Abstract
The mechanisms by which gammaherpesviruses maintain latency are unclear. Here we used a murine gammaherpesvirus model to show that previously uninfected B cells in immunocompetent mice can acquire virus during latency. In vivo depletion of T cells allowed viral reactivation, as measured by increased viral loads, but not enhanced transfer of virus to new cells. In the absence of both immune T cells and antibody following the transfer of latently infected cells into naïve animals, there was robust infection of new B cells. These data confirm that both T cells and antibody contribute to the control of gammaherpesvirus latency, reactivation, and spread.
TEXT
The hallmark of herpesvirus (HV) infections is the establishment of latency, a lifelong condition characterized by persistent viral genome maintenance and restricted, low-level viral gene expression. The ability to reactivate from latency, reenter the lytic life cycle, and generate infectious virions is responsible for many of the secondary pathological consequences of HV infections, including autoimmunity, cancer, immunoproliferative disorders, recrudescent skin lesions, and transplant rejection (15, 19). An unresolved issue in the field of herpesvirology is how latent infections are maintained in the host over time. In the case of alphaherpesvirus (αHV) latency, the viral genomes reside in nonreplicating cells such as neurons, and so latency is likely propagated by the mere survival of latently infected cells, with periodic de novo infections following reactivation events. βHV and γHV infections are different, as they establish latency in immune system cells, which, unlike neurons, not only can proliferate but also can express high levels of major histocompatibility complex (MHC) class I and are targets for T cell immunity. Since long-term latency persists even in the presence of functional virus-specific immunity, it is crucial to understand how viral latency is propagated in an immunocompetent host in order to develop rational strategies to control γHV infections.
Mechanisms for γHV latency persistence include viral immune evasion strategies; proliferation of latently infected B cells, in which latent viral episomes are replicated and transferred to the daughter cells; direct cell-to-cell transfer of infectious virus to naïve cells, in the absence of cell-free virus; and asymptomatic shedding of lytic virus, leading to the infection of new B cells. There is evidence in the literature for each of these mechanisms. For example, Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA-1) protein is associated with driving the proliferation of infected B cells (8). Furthermore, it has been shown that efficient EBV infection of epithelial cell lines requires cell-to-cell contact with virally infected cells in cocultures (3, 10), a process that is refractory to inhibition by blocking antibodies (Abs), suggesting that cell-free virus is not required for infection (22). In one study, long-term valacyclovir treatment did not affect the number of EBV DNA copies per B cell but did reduce the frequency of latently infected B cells, which is consistent with roles for both lytic viral replication and infected B cell half-life in maintaining viral loads during latency (9). There is also evidence for asymptomatic EBV shedding (17), and asymptomatic shedding has been noted in individuals infected with Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) (2, 16). Ganciclovir treatment of KSHV-seropositive HIV-infected patients versus placebo treatment resulted in reduced occurrences of KS indicative of a requirement for lytic replication in the etiology of KS, since ganciclovir does not inhibit latent virus (13). Additionally, in vitro evidence suggests that latent KSHV genomes are inefficiently maintained during tumorigenesis and that KSHV may consequently require periodic lytic replication to perpetuate viral latency (6). Thus, it is likely that gammaherpesvirus (γHV) latency in infected humans is perpetuated by a combination of infected cell proliferation and periodic de novo infections after reactivation. EBV and KSHV infections are highly species specific, however, making in vivo mechanistic studies of viral latency very difficult. Therefore, we took advantage of a well-characterized small-animal model—intranasal infection of C57BL/6 mice with murine gammaherpesvirus-68 (γHV68), a rodent pathogen closely related to EBV and KSHV (25)—to experimentally determine whether uninfected B cells could acquire virus during latency and to elucidate the roles of humoral and cellular immunity in the control of viral latency.
Spread of virus to naïve B cells during latency in immune mice.
