Abstract
Virus infection triggers interferon (IFN)-mediated innate immune defenses in part through viral nucleic acid interactions. However, the immune recognition mechanisms by which the host identifies incoming DNA viruses are still elusive. Here, we show that increased levels of Kaposi's sarcoma-associated herpesvirus (KSHV) persistency are observed in retinoic acid-inducible gene I (RIG-I)-deficient cells and that KSHV ORF64, a tegument protein with deubiqutinase (DUB) activity, suppresses RIG-I-mediated IFN signaling by reducing the ubiquitination of RIG-I, crucial for its activation. This study suggests that RIG-I plays a potential role in sensing KSHV infection and that KSHV ORF64 DUB counteracts RIG-I signaling.
TEXT
Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8 [HHV-8]) belongs to the gammaherpesvirus family, which includes Epstein-Barr virus (EBV), murine gammaherpesvirus 68 (MHV68), and herpesvirus Saimiri (HSV). KSHV is etiologically linked to Kaposi's sarcoma (KS) as well as two rare B-cell proliferative diseases, primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (3, 4). Recent studies have broadened our understanding of the mechanisms by which a number of innate immune sensors recognize herpesviruses and thereby induce innate immune responses (reviewed in references 7 and 21). Specifically, Toll-like receptor 3 (TLR3) and TLR4 are involved in antiviral responses against KSHV (17, 32). Moreover, alpha interferon (IFN-α) treatment has been shown to suppress KSHV lytic replication in PEL cells (20), and higher levels of MHV68 reactivation in IFN-α/β receptor-deficient cells than in wild-type (WT) cells have been reported (2). These studies suggest that IFN plays an important role in antiviral responses against gammaherpesviruses. To maintain persistent infection in hosts, KSHV employs a battery of genes (e.g., the genes encoding vIRFs, RTA, ORF45, and K-bZIP) to antagonize type I IFN signaling (reviewed in references 7 and 18). However, the precise details of how host intracellular pattern recognition receptors such as RIG-I-like receptors (RLRs) function in the recognition of incoming DNA viruses and how herpesviruses evade host RLR-mediated detection remain elusive.
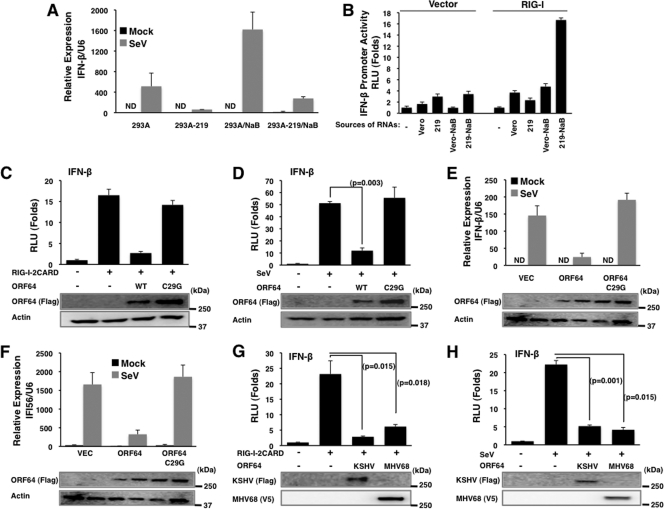
RIG-I is a cytosolic RNA sensor that recognizes viral RNA and is subsequently ubiquitinated by tripartite motif protein 25 (TRIM25) (10) to initiate the IFN signaling cascade. Recently, a growing body of evidence suggests that RIG-I is involved in the recognition of not only RNA viruses but also DNA viruses, including herpesviruses, further highlighting the importance of RIG-I as a viral sensor (1, 5, 6, 22–24). In order to investigate the role of RIG-I-mediated IFN signaling upon KSHV infection, we used a recombinant virus, rKSHV.219, which expresses red fluorescent protein (RFP) through the KSHV lytic PAN promoter and green fluorescent protein (GFP) through the EF-1α promoter (29). 293A cells carrying rKSHV.219 were mock treated or infected with Sendai virus (SeV) (100 hemagglutinating units [HAU]/ml) and then used for quantitative reverse transcription-PCR (qRT-PCR) to measure IFN-β mRNA. This showed that IFN-β mRNA levels were much lower in 293A.rKSHV.219 cells infected with SeV than in naïve 293A cells (Fig. 1A). Induction of KSHV lytic replication with sodium butyrate (NaB) treatment resulted in a minimal induction of IFN-β expression in 293A.rKSHV.219 cells compared to the level in naïve 293A cells (Fig. 1A). In contrast, transfection of total RNAs purified from Vero cells carrying lytically replicating KSHV, but not from Vero cells carrying latent KSHV, readily induced IFN-β promoter activation upon RIG-I overexpression (Fig. 1B), suggesting that RIG-I is capable of recognizing RNAs derived from KSHV replication to induce IFN signaling.
