROS production does not require TLR9 signaling.
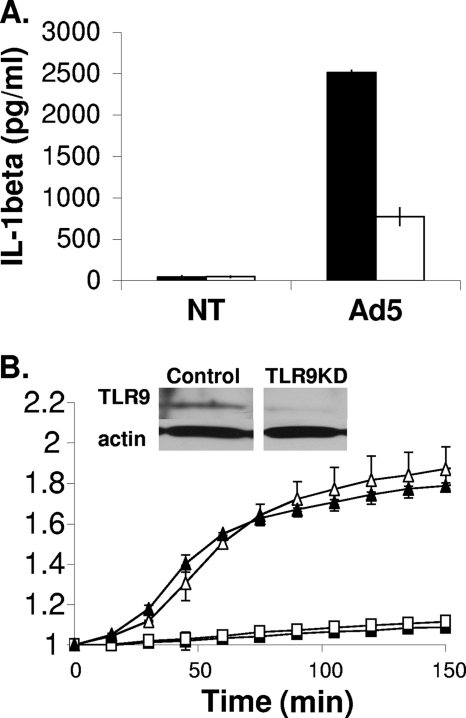
Several studies have reported that signaling through TLRs can lead to ROS production (24, 32, 47). We have shown that the DNA genome of Ad5 is recognized by TLR9 in PMA-differentiated THP-1 cells when the virus uncoats within endosomes and that TLR9 is required for IL-1β production during Ad5 infection (4). Using THP-1 cells stably expressing control or TLR9-specific shRNA, PMA-differentiated cells were loaded with DCF, infected with Ad5, and monitored for ROS-dependent fluorescence. While TLR9 knockdown leads to a decrease in IL-1β (Fig. 2 A), we found that TLR9 knockdown did not affect Ad5-induced ROS production, as indicated by a similar increase in DCF relative fluorescence intensity (RFI) between control and TLR9 knockdown cells (Fig. 2B).
Fig. 2.
ROS production in response to Ad5 infection in the presence or absence of TLR9 knockdown. (A) PMA-differentiated THP-1 control cells (black bars) and THP-1-TLR9KD cells (white bars) were serum starved for 3 h and either left untreated (NT) or infected with Ad5 at 30,000 ppc for 6 h. Secreted IL-1β was measured by ELISA. (B) PMA-differentiated THP-1 and TLR9 knockdown (KD) cells were serum starved for 3 h then incubated with 10 μM DCFDA for 30 min, washed, and then left untreated (open squares, THP-1 cells; closed squares, TLR9KD cells) or infected with Ad5 at 500,000 ppc (open triangles, THP-1 cells; closed triangles, TLR9KD cells). The graph depicts relative DCF fluorescence intensity (RFI) over time. (Inset) Western blot for TLR9 demonstrating efficient knockdown. Significance was determined by Student's t test (unpaired) where * indicates P < 0.005 and ** indicates P < 0.05.
Knockdown of the NADPH oxidase subunit gp91phox does not affect Ad5-induced ROS.
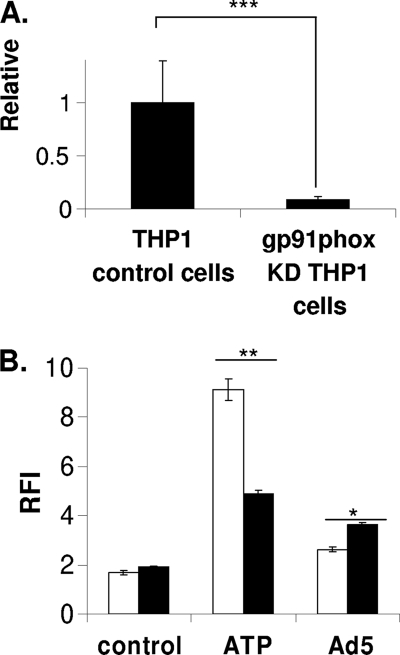
Previous studies have highlighted the existence of TLR-independent innate immune responses to Ad5, which may be involved in ROS production (30, 50). Several studies have highlighted the role for phagosomal NADPH oxidase in ROS production in response to innate immune signaling (15, 38). Furthermore, there are numerous examples implicating a role for phagosomal NADPH oxidase in the production of ROS upon infection, particularly during infection with intracellular pathogens such as bacteria (33, 35). The heme-binding subunit of NADPH oxidase, gp91phox, is a critical for the production of ROS by this enzyme (19, 35). To determine whether Ad5-induced ROS requires NADPH oxidase, we stably knocked down expression of an enzymatic subunit of phagosomal NADPH oxidase, gp91phox, in THP-1 cells by RNA interference (RNAi). Knockdown was quantified by quantitative reverse transcription-PCR (RT-qPCR) (Fig. 3 A). We treated PMA-differentiated THP-1 cells stably expressing control or gp91phox shRNA with Ad5 or ATP, a known activator of phagosomal NADPH oxidase, and measured DCF fluorescence over time as an indication of ROS production (Fig. 3B) (21, 31). Our data indicate that while knockdown of gp91phox attenuates ATP-induced ROS, it does not attenuate Ad5-induced ROS. Rather, gp91phox knockdown enhances Ad5-induced ROS. Although this increase in Ad5-induced ROS is statistically significant, it is unclear what role gp91phox might play in Ad5-induced ROS. These findings suggest that ROS produced in response to Ad5 infection do not require phagosomal NADPH oxidase.