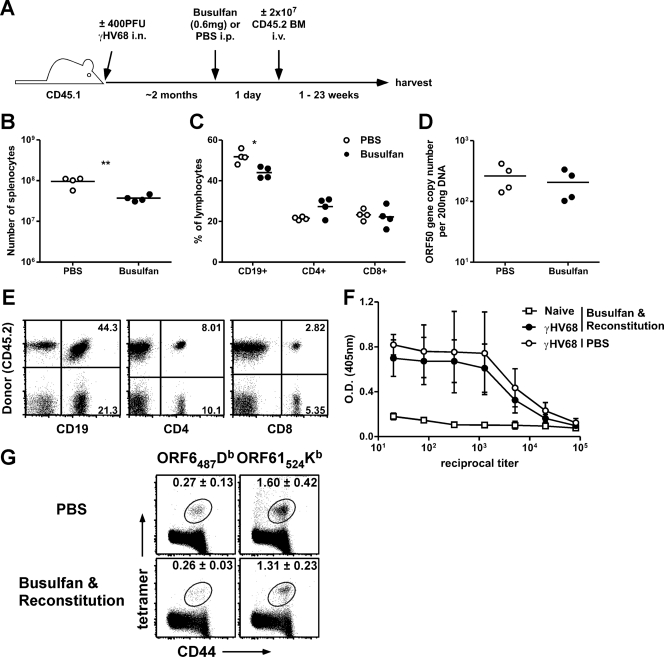
To generate a population of uninfected B cells that could be tracked during latency, we intraperitoneally treated latently infected mice with 0.6 mg of the chemotherapeutic bone marrow ablation agent busulfan (Busulfex; Otsuka America Pharmaceuticals), followed by lymphocyte reconstitution with 2 × 107 bone marrow cells from naïve congenic mice (Fig. 1 A). By selectively depleting cells of the bone marrow, busulfan treatment allows the establishment of partial hematopoietic chimerism without irradiation while leaving peripheral immunity largely intact; this approach has been used previously in studies of mice undergoing persistent viral infections (11, 21, 24).
Fig. 1.
Partial mixed bone marrow chimera approach. (A) Experimental design. Host mice were B6.SJL-PtprcaPepcb/Boy (CD45.1+Thy1.2+), and donor mice were C57BL/6 (CD45.2+Thy1.2+). BM, bone marrow. i.n., intranasal; i.p., intraperitoneal; i.v., intravenous. (B and C) At 1 week after busulfan treatment, spleens were harvested, labeled with fluorochrome-conjugated anti-CD4, anti-CD8, and anti-CD19 antibodies, and subjected to enumeration by flow cytometry. (B) Total numbers of splenocytes (n = 4/group; **, P < 0.01 [Student's t test]). (C) Percentages of each lymphocyte subset (n = 4/group; *, P < 0.05 [Student's t test]). (D) At 1 week after busulfan treatment (without reconstitution), DNA was isolated from B cells sorted from splenocytes and analyzed by a real-time PCR assay to determine copy numbers of the γHV68 ORF50 gene (n = 4/group). (E) At 5 weeks after bone marrow reconstitution, the donor and host percentages of each lymphocyte subset were enumerated by flow cytometry. Numbers in the quadrants indicate the percentages of total lymphocytes that are marker positive and host or donor derived. The mean percentages (± standard deviations [SD]) of each subset that were donor derived were as follows: for CD19+ cells, 63.32 ± 6.585; for CD4+ cells, 43.38 ± 5.658; and for CD8+ cells, 33.06 ± 5.331 (n = 5; data are representative of the results of at least 5 individual experiments). (F) Amounts of antiviral IgG antibody (±SD) were quantified by ELISA for naïve or γHV68-infected chimeras or nonchimeric mice 4 months p.i. (2 months posttransfer; n = 2 to 3/group). O.D., optical density. (G) Host splenocytes were isolated and labeled with anti-CD8 and anti-CD44 antibodies and tetramers specific for the ORF6487/Db (AGPHNDMEI) or ORF61524/Kb (TSINFVKI) epitope. The mean percentages (±SD) of CD8+ T cells specific for each tetramer are shown (n = 3 to 4/group; data are representative of the results of at least 4 experiments).