Fig. 1.
Inhibition of RIG-I-mediated signaling by KSHV ORF64. (A) 293A and 293A-rKSHV.219 cells were mock treated or treated with sodium butyrate (NaB; 3 mM) to induce lytic replication. After Sendai virus (SeV) infection, IFN-β mRNA levels were analyzed by qPCR. Values were normalized to U6 RNA transcript levels. ND, nondetectable. (B) Vero or Vero-rKSHV.219 (219) cells were mock treated or treated with sodium butyrate (NaB) to induce lytic reactivation. Total RNAs were prepared from cells by use of TRIzol, followed by treatment of DNase I. Purified total RNAs were transfected with reporter plasmids into HEK293T cells and subjected to an IFN-β promoter luciferase assay. (C and D) Effects of KSHV ORF64 and the ORF64-C29G mutant on IFN-β promoter activity induced by RIG-I–2CARD (C) or SeV infection (D) were analyzed by a dual luciferase assay. Values were normalized by TK-Renilla luciferase values. P values were calculated by a two-tailed t test. RLU, relative luciferase units. (E and F) Effects of KSHV ORF64 and the ORF64-C29G (C29G) mutant on IFN-β (E) and IFI56 (F) transcription upon SeV infection were analyzed by qRT-PCR. (G and H) The effect of MHV68 ORF64 on IFN-β promoter activity induced by RIG-I–2CARD (G) or SeV infection (H) was analyzed by a dual-luciferase assay. P values were calculated by a two-tailed t test. Expression of ORF64 and the ORF64-C29G mutant was confirmed by immunoblot assays (C to H).
A number of viruses encode deubiquitination enzymes (DUBs) to manipulate cellular processes, and several virally encoded DUBs have been shown to antagonize IFN signaling (13, 28, 30, 34). Recently, a number of studies have shown that herpesviridae have large tegument proteins with a DUB domain embedded at their N terminus, such as herpes simplex virus 1 (HSV-1) UL36USP, mouse cytomegalovirus (MCMV) M48, human cytomegalovirus (HCMV) UL48, EBV BPLF1, and KSHV and MHV68 ORF64 (11, 14, 25, 26, 31). These viral tegument DUBs can cleave either Lys48- or Lys63-linked polyubiquitin chains in in vitro deubiquitination assays (11, 16, 26, 33). Since ubiquitination plays a critical role in RIG-I signaling (10), we tested whether KSHV ORF64 inhibits RIG-I function. Ectopic expression of KSHV ORF64 (a kind gift from B. Damania) readily suppressed RIG-I–2CARD-induced IFN-β promoter activation (Fig. 1C) as well as SeV infection-induced IFN-β promoter activation (Fig. 1D). However, the KSHV ORF64-C29G mutant, which is enzymatically defective due to the mutation of its conserved cysteine (11), showed much weaker or no inhibition, suggesting that DUB activity is required for ORF64 to inhibit RIG-I–2CARD- or SeV infection-induced IFN-β promoter activation (Fig. 1C and D). Moreover, KSHV ORF64 blocked IFN-β and IFI56 mRNA production induced by SeV infection, whereas the ORF64-C29G mutant did not (Fig. 1E and F). As seen with KSHV ORF64, MHV68 ORF64 was also able to inhibit RIG-I–2CARD- and SeV-induced IFN-β promoter activation, indicating that the ability of this tegument DUB to inhibit RIG-I function is conserved between KSHV and MHV68 (Fig. 1G and H).