Fig. 3.
ROS production in response to Ad5 in the presence or absence of gp91phox (Nox2) knockdown. (A) RNA was isolated from THP-1 control cells and THP-1-gp91phox knockdown cells, and subjected to qRT-PCR using primer sets for gp91phox and β-actin. Relative gp91phox expression was generated using the 2−ΔΔCT method where the fold change in gp91phox expression was normalized to the housekeeping gene β-actin and gp91phox expression in the THP-1 control cells is set to 1. (B) PMA-differentiated THP-1 control cells (white bars) and THP-1-gp91phox knockdown cells (black bars) were serum starved for 3 h then incubated with 10 μM DCFDA for 30 min, washed, and left untreated, treated with Ad5 at 100,000 ppc, or treated with 5 mM ATP. DCF fluorescence was measured on a fluorescent plate reader. Graph depicts relative fluorescence intensity at 6 h postinfection normalized to time zero. Significance was determined by Student's t test (unpaired), where * indicates P < 0.001, ** indicates P < 0.005, and *** indicates P < 0.01.
Mitochondrial membrane destabilization contributes to Ad5-induced ROS.
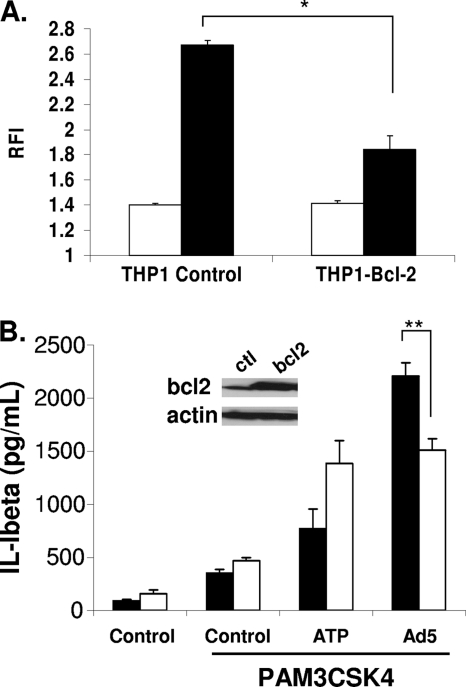
Based on the studies above, roles for TLR9 and phagosomal NADPH oxidase in Ad5-induced ROS production have been excluded. However, based on experiments with ts1, it appears endosomal membrane penetration by Ad5 is required for ROS production. Recent evidence demonstrates that mitochondrial membrane stress resulting from a loss of outer mitochondrial membrane integrity and loss of inner mitochondrial membrane potential (Ψm) contributes to ROS production in the context of infection (12). Furthermore, this mitochondrial stress is thought to contribute to the innate immune response, particularly NLRP3 inflammasome activation (49). Upon discovering that Ad5-induced ROS requires membrane disruption and does not involve TLR9 or phagosomal NADPH oxidase, we next investigated whether mitochondrial membrane permeability contributes to Ad5-induced ROS. To attenuate mitochondrial membrane permeability, we stably overexpressed Bcl-2 in THP-1 cells (THP-1-Bcl-2 cells) by retroviral transduction. When overexpressed, the antiapoptotic protein Bcl-2 associates with proapoptotic Bcl-2 family members on the mitochondrial membrane and prevents pore formation that leads to cytochrome c release and eventual loss of Ψm (6, 39). We subjected our THP-1-Bcl-2 and THP-1-control cells to the DCF assay as described above and assessed whether ROS production was attenuated when mitochondrial membrane disruption was inhibited. As expected, we found that, upon Ad5 infection, THP-1-Bcl-2 cells exhibited an ∼50% decrease in ROS production compared to THP-1-control cells, implicating mitochondrial membrane disruption as the key source for Ad-induced ROS (Fig. 4 A). Furthermore, this reduction in ROS production in THP-1-Bcl-2 cells correlated with decreased NLRP3 inflammasome activation in THP-1-Bcl-2 cells compared to THP-1-control cells (Fig. 4B). Additionally, these observed decreases in ROS and IL-1β production in the THP-1-Bcl-2 cells are not due to decreased infectivity upon Bcl-2 overexpression, as indicated by the presence of no significant difference in relative luciferase activities between THP-1-control cells and THP-1-Bcl-2 cells upon infection with an Ad5 luciferase reporter virus (data not shown). These results suggest that mitochondrial stress contributes to Ad5-induced ROS production.
