Abstract
Hepatocyte transduction following intravenous administration of adenovirus 5 (Ad5) is mediated by interaction between coagulation factor X (FX) and the hexon. The FX serine protease (SP) domain tethers the Ad5/FX complex to hepatocytes through binding heparan sulfate proteoglycans (HSPGs). Here, we identify the critical HSPG-interacting residues of FX. We generated an FX mutant by modifying seven residues in the SP domain. Surface plasmon resonance demonstrated that mutations did not affect binding to Ad5. FX-mediated, HSPG-associated cell binding and transduction were abolished. A cluster of basic amino acids in the SP domain therefore mediates surface interaction of the Ad/FX complex.
TEXT
Adenoviruses are nonenveloped, double-stranded DNA viruses belonging to the Adenoviridae family, of which there are 54 different serotypes divided into species based on their phylogenic origin and hemagglutination properties. Adenoviruses can infect a wide variety of cell types and are extensively used as vectors for gene transfer applications, from use in vitro or in vivo in the laboratory setting to gene therapy clinical protocols. Species C adenovirus serotype 5 (Ad5) is the most commonly used vector for clinical gene therapy and vaccination applications. However, liver sequestration after intravenous administration of Ad5 results in high-level gene transfer in hepatocytes, which can greatly reduce the efficacy of the vector for the target organ or disease site. Recent studies have shown that hepatocyte transduction by Ad5 is mediated by a high-affinity interaction between coagulation factor X (FX) and the Ad5 trimeric hexon protein (4, 11, 13–15), which is the most abundant protein in the adenovirus capsid. FX is a zymogen of a vitamin K-dependent serine protease that is primarily synthesized in the liver and circulates in the bloodstream at 8 μg/ml. It is composed of a light chain consisting of a γ-carboxylated glutamic acid (Gla) and two epidermal growth factor (EGF)-like domains that are disulfide linked to the serine protease (SP) heavy chain. While the FX Gla domain has been shown to bind hexon proteins in the Ad5 capsid, the FX SP domain bridges the Ad5/FX complex to the hepatocyte surface (2, 11, 15). Previous studies have shown soluble heparin or heparan sulfate can completely ablate FX-mediated Ad5 uptake in vitro and in vivo, indicating the critical role of heparan sulfate proteogylcans (HSPGs) for Ad5 cellular binding (2). FX has been shown to bind to at least 14 other Ad serotypes, indicating that this interaction is conserved (15). These findings have highlighted the importance of understanding the precise mechanisms governing cellular infectivity and tropism in order for adenoviruses to be successfully used as therapeutic gene delivery vectors.
Our previous studies demonstrated that the anticoagulants nematode anticoagulant peptide 2 (NAPc2) and Ixolaris can block FX-mediated Ad5 transduction in vitro (7, 8, 15). Ixolaris has been shown to bind residues within the heparin binding exosite of FXa, previously defined as R93, K96, R125, R165, K169, K236, and R240 (12). The crystal structure of NAPc2 with FXa indicates it also binds to this exosite and residues R93, R125, K236, and R240 (8). Moreover, preincubation of FX with each anticoagulant prior to injection into coagulation factor-depleted mice resulted in a significant decrease in Ad5 hepatic gene transfer (15). Although this study implicated a role for the FX SP domain in Ad5 transduction, the precise mechanism and amino acid residues responsible for FX-mediated Ad5 attachment to HSPGs have not been reported. Here, we demonstrate that seven basic amino acid residues in the FX heparin binding proexosite (HBPE) are responsible for this fundamentally important, tropism-determining interaction.