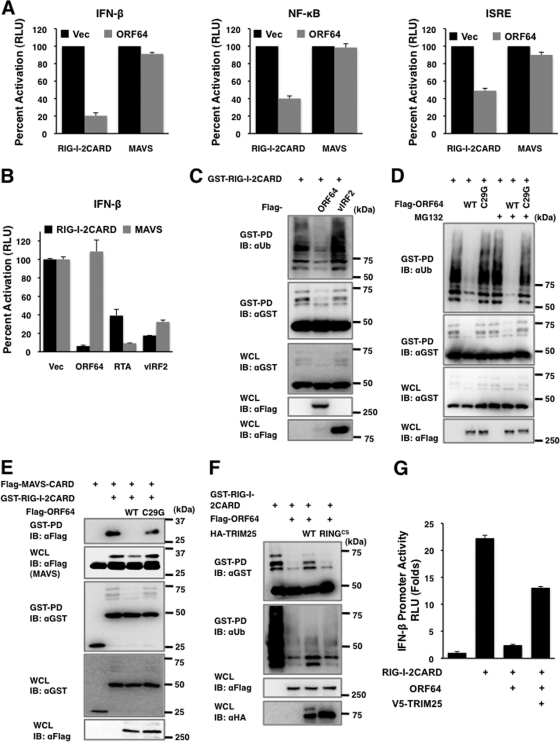
Upon viral infection, the N-terminal CARDs of RIG-I undergo K63-linked ubiquitination induced by E3 ligase TRIM25, which is critical for interaction of RIG-I with its downstream signaling partner MAVS/VISA/IPS-1/Cardif, which ultimately leads to RIG-I-mediated IFN responses (15, 19). Interestingly, despite its robust ability to block RIG-I function, ORF64 was unable to suppress MAVS-mediated activation of IFN-β, NF-κB, and ISRE promoter activities (Fig. 2A). In contrast, the KSHV RTA and vIRF2 proteins were able to suppress both RIG-I–2CARD and MAVS induction of IFN-β promoter activation (Fig. 2B), indicating that, as previously shown (8, 35), RTA and vIRF2 target distinct steps of the IFN pathway downstream of RIG-I and MAVS. To test whether KSHV utilizes ORF64 DUB activity to specifically target RIG-I ubiquitination and thereby block IFN signaling, we examined the levels of RIG-I–2CARD ubiquitination upon coexpression with WT ORF64 or its C29G mutant in 293T cells. vIRF2 was included as a negative control. Ectopic expression of WT ORF64 severely reduced the ubiquitination of RIG-I–2CARD, whereas the ORF64-C29G mutant did not do so under the same conditions (Fig. 2C and D). Furthermore, treatment of MG132 proteosomal inhibitor (20 μM for 4 h) showed little or no effect on glutathione S-transferase (GST)–RIG-I–2CARD levels, indicating that ORF64 markedly downregulates RIG-I ubiquitination without affecting its expression. In contrast, vIRF2 did not show any effect on RIG-I–2CARD ubiquitination (Fig. 2C). Furthermore, due to its efficient downregulation of RIG-I ubiquitination, which is essential for its interaction with the downstream MAVS signaling molecule, KSHV ORF64 effectively blocked the interaction between RIG-I–2CARD and MAVS-CARD (Fig. 2E). Finally, the inhibitory effects of ORF64 on RIG-I-mediated induction of IFN signaling were partially reversed by the overexpression of TRIM25; ectopic expression of WT TRIM25, but not the E3 ligase mutant TRIM25 RINGCS (10), restored not only RIG-I ubiquitination but also RIG-I-mediated induction of IFN-β promoter activity (Fig. 2F and G). These results demonstrate that KSHV ORF64 targets TRIM25-mediated RIG-I ubiquitination, contributing to inhibition of IFN signaling.