Fig. 4.
Ad5-induced ROS production and IL-1β release in the presence or absence of Bcl-2 overexpression. (A) PMA-differentiated THP-1 control cells and THP-1-Bcl-2 cells were serum starved for 3 h and then incubated with 10 μM DCFDA for 30 min, washed, and left untreated (white bars) or infected with Ad5 at 500,000 ppc (black bars). DCF fluorescence was measured on a fluorescent plate reader. Graph depicts relative fluorescence intensity at 6 h postinfection normalized to time zero. (B) PMA-differentiated THP-1 control cells (black bars) and THP-1-Bcl-2 cells (white bars) were serum starved for 3 h, pretreated with the TLR2 ligand PAM3CSK4, and infected with Ad5 at 50,000 ppc for 2 h. Secreted IL-1β was measured by ELISA. (Inset) Western blot demonstrating Bcl-2 protein levels. ctl, control. Significance was determined by Student's t test (unpaired), where * indicates P < 0.0005 and ** indicates P < 0.05.
Ad5 endosomal membrane penetration and cathepsin activity induce mitochondrial membrane depolarization.
We have so far implicated Ad5 penetration of endosomal membranes and mitochondrial stress in the production of ROS upon Ad5 infection. We have previously shown that during Ad5 cell entry, viral rupture of endosomes releases catalytically active lysosomal cathepsin B into the cytoplasm and, furthermore, this event is required to activate the NLRP3 inflammasome (3, 4). Cytosolic cathepsin B has previously been implicated in the destabilization of the mitochondrial outer membrane and loss of Ψm (14, 48). Thus, cathepsin B release, upon Ad5 membrane rupture, may play a role in mitochondrial membrane disruption during infection.
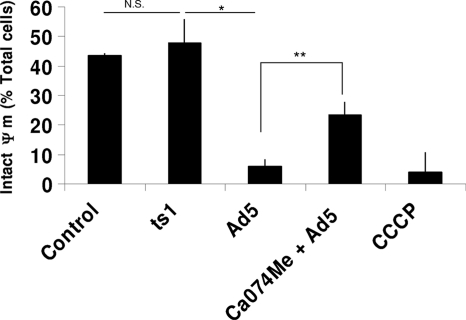
To investigate the loss of Ψm during Ad5 infection, we utilized the fluorophore JC-1, which accumulates in mitochondria and emits red fluorescence when Ψm is intact (2). Upon loss of Ψm, JC-1 remains diffuse in the cytoplasm and fluoresces green. After treating PMA-differentiated THP-1 cells with Ad5 or the ts1 mutant, we incubated the cells with the JC-1 reagent and assessed JC-1 fluorescence by microscopy 30 min postinfection to monitor the status of the mitochondrial membrane. We found that similar to our positive control, the uncoupling agent carbonyl cyanide m-chlorophenyl hydrazone (CCCP), Ad5 treatment reduces the percentage of cells with intact Ψm by nearly 10-fold compared to the negative control (Fig. 5). In contrast, infection with the ts1 mutant does not result in a significant decrease in the percentage of cells with intact Ψm (Fig. 5). These results confirm that Ad5 penetration of endosomal membranes is required to destabilize the mitochondrial membrane leading to ROS production.