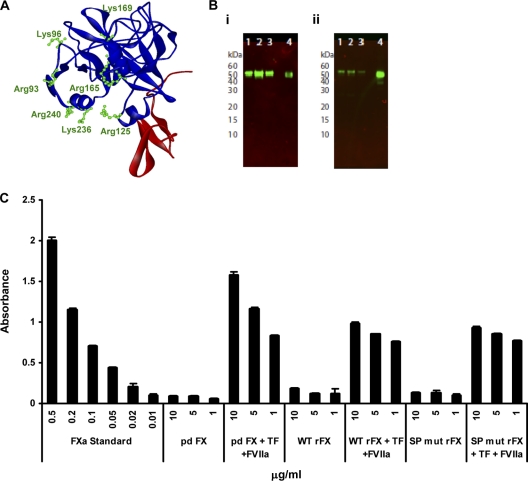
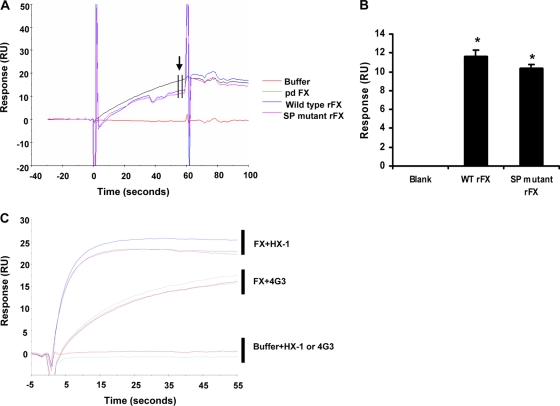
To assess the importance of the HBPE on Ad5/FX complex binding to HSPGs, we generated an FX construct in which the seven basic residues previously shown to bind heparin (12) were mutated (SP mutant rFX). Charge to alanine site-directed PCR mutagenesis was performed at the seven codons encoding basic residues R93, K96, R125, R165, K169, K236, and R240 (amino acids are numbered from the amino-terminal residue of the mature FX) in the FX construct (Fig. 1A). Mutagenesis and the entire FX coding sequence were verified by DNA sequence analysis. The wild-type (WT) and SP mutant FX-encoding cDNAs were cloned into a mammalian expression vector (human prothrombin signal sequence and propeptide followed by the sequence for the factor X derivative pCMV4-ss-pro-II-FX) (3). Constructs were transfected into 293T cells, and stable cell lines were generated based on zeocin resistance (200 μg/ml). Conditioned medium from cells cultured in serum-free media supplemented with 5 μg/ml vitamin K was affinity purified using the 4G3 mouse monoclonal antibody coupled to Sepharose as previously described (5). The recombinant protein was analyzed by paired antibody kit enzyme-linked immunosorbent assay (ELISA) (Affinity Biologicals, Canada) for human FX, SDS-PAGE, activation assay, and surface plasmon resonance (SPR). Each recombinant FX protein migrated as a single band on nonreduced SDS-PAGE with an apparent molecular mass of 59 kDa, similar to purified plasma-derived FX (pd FX) (Hematologic Technologies), as assessed by Western blot analysis (Fig. 1B). The amidolytic activity of FXa was measured using 1 mM chromogenic substrate S-2765 (Quadratech, United Kingdom) in the absence and presence of 100 nM tissue factor, 100 nM factor VIIa, and 6 mM CaCl2. All measurements were determined kinetically by measuring absorbance at 405 nm (9). Recombinant FX (rFX) proteins were converted to active forms (FXa) in the presence of tissue factor and FVIIa, confirming their biological activity (Fig. 1C). SPR was performed using a Biacore T100 (GE Healthcare). Ad5 hexon was covalently immobilized (1036RU) onto the flow cell of a CM5 biosensor chip by amine coupling according to the manufacturer's instructions and as described previously (15). Injections of purified human plasma-derived FX (pd FX), WT, or SP mutant rFX conditioned media were immediately followed by the monoclonal anti-FX antibodies HX-1 and 4G3 prior to regeneration of the sensor chip surface. SPR analysis indicated that the conditioned media of cells transfected with either WT or SP mutant FX bound to hexon in a calcium-dependent manner, and this was confirmed to be FX by subsequent injection of two monoclonal antibodies against human FX (HX-1 and 4G3) (Fig. 2A to C).
Fig. 1.
Validation of rFX protein. (A) Ribbon diagram of the FX serine protease-epidermal growth factor 2 (EGF2) domains (1HCG; Accelrys ViewerLite software) (10). SP is in blue, and EGF2 is in red. The diagram highlights amino acid residues (green) targeted for mutagenesis studies. (B) Western blot analysis of rFX was performed under nonreducing conditions (10% Bis-Tris mini gels; Novex), and rFX was probed with a primary monoclonal human anti-FX clone HX1 antibody (1:1,000 dilution) and a goat anti-mouse infrared (IR) dye (800CW)-labeled secondary antibody (LI-COR). The blot was visualized using an Odyssey infrared imaging system (LI-COR). (i) Lanes 1 to 3, SP mutant rFX purified by 4G3 column; lane 4, pd FX control. (ii) Lanes 1 to 3, WT rFX purified by 4G3 column; lane 4; pd FX control. (C) Activation of WT rFX and SP mutant rFX (SP mut rFX). The amidolytic activity of FXa was measured using 1 mM chromogenic substrate S-2765 (Quadratech, Surrey, United Kingdom) in the absence and presence of 100 nM tissue factor (TF), 100 nM factor VIIa (FV119), and 6 mM CaCl2. All measurements were determined kinetically at 405 nm. Error bars represent standard errors of the means (n = 3).