Fig. 2.
Reduction of RIG-I ubiquitination induced by KSHV ORF64. (A) HEK293T cells were transfected with vector (Vec), RIG-I–2CARD, or MAVS together with vector or KSHV ORF64. IFN-β, NF-κB, and ISRE promoter activities were assessed by dual-luciferase assays. (B) Vector, ORF64, RTA, or vIRF2 was transfected together with RIG-I–2CARD and reporter plasmids. IFN-β promoter activity was assessed by a dual-luciferase assay. (C) GST-RIG-I–2CARD was transfected with vector, ORF64 (WT), or vIRF2 into HEK293T cells. RIG-I–2CARD ubiquitination was analyzed by GST pulldown (GST-PD), followed by immunoblotting (IB) with antibodies as indicated. Whole-cell lysates (WCL) were analyzed by IB to show expression levels. aUB, anti-Ub. (D) GST–RIG-I–2CARD was transfected with vector, ORF64 (WT), or the ORF64-C29G mutant (C29G) into HEK293T cells, followed by treatment with dimethyl sulfoxide (DMSO) or MG132 (20 μM) for 4 h before harvest. RIG-I–2CARD ubiquitination was analyzed as described for panel C. (E) GST–RIG-I–2CARD and Flag-MAVS-CARD were transfected with vector, ORF64 (WT), or the ORF64-C29G mutant (C29G) into HEK293T cells as indicated. Cell lysates were subjected to GST-PD, followed by IB with antibodies as indicated. (F) GST–RIG-I–2CARD was transfected with vector or ORF64. TRIM25 (WT) or the TRIM25-RINGCS mutant (RINGCS) was transfected together as indicated. RIG-I–2CARD ubiquitination was analyzed as described for panel C. HA, hemagglutinin. (G) The effect of TRIM25 on ORF64 was analyzed by a luciferase assay. Plasmids were transfected as indicated. The experimental procedures were as described for panel A.
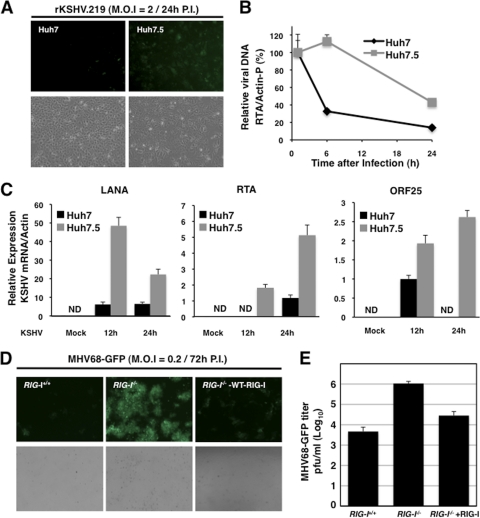
Since KSHV utilizes ORF64 DUB activity to specifically counteract RIG-I-mediated IFN signaling, we then examined the exact role of RIG-I in KSHV infection and replication. To this end, we utilized Huh7 human hepatocellular carcinoma cells and their derivative, Huh7.5 cells carrying RIG-I with a T55I mutation at its 1st CARD that abolishes TRIM25 interaction and thereby disrupts the signaling function of RIG-I to induce antiviral IFN production, leading to a high level of permissiveness to hepatitis C virus (HCV) replication (9, 27). At 24 h after infection with rKSHV.219, significantly higher GFP signal, a marker of rKSHV .219 infection, was observed in Huh7.5 cells than in Huh7 cells (Fig. 3A). While the loss of intracellular KSHV DNA content was observed in both infected Huh7 and infected Huh7.5 cells over a 24-h incubation period, it was markedly faster in Huh7 cells than in Huh7.5 cells (Fig. 3B). qRT-PCR revealed that the levels of viral transcripts, including LANA (latent gene), RTA (immediate-early gene), and ORF25 (late gene), were considerably higher in Huh7.5 cells than in Huh7 cells (Fig. 3C). Similarly, recombinant MHV68-GFP replicated in RIG-I−/− mouse embryonic fibroblasts (MEFs) at markedly higher levels than in RIG-I+/+ MEFs: RIG-I−/− MEFs showed ∼230-fold-higher virus loads at a multiplicity of infection (MOI) of 2 than RIG-I+/+ MEFs (Fig. 3D and E). In contrast, the complementation of RIG-I−/− MEFs with wild-type RIG-I led to a significant reduction of MHV68-GFP replication (Fig. 3D and E). These results indicate a potential role for RIG-I in recognizing incoming gammaherpesviral DNAs.