Busulfan treatment alone, without congenic bone marrow reconstitution, resulted in a decrease in the total numbers of splenocytes (Fig. 1B) and a decrease in the percentages of splenic CD19+ B cells but not of CD4+ or CD8+ T cells (Fig. 1C). Importantly, latent viral loads in B cells sorted from the spleen were similar 1 week after busulfan or phosphate-buffered saline (PBS) control treatment, as measured by real-time PCR (Fig. 1D) (23), demonstrating that busulfan treatment did not disrupt the viral loads of latently infected mice. This was probably a result of the low frequency of infected B cells being largely unaffected by the relatively slight decrease in B cell numbers. After busulfan treatment and reconstitution with congenic bone marrow, we observed considerable chimerism by 5 weeks, with the largest proportion of donor-derived lymphoid cells in the CD19+ B cell compartment, likely because the donor-derived T cells required maturation in the thymus before entrance into the periphery (Fig. 1E). Although the results showing the extent of chimerism differed among individual mice, most mice exhibited at least 50% chimerism in all lymphocyte subsets by 8 weeks after reconstitution (data not shown). Both antiviral antibody and T cell responses have been shown to be important for controlling infection and preventing viral recrudescence during γHV68 latency (1, 12), so to ensure that the partial bone marrow chimeras maintained virus-specific immunity, we measured the amount of antiviral IgG antibody by an enzyme-linked immunosorbent assay (ELISA) (Fig. 1F) and the percentages of host CD8+ T cells in the spleen specific for two immunodominant epitopes (ORF6487–495/Db and ORF61524–531/Kb) by tetramer staining (Fig. 1G). Neither humoral nor cellular antiviral immunity was impaired following busulfan treatment and reconstitution.
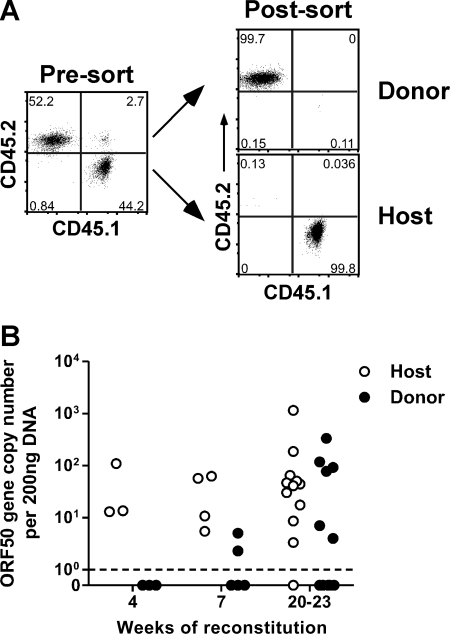
The donor-derived B cells in the resulting partial bone marrow chimeras were initially uninfected, so any detectable virus they acquired was a direct consequence of de novo infection. Therefore, we sorted B cells from chimeras on host or donor congenic markers (Fig. 2 A) and measured viral genomic DNA in host and donor cells after 4, 7, or 20 to 23 weeks of reconstitution. Although we failed to detect viral DNA in donor B cells at 4 weeks, viral DNA was detectable in donor B cells in 2 out of 5 mice (40%) at 7 weeks and 6 out of 12 mice (50%) at 20 to 23 weeks (Fig. 2B). These data conclusively demonstrate that naïve B cells can progressively acquire virus during γHV68 latency in immunocompetent mice.
Fig. 2.
Virus is transferred to donor-derived B cells during γHV68 latency. (A) Representative dot plots showing the purity of sorted donor and host populations after busulfan treatment and bone marrow reconstitution. Host mice were B6.SJL-PtprcaPepcb/Boy (CD45.1+Thy1.2+); donor mice were C57BL/6 (CD45.2+Thy1.2+). (B) At the indicated times after reconstitution, spleens were harvested, sorted into donor and host B cell populations, and analyzed by real-time PCR to determine γHV68 ORF50 gene copy numbers (n = 3 to 12/group).
Robust infection of donor-derived B cells following reactivation.