Fig. 5.
Mitochondrial membrane destabilization upon Ad5 infection in the presence or absence of the cathepsin B inhibitor Ca074me. PMA-differentiated THP-1 cells were serum starved for 3 h and pretreated with 100 μM Ca074me for 20 min or left untreated. Cells were then incubated with CCCP, infected with Ad5, or infected with the ts1 mutant adenovirus for 30 min, washed, and incubated with JC-1 for 15 min. Following two washes, cells were visualized by fluorescence microscopy and counted. Significance was determined by Student's t test (unpaired), where * indicates P < 0.0005 and ** indicates P < 0.05. N.S., not significant.
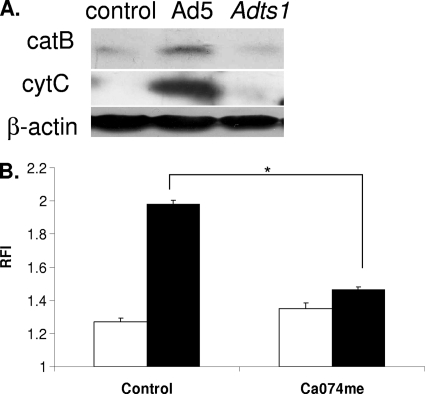
Since Ad5 endosomal membrane rupture is required for mitochondrial membrane destabilization, we next asked whether endosomal cathepsin B release into the cytoplasm and mitochondrial membrane destabilization correlate with Ad5-induced endosomal membrane rupture. Again, cytosolic cathepsins have been shown to contribute to mitochondrial membrane stress (14, 48). Thus, Ad5-induced cathepsin release may facilitate this observed loss in Ψm. We assessed both cathepsin B and cytochrome c release into the cytoplasm upon Ad5 infection or infection with the ts1 mutant. Cytosolic cathepsin B indicates endosomal Ad5 membrane rupture, while cytochrome c indicates mitochondrial membrane disruption (6). Thirty minutes after viral infection, differentiated THP-1 cells were subjected to selective digitonin permeabilization of the plasma membrane to extract cytoplasm for Western blot analysis of the cytoplasmic levels of cathepsin B and cytochrome c. Following Ad5 infection, cathepsin B and cytochrome c levels in the cytoplasm increased significantly compared to those in untreated control cells (Fig. 6 A). In contrast to Ad5 infection, ts1 infection did not induce release of cathepsin B or cytochrome c into the cytoplasm, indicating that membrane disruption was required for the release of cathepsin B and cytochrome c into the cytoplasm (Fig. 6A).
Fig. 6.
Cathepsin B and cytochrome c release during Ad5 infection and Ad5-induced ROS in the presence of the cathepsin B inhibitor Ca074me. (A) Two million PMA-differentiated THP-1 cells were left untreated, infected with Ad5 at 100,000 ppc, or infected with ts1 mutant adenovirus at 100,000 ppc for 30 min. Cell membranes were selectively lysed with cytosolic extraction buffer containing 50 μg/ml digitonin. Cell lysates were run on SDS-PAGE and transferred to a nitrocellulose membrane. Western blotting detected cytoplasmic cathepsin B (catB) and cytochrome c (cytC). (B) PMA-differentiated THP-1 cells were serum starved for 3 h and then incubated with 10 μM DCFDA for 30 min, washed, pretreated with the cathepsin inhibitor Ca074me (100 μM) or DMF control for 1 h, and then left untreated (white bars) or incubated with Ad5 at 250,000 ppc (black bars). DCF fluorescence was measured on a fluorescent plate reader. The graph depicts relative fluorescence intensity at 6 h postinfection normalized to time zero. Significance was determined by Student's t test (unpaired), where * indicates P < 0.0001.
We asked directly whether cathepsin B activity was required for Ad5-induced mitochondrial inner membrane depolarization by pretreating cells with the cathepsin B inhibitor Ca074me and infecting them with Ad5 in the continued presence of drug. As expected, we found that inhibiting cathepsin B activity prevented the loss of Ψm during Ad5 infection (Fig. 5). One alternative explanation of these results could be that Ca074me inhibits the adenovirus 23K protease. We have previously reported that Ca074me does not inhibit Ad5 transduction of THP-1 cells (4). Since the 23K protease is required for Ad5 transduction (17), we conclude that Ca074me does not inhibit the adenovirus 23K protease. These results support our hypothesis that Ad5-induced membrane disruption facilitates cathepsin-dependent mitochondrial membrane permeabilization.