Fig. 3.
Increased susceptibility of RIG-I deficient cells to KSHV and MHV68 infection. (A) Huh7 and Huh7.5 cells were infected with rKSHV.219 virus at multiplicity of infection (MOI) of 2. Pictures were taken at 24 h after infection. (B) Intracellular KSHV DNA amounts. At the indicated time points after rKSHV.219 infection, total DNAs were purified using a genomic DNA purification kit and KSHV DNA amounts were measured by qPCR using an RTA primer set. Values were normalized to the actin promoter DNA value. The DNA amount at 1 h after infection is set to 100%. (C) The mRNA levels of LANA, RTA, and ORF25 were measured from mock-infected or KSHV-infected Huh7 and Huh7.5 cells by qPCR. Values were normalized to the U6 housekeeping gene transcript value. ND, nondetectable. (D) RIG-I+/+, RIG-I−/−, and RIG-I-reconstituted RIG-I−/− (RIG-I−/−-WT-RIG-I) MEFs were infected with MHV68-GFP (MOI = 0.2). (E) Viral titers of MHV68-GFP from infected RIG-I+/+, RIG-I−/−, and RIG-I-reconstituted RIG-I−/− (RIG-I−/−-WT-RIG-I) MEFs were determined by a plaque assay.
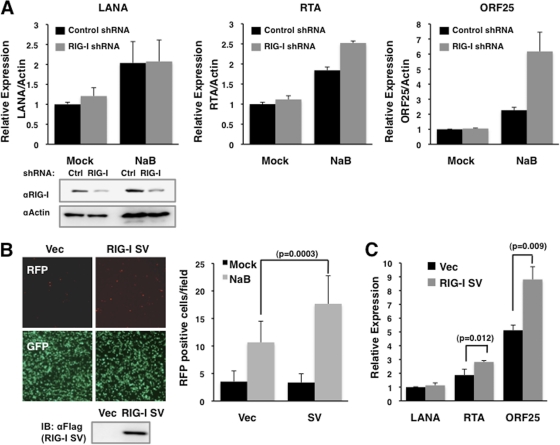
Next, the effect of RIG-I activity on KSHV lytic reactivation was examined by short hairpin RNA (shRNA)-mediated depletion of RIG-I expression (RHS3979-99220020; Open Biosystems) in 293A.rKSHV.219 cells. RIG-I depletion resulted in increased RTA and ORF25 mRNA levels with or without NaB treatment (Fig. 4A). The RIG-I splicing variant (RIG-I SV), which has a short deletion (amino acids 36 to 80) within its 1st CARD, not only loses TRIM25-mediated ubiquitination but also effectively suppresses RIG-I-mediated IFN-β production (9). RIG-I SV was transfected into 293A.rKSHV.219 cells, followed by NaB (3 mM) treatment to induce the lytic reactivation of rKSHV.219. This showed that ectopic expression of RIG-I SV pronouncedly increased the numbers of RFP-positive cells, a marker for rKSHV .219 reactivation (Fig. 4B). Correlated with this, the levels of RTA and ORF25 transcripts were detectably higher in cells transfected with RIG-I SV than in cells transfected with vector alone (Fig. 4C). Collectively, these results suggest that RIG-I-mediated antiviral signaling is involved in suppression of KSHV lytic reactivation.