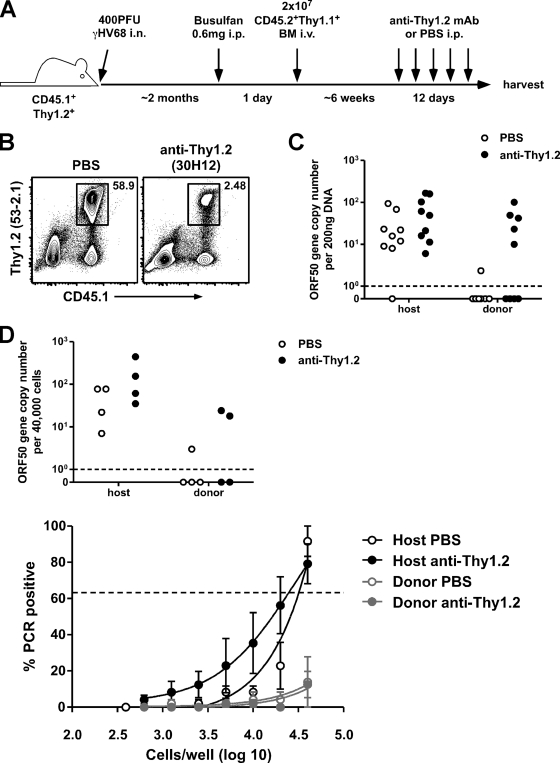
To investigate whether infection of naïve B cells could be enhanced in the absence of host cellular immunity, we first established partial mixed bone marrow chimeras by the use of host Thy1.2+ mice and donor Thy1.1+ mice and then depleted host T cells by treating the chimeras intraperitoneally with 250 μg of anti-Thy1.2 monoclonal Ab (MAb) (30H12) every 3 days for 12 days (Fig. 3 A). This procedure substantially reduced host T cell numbers (Fig. 3B) and resulted in an impact on viral control, as evidenced by a robust increase in viral genome copy numbers in donor-derived B cells (Fig. 3C), suggesting a critical role for T cells in maintaining γHV68 latency. There was a less dramatic effect of anti-Thy1.2 treatment on viral DNA in the host B cells—although there was a trend toward increased genome copies, the difference was not statistically significant (P = 0.1259 [Student's t test]). This was most likely due to complications stemming from the range of viral DNA numbers in the host B cells coupled with our inability to measure DNA changes within individual mice. Alternatively, it could have been the result of host B cells providing the viral DNA that accumulated in the donor B cells, of host B cells being less sensitive to reactivation stimuli, as ex vivo reactivation efficiency declines over time (26), or of host B cells being less susceptible to de novo infection, since they would include memory B cells and plasma cells but the donor B cell population would largely be composed of naïve and germinal center B cells (4, 20).
Fig. 3.
Infection of donor-derived B cells is enhanced following T cell depletion. (A) Experimental design. Host mice were B6.SJL-PtprcaPepcb/Boy (CD45.1+Thy1.2+); donor mice were B6.PL-Thy1a/Cy (CD45.2+Thy1.1+). (B) Representative contour plots showing the efficiency of Thy1.2 cell depletion following anti-Thy1.2 MAb (30H12) treatment. Splenocytes were harvested and labeled with anti-CD45.1 and anti-Thy1.2 (clone 53-2.1). (C) At 12 days after the start of anti-Thy1.2 MAb or PBS (control) treatment, spleens were harvested and sorted into donor and host B cell populations (see Fig. 2A), and DNA was isolated and analyzed by real-time PCR for determination of γHV68 ORF50 gene copy numbers (n = 9/group). (D) At 12 days after anti-Thy1.2 or PBS (control) treatment, spleens were harvested and sorted into donor and host B cell populations, and a 40,000-cell aliquot was analyzed by real-time PCR for determination of γHV68 ORF50 gene copy numbers (top panel). The rest of the sorted cells were subjected to LDA/PCR analysis to determine the frequency of viral genome-positive cells (bottom panel). The percentages (±SD) of wells that were PCR positive for each dilution are shown (n = 4/group). The dotted line in the bottom panel indicates 63.2%, which was the value that was used to calculate the frequency of viral genome-positive cells by Poisson distribution.
The increase in viral genome numbers in the donor-derived B cells after anti-Thy1.2 treatment could have been due to DNA replication within already-infected cells or to de novo infection of naïve B cells by virus reactivating from host cells or to a combination of the two. We investigated whether the increase in viral DNA numbers after anti-Thy1.2 MAb treatment was due to the infection of new donor-derived B cells by virus reactivating from host B cells by determining the frequency of infected cells with a limiting-dilution assay–nested PCR (LDA/PCR) (26). An increase in the frequency of infected B cells would indicate that previously uninfected cells had acquired viral DNA during the anti-Thy1.2 treatment period. We observed a trend toward increased copy numbers in both the host and donor B cells, but the frequency of infected host B cells did not change following anti-Thy1.2 MAb treatment, and the frequency of infected donor B cells remained very low—below 1 in 4 × 104 cells, our limit of detection for this particular assay (Fig. 3D). Because anti-Thy1.2 MAb-treated mice remain antibody sufficient (Fig. 1F), these data are consistent with previous findings reported from our laboratory indicating that virus-specific antibody is important for controlling viral recrudescence during latency (12). In addition, we failed to detect infectious virus in the spleens or lungs of chimeric mice by a plaque assay performed after anti-Thy1.2 MAb treatment (data not shown). These data support the hypothesis that the majority of reactivation observed in the absence of T cells is due to robust viral genome replication within already infected cells and not to widespread infection of new B cells, though it is likely that some de novo infection does occur during reactivation.