Fig. 2.
Proexosite mutations have no effect on rFX binding to Ad5 hexon by SPR. (A) Representative subtracted sensorgrams of pd FX, wild-type rFX and SP mutant rFX binding hexon, injected at time zero for 60 s at a flow rate of 30 μl/min. RU, response units. (B) Quantification of relative binding to hexon at the end of the injection, as indicated by the arrow. (C) Representative subtracted sensorgrams showing binding of anti-FX antibodies 4G3 or HX-1 to the chip following prior injection of running buffer, pd FX, and WT or mutant SP rFX, injected at a flow rate of 30 μl/min. *, P < 0.05. Error bars represent standard errors of the mean (n = 5).
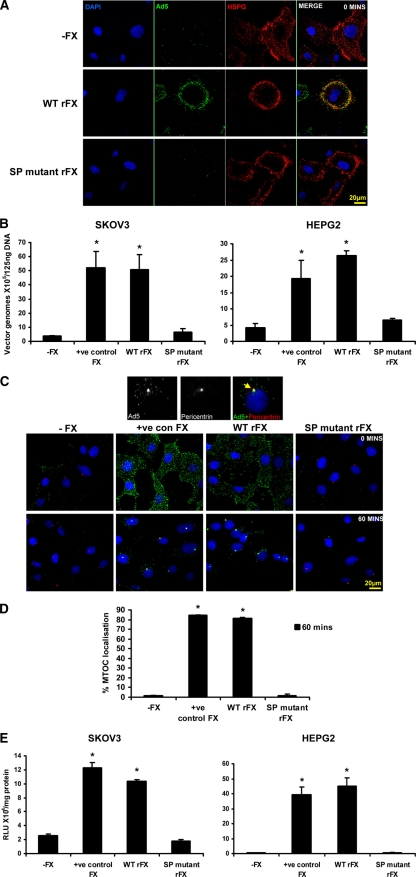
To assess the effects of the SP proexosite mutations on Ad5/FX-mediated cell binding and gene transfer, we used SKOV3 ovarian carcinoma and human HepG2 hepatoma cells since they can be readily transduced via the FX pathway (2). For experiments involving SKOV3 cells, which have low levels of coxsackievirus and adenovirus receptor (CAR) expression (6), we utilized a β-galactosidase-expressing Ad5, and for experiments involving HepG2 cells, which have higher CAR expression, we utilized Ad5KO1 (a CAR binding mutated Ad5). First, since cell surface binding of Ad5/FX complexes is mediated by HSPGs (2, 11), we evaluated the interaction of fluorescently labeled Ad5 with HSPGs when complexed with WT or the SP mutant rFX. Ad5 was fluorescently labeled using an Alexa Fluor-488 protein labeling kit according to the manufacturer's instructions (Invitrogen). Cells were incubated with 1 × 104 virus particles (vp)/cell of Alexa 488-labeled Ad5 in serum-free Dulbecco's modified Eagle's medium (SFDMEM) (Invitrogen) for 1 h on ice in the absence of FX or in the presence of WT or SP mutant rFX prior to fixation. Localization of Ad5 at the cell surface was characterized by staining with the mouse monoclonal pan-heparan sulfate antibody (clone 10E4) (AMS Biotechnology, Oxford, United Kingdom). Cell nuclei were counterstained using DAPI (4′,6-diamidino-2-phenylindole). Images were taken using the Zeiss confocal imaging system (LSM500). Following pretreatment with WT rFX, Ad5 complexes were clearly visible on the cell surface and colocalized with HSPGs (Fig. 3A). In contrast, no colocalization with HSPGs was observed when Ad5 was pretreated with SP mutant rFX. For binding and transduction assays, cells were incubated with 1,000 vp/cell of Ad5 in SFDMEM in the absence or presence of 1 μg/ml pd FX or WT or SP mutant rFX. Quantification of Ad5 cell surface binding by qPCR (1) revealed that WT rFX caused a significant ∼14-fold increase compared to cells not treated with WT rFX or pd human FX (positive control FX) (Fig. 3B), whereas no significant increase was observed when SP mutant rFX was used at an equivalent concentration to WT rFX (Fig. 3B). To analyze the effects of FX mutagenesis on Ad5 cell binding, internalization, and cytosolic transport, trafficking assays using fluorescently labeled Ad5 were performed in SKOV3 cells. In the presence of WT rFX or pd human FX, Ad5 bound the cell surface and after 1 h of incubation at 37°C efficiently trafficked to the nucleus, where it colocalized with the microtubule organizing center (MTOC), which was characterized by staining with a polyclonal rabbit pericentrin antibody (Abcam, United Kingdom) (Fig. 3C). However, when performed using the SP mutant FX, no cell surface binding or trafficking was observed (Fig. 3C and D). Ad5-MTOC colocalization was quantified as previously described (2). These data were confirmed by analyzing expression of the β-galactosidase transgene: both WT rFX and pd FX mediated a significant increase in gene expression, whereas the SP mutant rFX resulted in no significant change in SKOV3 or HepG2 cells (Fig. 3E).