Fig. 4.
Increased lytic replication of KSHV by suppression of RIG-I function or expression. (A) 293A.rKSHV.219 cells were transfected with control or RIG-I silencing shRNAs. Cells treated with NaB for 48 h were subjected to qRT-PCR analysis to measure the mRNA levels of viral and RIG-I genes. Values were normalized to actin mRNA values. (B) 293A.rKSHV.219 cells were transfected with vector or the RIG-I splicing variant (RIG-I SV) and treated with sodium butyrate (NaB; 3 mM) to induce lytic replication. The numbers of RFP-positive cells were counted and are presented as a bar graph (right panel). (C) At 48 h after NaB treatment, the levels of LANA, RTA, and ORF25 mRNA were measured from cells transfected with either vector or RIG-I SV by qRT-PCR. Values were normalized to actin mRNA values. P values were calculated by a two-tailed t test.
The discovery of a ubiquitin (Ub)-specific cysteine protease encoded within the HSV-1 UL36 tegument protein was a seminal finding (14). Since then, a number of herpesvirus ubiquitin-specific proteases have been studied for their functions in the viral life cycle (11, 25, 26, 31). Specifically, recombinant MHV68 carrying an enzymatically inactive ORF64 protein was cleared faster than revertant viruses in an in vivo mouse infection model (12), an HCMV UL48 mutant virus produced 10-fold-lower progeny virus (16), and KSHV ORF64 depletion resulted in decreased viral lytic transcription and lytic-protein expression (11). Here, we demonstrate that increased levels of KSHV persistency are observed in RIG-I-deficient or -depleted cells and that the KSHV ORF64 DUB enzyme specifically targets and suppresses RIG-I-mediated signaling, suggesting that RIG-I plays a potential role in detecting KSHV infection and that KSHV ORF64 counteracts RIG-I signaling. However, it remains elusive what component(s) of KSHV is sensed by RIG-I. It has been reported that EBER1 and EBER2, the most abundant noncoding RNAs produced during EBV infection, activate the RIG-I pathway (23). However, we found that, unlike EBV EBER1/2, KSHV PAN RNA (T1.1/nut-1), the most abundant noncoding RNA produced during KSHV infection (36, 37), did not induce RIG-I activation (data not shown). Further study is thus necessary to elucidate how RIG-I senses KSHV infection. Finally, since another tegument protein, ORF45, encoded by KSHV, has been shown to block host IFN signaling by inhibiting IRF7 activity (38, 39), KSHV utilizes two virion-associated ORF45 and ORF64 tegument proteins to mitigate intracellular RIG-I-mediated antiviral responses to enhance KSHV infectivity and persistency.
Acknowledgments
This work was partly supported by grants CA82057, CA91819, CA31363, CA115284, CA147868, CA148616, DE019085, AI073099, and AI083025, the Hastings Foundation, the Fletcher Jones Foundation, and a National Agenda Project grant from the Korea Research Council of Fundamental Science & Technology.
We thank Blossom Damania for reagents and Stacy Lee for manuscript preparation.