Latently infected B cells reactivate efficiently in the absence of immunosurveillance.
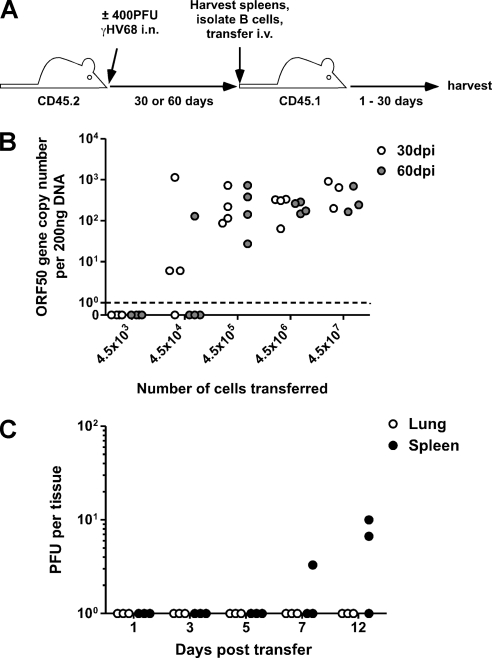
To confirm that latent virus efficiently reactivates in the complete absence of immune protection, we adoptively transferred graded numbers of purified B cells from latently infected mice (30 and 60 days postinfection [p.i.]) into naïve congenic hosts (Fig. 4 A). Reactivation, as indicated by an increase in genome copy numbers in host splenic B cells 30 days after transfer, occurred upon transfer of as few as 4.5 × 104 cells from mice 30 days postinfection (Fig. 4B). The numbers of transferred cells required for infection of the host corresponded to the frequency of latently infected cells present at the two time points—∼1 in 1.5 × 103 in the 30-day mice as determined by LDA/PCR analysis of the pool of transferred cells (a maximum of ∼300 latently infected cells transferred; data not shown) and ∼1 in 5 × 104 in the 60-day mice (a maximum of ∼9 latently infected cells transferred; data not shown). Importantly, the in vivo reactivation efficiencies of the latently infected cells were comparable at 30 and 60 days p.i., in contrast to the reactivation efficiency seen in vitro, which has been shown to drop dramatically after 30 days p.i. (26). Thus, transferring latently infected B cells into naïve recipient mice (which lack specific antiviral immunity) allows virus reactivation in the donor B cells and leads to de novo infection of host B cells, confirming clinical observations of virus-seronegative patients becoming seropositive after organ transplant (7). Stimulation of latently infected B cells by treatment with anti-Ig/anti-CD40 antibodies or various Toll-like receptor ligands has been demonstrated to induce γHV68 reactivation from latency (5, 14, 18), suggesting that inadvertent B cell stimulation during the transfer process could facilitate virus reactivation. However, the kinetics of reactivation after transfer argued against the likelihood that lytic virus or reactivating B cells were transferred, as we were unable to detect lytic virus in the spleens and lungs of recipient mice until 7 days after transfer (Fig. 4C).
Fig. 4.
Latently infected B cells reactivate efficiently in vivo in the absence of immunosurveillance. (A) Experimental design. Donor mice were C57BL/6 (CD45.2+Thy1.2+); recipient mice were B6.SJL-PtprcaPepcb/Boy (CD45.1+Thy1.2+). (B) B cells from mice latently infected for 30 days or 60 days were adoptively transferred i.v. into naïve congenic recipients. At 30 days after transfer, spleens from recipient mice were harvested. DNA was isolated from sorted B cells and analyzed by real-time PCR for determination of viral genome copy numbers (n = 3 to 4/group). (C) At the indicated days after transfer with 4.5 × 107 latently infected B cells, spleens and lungs were harvested and analyzed for determination of levels of lytic virus by a plaque assay (n = 3/tissue/time point).