Adenovirus-induced ROS production requires cathepsin B activity.
We next directly investigated whether cathepsin activity is required for ROS produced in response to infection. We hypothesize that Ad5 membrane penetration leads to the disruption of local lysosomal membranes and, in turn, the release of cathepsins from the lysosome. Furthermore, we hypothesize that cathepsin B release facilitates cathepsin B-dependent mitochondrial membrane disruption, leading to the release of ROS from the damaged mitochondria. To address whether Ad5-induced ROS required cathepsin activity, DCF-loaded, PMA-differentiated THP-1 cells were pretreated with Ca074me and infected with Ad5 in the continued presence of drug, and then DCF fluorescence was monitored 1 h postinfection. We found that Ca074me treatment attenuated ROS production upon Ad5 infection (Fig. 6B). These results are consistent with our data indicating cathepsin activity is required for mitochondrial membrane disruption and further demonstrate that cathepsin activity contributes to Ad5-induced ROS. Overall, we demonstrate that cathepsin B activity contributes to Ad5-induced ROS by facilitating mitochondrial membrane disruption that leads to the release of ROS into the cytoplasm of the infected cell.
DISCUSSION
ROS are key players in the innate immune response to pathogens. Following bacterial infection, ROS are known to be involved in the activation of transcription factors IRF-3 and NF-κB (10, 15). While the role for ROS in the inflammatory response to extracellular bacteria is better characterized, recent studies have implicated ROS as a mediator of inflammatory signals in response to viruses. For example, ROS are known to enhance RIG-I-dependent IRF-3 activation in response to respiratory syncytial virus (RSV) infection (38). We have recently demonstrated that ROS contribute to NLRP3 inflammasome activation during Ad5 infection (4). It is unclear how ROS affect other innate immune pathways in response to Ad5 infection. In this study, we demonstrate that Ad5-induced ROS contributes to the production of TNF-α and that enhanced mitochondrial ROS augments this process. These findings highlight the importance of ROS as a key mediator in innate immune activation. Furthermore, these data implicate a role for mitochondrial ROS in the innate immune response to adenovirus. To better understand the role for ROS in generating the proinflammatory response to Ad5, we addressed how ROS are generated during adenovirus infection.
Inflammatory responses to adenovirus have been tightly linked to cell entry and endosomal escape. During viral entry following receptor-mediated endocytosis, TLR9 activation in response to the Ad dsDNA genome leads to NF-κB activation, while endosomal escape activates IRF-3, although the exact mechanism remains poorly defined (8, 29). Furthermore, both Ad cell entry and endosomal escape contribute to NLRP3 activation (3, 4, 13). ROS-dependent signaling reportedly enhances much of these signaling pathways, which are also activated during the early phases of Ad infection (30, 51). While contributions of Ad5-induced ROS to NF-κB and IRF-3-dependent transcription have not yet been elucidated, previous observations that the ROS-p38-NF-κB (24), ROS/Jun N-terminal protein kinase (JNK) (41), and JNK/IRF-3 (28) signaling pathways contribute to proinflammatory signaling in response to other stimuli could explain observations of the involvement of p38 and JNK MAPKs in innate immune responses to Ad5 infection (29). Interestingly, previous studies have shown that coadministration of reducing agents such as N-acetyl-cysteine with Ad5 vectors in the lungs reduces the inflammatory response in mice and augments the level and duration of transgene expression (18). Our current studies provide additional insight into ROS-dependent innate immune responses and have identified molecules whose contributions to innate immune signaling during Ad5 cell entry can now be further explored.