Fig. 3.
Critical role of the heparin binding proexosite in the FX SP domain in Ad5 cell binding and gene transfer in vitro. (A) Alexa 488-labeled Ad5 (green) at 10,000 vp/cell was allowed to bind SKOV3 cells for 1 h at 4°C in the absence of FX or the presence of WT rFX or SP mutant rFX. Cells were then stained with a pan-heparan sulfate antibody (clone 10E4) (red). Nuclei were counterstained using DAPI. Images were captured on a confocal microscope at 63× objective. (B) Binding of Ad5 at 1,000 vp/cell to SKOV3 cells or Ad5KO1 at 1,000 vp/cell to HepG2 cells for 1 h at 4°C in the presence or absence of pd FX (positive control FX), WT rFX, or SP mutant rFX was quantified. Vector genomes were detected by quantitative SYBR green PCR. (C) Alexa 488-labeled Ad5 (green) at 10,000 vp/cell was allowed to bind SKOV3 cells for 1 h at 4°C in the absence or presence of pd FX, WT rFX, or SP mutant rFX. Cells were then incubated at 37°C for 0 or 60 min prior to fixation and staining for the MTOC marker pericentrin (red). Nuclei were counterstained using DAPI. Images were captured on a confocal microscope at 63× objective. (D) The percentage of cells with colocalization of fluorescently labeled Ad5 with the MTOC marker pericentrin was calculated by analyzing at least five separate 40× microscope fields per experimental condition. (E) SKOV3 and HepG2 cell transduction at 48 h postinfection after a 3-h exposure to Ad5 or Ad5KO1 in the presence or absence of pd FX, WT rFX, or SP mutant rFX conditions. RLU, relative light units. Error bars represent standard errors of the means.
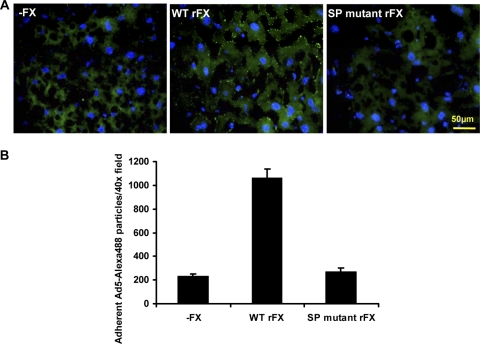
To confirm the importance of the SP proexosite, we characterized the effect of the SP mutations ex vivo by examining fluorescently labeled Ad5 binding to mouse liver sections (Fig. 4). Frozen liver sections from MF-1 mice were incubated with 2 × 109 vp of Alexa 488-labeled Ad5 in SFDMEM in the absence or presence of WT or SP mutant rFX for 1 h on ice followed by 1 h at 37°C. Sections were counterstained using DAPI and imaged using an Olympus Cell M imaging system. To quantify adherent Ad5, 40× microscope fields were processed using Image J counting software. Under SP mutant rFX conditions, there was ∼5-fold less adherent Ad5 particles compared to under WT rFX conditions.
Fig. 4.