Taken together, our data show that γHV68 latency is a dynamic process in which humoral and cellular immunity contribute to the control of viral reactivation and the prevention of recrudescence. Over time, the virus can escape immune surveillance, leading to the de novo infection of B cells. We propose a model in which gammaherpesviruses persist not only by viral transfer to new B cells during latency, either by cell-to-cell contact or virus release, but also by enhanced intracellular viral replication during periodic reactivation events, thereby increasing the pool of transferrable virus.
Acknowledgments
We thank Jacob E. Kohlmeier for critical reading of the manuscript and Otsuka America Pharmaceuticals for the generous gift of Busulfex.
This project was funded by NIH grants F32 AI084327 (to M.L.F.), T32 AI049823 (to D.L.W.), and AI042927, AI082919, and CA148250 (to M.A.B.) and by funds from the Trudeau Institute.
Footnotes
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Cardin R. D., Brooks J. W., Sarawar S. R., Doherty P. C. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casper C., et al. 2007. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J. Infect. Dis. 195:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang Y., et al. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 73:8857–8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaño E., Kim I. J., Woodland D. L., Blackman M. A. 2002. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J. Exp. Med. 196:1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gargano L. M., Forrest J. C., Speck S. H. 2009. Signaling through Toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J. Virol. 83:1474–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grundhoff A., Ganem D. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 113:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho M., et al. 1988. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation 45:719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong M., et al. 2006. Suppression of Epstein-Barr nuclear antigen 1 (EBNA1) by RNA interference inhibits proliferation of EBV-positive Burkitt's lymphoma cells. J. Cancer Res. Clin. Oncol. 132:1–8 [DOI] [PubMed] [Google Scholar]
- 9. Hoshino Y., et al. 2009. Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected B cells but not the number of EBV DNA copies per B cell in healthy volunteers. J. Virol. 83:11857–11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imai S., Nishikawa J., Takada K. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kemball C. C., et al. 2005. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J. Immunol. 174:7950–7960 [DOI] [PubMed] [Google Scholar]
- 12. Kim I. J., Flano E., Woodland D. L., Blackman M. A. 2002. Antibody-mediated control of persistent gamma-herpesvirus infection. J. Immunol. 168:3958–3964 [DOI] [PubMed] [Google Scholar]
- 13. Martin D. F., et al. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 340:1063–1070 [DOI] [PubMed] [Google Scholar]
- 14. Moser J. M., Upton J. W., Gray K. S., Speck S. H. 2005. Ex vivo stimulation of B cells latently infected with gammaherpesvirus 68 triggers reactivation from latency. J. Virol. 79:5227–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niller H. H., Wolf H., Minarovits J. 2008. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity 41:298–328 [DOI] [PubMed] [Google Scholar]
- 16. Pauk J., et al. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369–1377 [DOI] [PubMed] [Google Scholar]
- 17. Perera R. A., Samaranayake L. P., Tsang C. S. 2010. Shedding dynamics of Epstein-Barr virus: A type 1 carcinogen. Arch. Oral Biol. 55:639–647 [DOI] [PubMed] [Google Scholar]
- 18. Ptaschinski C., Wilmore J., Fiore N., Rochford R. 2010. In vivo activation of toll-like receptor-9 induces an age-dependent abortive lytic cycle reactivation of murine gammaherpesvirus-68. Viral Immunol. 23:547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rickinson A. B., Kieff E. 2001. Epstein-Barr virus, p. 2575–2627 In Howley P. M., Knipe D. M. (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 20. Siegel A. M., Rangaswamy U. S., Napier R. J., Speck S. H. 2010. Blimp-1-dependent plasma cell differentiation is required for efficient maintenance of murine gammaherpesvirus latency and antiviral antibody responses. J. Virol. 84:674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snyder C. M., et al. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speck P., Longnecker R. 2000. Infection of breast epithelial cells with Epstein-Barr virus via cell-to-cell contact. J. Natl. Cancer Inst. 92:1849–1851 [DOI] [PubMed] [Google Scholar]
- 23. Usherwood E. J., Ward K. A., Blackman M. A., Stewart J. P., Woodland D. L. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283–8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vezys V., et al. 2006. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 203:2263–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Virgin H. W., et al. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weck K. E., Kim S. S., Virgin H. W., Speck S. H. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]