Perhaps the most striking finding of these studies was the observation that ROS produced in response to adenoviral infection were derived from mitochondrial sources, independently of TLR signaling or phagosomal NADPH oxidase—both known to contribute to ROS production in response to infection (15, 32). Recent observations have highlighted an important role for mitochondria in antiviral innate immune signaling through RIG-I-like receptors that recognize viral RNA genomes and the inflammasome, with many signaling intermediates localizing to mitochondria upon viral infection (7, 25, 38, 41). Additionally, mitochondrial stress induced by incoming virions (11) or via RIG-I recognition of viral RNA (9) has also been linked to an apoptotic response, which is thought to limit viral replication. We have shown a novel manner by which a nonenveloped virus triggers an innate immune response by activating a well-established danger signal in cells: the permeabilization of lysosomal membranes. Taken together, mitochondria are capable of responding to a diverse array of virally associated molecular patterns and danger signals to elicit an innate immune response.
During Ad5 cell entry, lysosomal rupture is a danger signal that triggers ROS production. The mode of membrane rupture could influence this response. For example, we have previously shown that reovirus endosomal escape does not activate the NLRP3 inflammasome to the extent of Ad5 despite enhanced colocalization with cathepsin-enriched lysosomes (3). Additionally, the extent to which Ad vectors colocalize with cathepsin-enriched lysosomes was shown to influence the inflammatory response to vectors (3). These observations have many implications for gene therapy and vaccine development. Since release of lysosomal cathepsins leads to ROS production, altering Ad intracellular trafficking by modifying the receptors used for cell entry could alter this ROS-dependent inflammatory response. It is not yet clear that altering Ad membrane lytic activity could attenuate or enhance the inflammatory response.
Cathepsins have been shown to cleave the proapoptotic factor Bid to facilitate mitochondrial membrane permeabilization and caspase-dependent apoptosis (14, 48). Our results demonstrate that stabilization of mitochondrial membrane potential by overexpressing Bcl-2 reduces Ad5-induced ROS production and IL-1β secretion. The gene coding for Ad5 E1B-19K is a Bcl-2 family member previously shown to inhibit NF-κB transcriptional activity (36). Although E1B-19K is important for preventing apoptosis in response to viral infection, it is interesting to consider that this protein, detectable several hours postinfection, may function in some capacity to dampen the inflammatory response elicited by incoming virions or during viral replication. Further study is required to explore this possibility.
As a whole, our data indicate that Ad5 membrane rupture and subsequent cathepsin B activity in the cytoplasm contribute to mitochondrial stress and the production of ROS during infection. This mechanism of Ad5-induced ROS is likely a common response to pathogens that rupture membranes, such as bacteria that replicate in the cytoplasm. ROS is a key component in the antiviral and proinflammatory response to both bacterial and viral infections. These results will help with future studies of innate immune responses to Ad5. Additionally, our understanding of this process could allow us to either enhance or attenuate the innate immune response to adenovirus to generate novel vectors for gene therapy and vaccination.
ACKNOWLEDGMENTS
C.M.W. acknowledges financial assistance from the NIH (AI082430) and the American Heart Association (2261306). K.A.M. acknowledges financial support from the NIH (AI007508).
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Appledorn D. M., et al. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134–2144 [DOI] [PubMed] [Google Scholar]
- 2. Arnoult D. 2008. Apoptosis-associated mitochondrial outer membrane permeabilization assays. Methods 44:229–234 [DOI] [PubMed] [Google Scholar]
- 3. Barlan A. U., Danthi P., Wiethoff C. M. 2011. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology 412:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. 2011. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borgland S. L., Bowen G. P., Wong N. C., Libermann T. A., Muruve D. A. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-kappaB. J. Virol. 74:3941–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunelle J. K., Letai A. 2009. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 122:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castanier C., Garcin D., Vazquez A., Arnoult D. 2010. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 11:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerullo V., et al. 2007. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15:378–385 [DOI] [PubMed] [Google Scholar]
- 9. Chattopadhyay S., et al. 2010. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 29:1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiang E., et al. 2006. Cutting edge: apoptosis-regulating signal kinase 1 is required for reactive oxygen species-mediated activation of IFN regulatory factor 3 by lipopolysaccharide. J. Immunol. 176:5720–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danthi P., et al. 2008. Reovirus apoptosis and virulence are regulated by host cell membrane penetration efficiency. J. Virol. 82:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng L., et al. 2008. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3-dependent pathway. J. Virol. 82:10375–10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Paolo N. C., et al. 2009. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Droga-Mazovec G., et al. 2008. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 283:19140–19150 [DOI] [PubMed] [Google Scholar]