Critical role of the heparin binding proexosite in the FX SP domain in Ad5 cell binding ex vivo. (A) Liver slices from MF1 mice were incubated with 2 × 109 vp of Alexa 488-labeled Ad5 in the presence of WT or SP mutant rFX. (B) Attachment of Ad5 particles to liver slices was quantified using ImageJ counting software. Data represent the average number of particles by analyzing at least 6 separate 40× microscope fields per experimental condition. *, P < 0.05. Error bars represent standard errors of the mean.
This study investigates the precise mechanism by which the Ad/FX complex interacts with HSPGs by demonstrating the critical importance of the amino acid residues of the HBPE of FX. Here we mutated the seven proexosite residues. Of interest, the four residues Arg165, Lys169, Lys236, and Arg240 are conserved in mouse and human FX. The remaining three residues are charge conserved (i.e., Arg-Lys or Lys-Arg), thus maintaining a positive charge. Future studies could aim to further dissect the individual residues critical to Ad5 binding with HSPGs. Using surface plasmon resonance of recombinant protein, we demonstrated that the SP mutations had no effect on FX-specific binding to the Ad5 hexon; however, FX-mediated binding and transduction were ablated. Taken together, this study uncovers the basic residues (R93, K96, R125, R165, K169, K236, and R240) in the SP domain of FX that have a fundamental role in FX-mediated Ad5 complex engagement with HSPGs at the surface of target cells.
Acknowledgments
This work was funded by the Scottish Universities Life Sciences Alliance, the British Heart Foundation, and the Biotechnology and Biological Sciences Research Council.
We thank Nicola Britton and Gregor Aitchison of the British Heart Foundation Glasgow Cardiovascular Research Centre for technical assistance.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Alba R., et al. 2009. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood 114:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradshaw A. C., et al. 2010. Requirements for receptor engagement during infection by adenovirus complexed with blood coagulation factor X. PLoS Pathog. 6:e1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camire R. M., Larson P. J., Stafford D. W., High K. A. 2000. Enhanced γ-carboxylation of recombinant factor X using a chimeric construct containing the prothrombin propeptide. Biochemistry 39:14322–14329 [DOI] [PubMed] [Google Scholar]
- 4. Kalyuzhniy O., et al. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. U. S. A. 105:5483–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim D. J., James H. L. 1994. Expression of human factor X with normal biological activity in human embryonic kidney cells. Biotechnol. Lett. 16:549–554 [Google Scholar]
- 6. Kim M., et al. 2002. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur. J. Cancer 38:1917–1926 [DOI] [PubMed] [Google Scholar]
- 7. Monteiro R. Q., Rezaie A. R., Ribeiro J. M., Francischetti I. M. 2005. Ixolaris: a factor Xa heparin-binding exosite inhibitor. Biochem. J. 387:871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murakami M. T., et al. 2007. Intermolecular interactions and characterization of the novel factor Xa exosite involved in macromolecular recognition and inhibition: crystal structure of human Gla-domainless factor Xa complexed with the anticoagulant protein NAPc2 from the hematophagous nematode Ancylostoma caninum. J. Mol. Biol. 366:602–610 [DOI] [PubMed] [Google Scholar]
- 9. O'Brien D. P., et al. 1994. Surface plasmon resonance studies of the interaction between factor VI1 and tissue factor. Demonstration of defective tissue factor binding in a variant FVII molecule (FVII-R79Q). Biochemistry 33:14162–14169 [DOI] [PubMed] [Google Scholar]
- 10. Padmanabhan K., et al. 1993. Structure of human des(1-45) factor Xa at 2.2 A resolution. J. Mol. Biol. 232:947–966 [DOI] [PubMed] [Google Scholar]
- 11. Parker A. L., et al. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes in vitro and in vivo. Blood 108:2554–2561 [DOI] [PubMed] [Google Scholar]
- 12. Rezaie A. R. 2000. Identification of basic residues in the heparin-binding exosite of factor Xa critical for heparin and factor Va binding. J. Biol. Chem. 275:3320–3327 [DOI] [PubMed] [Google Scholar]
- 13. Shayakhmetov D. M., Gaggar A., Ni S., Li Z. Y., Lieber A. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vigant F., et al. 2008. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol. Ther. 16:1474–1480 [DOI] [PubMed] [Google Scholar]
- 15. Waddington S. N., et al. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]