- 15. Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. 2008. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J. Immunol. 180:6911–6922 [DOI] [PubMed] [Google Scholar]
- 16. Ginsberg H. S., Prince G. A. 1994. The molecular basis of adenovirus pathogenesis. Infect. Agents Dis. 3:1–8 [PubMed] [Google Scholar]
- 17. Greber U. F., Webster P., Weber J., Helenius A. 1996. The role of the adenovirus protease on virus entry into cells. EMBO J. 15:1766–1777 [PMC free article] [PubMed] [Google Scholar]
- 18. Jornot L., Morris M. A., Petersen H., Moix I., Rochat T. 2002. N-acetylcysteine augments adenovirus-mediated gene expression in human endothelial cells by enhancing transgene transcription and virus entry. J. Gene Med. 4:54–65 [DOI] [PubMed] [Google Scholar]
- 19. Katsuyama M. 2010. NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J. Pharmacol. Sci. 114:134–146 [DOI] [PubMed] [Google Scholar]
- 20. Leon A. J., et al. 2009. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009:580450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lister M. F., et al. 2007. The role of the purinergic P2X7 receptor in inflammation. J. Inflamm. (Lond.) 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Maier O., Galan D. L., Wodrich H., Wiethoff C. M. 2010. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology 402:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuzawa A., et al. 2005. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 6:587–592 [DOI] [PubMed] [Google Scholar]
- 25. Moore C. B., et al. 2008. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451:573–577 [DOI] [PubMed] [Google Scholar]
- 26. Moyer C. L., Wiethoff C. M., Maier O., Smith J. G., Nemerow G. R. 2011. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J. Virol. 85:2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muruve D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157–1166 [DOI] [PubMed] [Google Scholar]
- 28. Muruve D. A., et al. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103–107 [DOI] [PubMed] [Google Scholar]
- 29. Nociari M., Ocheretina O., Murphy M., Falck-Pedersen E. 2009. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J. Virol. 83:4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nociari M., Ocheretina O., Schoggins J. W., Falck-Pedersen E. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noguchi T., et al. 2008. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 283:7657–7665 [DOI] [PubMed] [Google Scholar]
- 32. Park H. S., et al. 2004. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 173:3589–3593 [DOI] [PubMed] [Google Scholar]
- 33. Rada B., et al. 2008. Role of Nox2 in elimination of microorganisms. Semin. Immunopathol. 30:237–253 [DOI] [PubMed] [Google Scholar]
- 34. Roelvink P. W., et al. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sadikot R. T., et al. 2004. p47phox deficiency impairs NF-kappa B activation and host defense in Pseudomonas pneumonia. J. Immunol. 172:1801–1808 [DOI] [PubMed] [Google Scholar]
- 36. Schmitz M. L., et al. 1996. The dual effect of adenovirus type 5 E1A 13S protein on NF-kappaB activation is antagonized by E1B 19K. Mol. Cell. Biol. 16:4052–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shirakawa T. 2008. The current status of adenovirus-based cancer gene therapy. Mol. Cells 25:462–466 [PubMed] [Google Scholar]
- 38. Soucy-Faulkner A., et al. 2010. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 6:e1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tait S. W., Green D. R. 2010. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11:621–632 [DOI] [PubMed] [Google Scholar]
- 40. Tal M. C., et al. 2009. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. U. S. A. 106:2770–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tattoli I., et al. 2008. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tibbles L. A., et al. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309–319 [DOI] [PubMed] [Google Scholar]
- 44. Wiethoff C. M., Wodrich H., Gerace L., Nemerow G. R. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 79:1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi T., et al. 2007. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 18:753–762 [DOI] [PubMed] [Google Scholar]
- 46. Yang C. S., et al. 2007. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J. Neuroinflammation 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang C. S., et al. 2008. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell. Microbiol. 10:741–754 [DOI] [PubMed] [Google Scholar]
- 48. Zhang H., Zhong C., Shi L., Guo Y., Fan Z. 2009. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to necroptosis. J. Immunol. 182:6993–7000 [DOI] [PubMed] [Google Scholar]
- 49. Zhou R., Yazdi A. S., Menu P., Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225 [DOI] [PubMed] [Google Scholar]
- 50. Zhu J., Huang X., Yang Y. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81:3170–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zsengeller Z., Otake K., Hossain S. A., Berclaz P. Y., Trapnell B. C. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655–9667 [DOI] [PMC free article] [PubMed] [Google Scholar]