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Ablasser A., et al. 2009. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton E. S., Lutzke M. L., Rochford R., Virgin IV H. W. 2005. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79:14149–14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cesarman E., Chang Y., Moore P. S., Said J. W., Knowles D. M. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 4. Chang Y., et al. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 5. Cheng G., Zhong J., Chung J., Chisari F. V. 2007. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. U. S. A. 104:9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu Y. H., Macmillan J. B., Chen Z. J. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coscoy L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat. Rev. Immunol. 7:391–401 [DOI] [PubMed] [Google Scholar]
- 8. Fuld S., Cunningham C., Klucher K., Davison A. J., Blackbourn D. J. 2006. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 80:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gack M. U., et al. 2008. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc. Natl. Acad. Sci. U. S. A. 105:16743–16748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gack M. U., et al. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916–920 [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez C. M., Wang L., Damania B. 2009. Kaposi's sarcoma-associated herpesvirus encodes a viral deubiquitinase. J. Virol. 83:10224–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gredmark-Russ S., et al. 2009. A gammaherpesvirus ubiquitin-specific protease is involved in the establishment of murine gammaherpesvirus 68 infection. J. Virol. 83:10644–10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang J., Tang H. 2010. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein. Protein Cell 1:1106–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kattenhorn L. M., Korbel G. A., Kessler B. M., Spooner E., Ploegh H. L. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547–557 [DOI] [PubMed] [Google Scholar]
- 15. Kawai T., et al. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981–988 [DOI] [PubMed] [Google Scholar]
- 16. Kim E. T., Oh S. E., Lee Y. O., Gibson W., Ahn J. H. 2009. Cleavage specificity of the UL48 deubiquitinating protease activity of human cytomegalovirus and the growth of an active-site mutant virus in cultured cells. J. Virol. 83:12046–12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lagos D., et al. 2008. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4:470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee H. R., Lee S., Chaudhary P. M., Gill P., Jung J. U. 2010. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Future Microbiol. 5:1349–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meylan E., et al. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172 [DOI] [PubMed] [Google Scholar]
- 20. Monini P., et al. 1999. Alpha interferon inhibits human herpesvirus 8 (HHV-8) reactivation in primary effusion lymphoma cells and reduces HHV-8 load in cultured peripheral blood mononuclear cells. J. Virol. 73:4029–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paludan S. R., Bowie A. G., Horan K. A., Fitzgerald K. A. 2011. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rasmussen S. B., et al. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 90:74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samanta M., Iwakiri D., Kanda T., Imaizumi T., Takada K. 2006. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25:4207–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samanta M., Iwakiri D., Takada K. 2008. Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene 27:4150–4160 [DOI] [PubMed] [Google Scholar]
- 25. Schlieker C., Korbel G. A., Kattenhorn L. M., Ploegh H. L. 2005. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 79:15582–15585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlieker C., et al. 2007. Structure of a herpesvirus-encoded cysteine protease reveals a unique class of deubiquitinating enzymes. Mol. Cell 25:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sumpter R., Jr., et al. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun Z., Chen Z., Lawson S. R., Fang Y. 2010. The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. J. Virol. 84:7832–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vieira J., O'Hearn P. M. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225–240 [DOI] [PubMed] [Google Scholar]
- 30. Wang D., et al. 2011. The leader proteinase of foot-and-mouth disease virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. Virol. 85:3758–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J., Loveland A. N., Kattenhorn L. M., Ploegh H. L., Gibson W. 2006. High-molecular-weight protein (pUL48) of human cytomegalovirus is a competent deubiquitinating protease: mutant viruses altered in its active-site cysteine or histidine are viable. J. Virol. 80:6003–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West J., Damania B. 2008. Upregulation of the TLR3 pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. J. Virol. 82:5440–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitehurst C. B., et al. 2009. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 83:4345–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wojdyla J. A., et al. 2010. Papain-like protease 1 from transmissible gastroenteritis virus: crystal structure and enzymatic activity toward viral and cellular substrates. J. Virol. 84:10063–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu Y., Wang S. E., Hayward G. S. 2005. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity 22:59–70 [DOI] [PubMed] [Google Scholar]
- 36. Zhong W., Ganem D. 1997. Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J. Virol. 71:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong W., Wang H., Herndier B., Ganem D. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. U. S. A. 93:6641–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu F. X., King S. M., Smith E. J., Levy D. E., Yuan Y. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. U. S. A. 99:5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu F. X., Sathish N., Yuan Y. 2010. Antagonism of host antiviral responses by Kaposi's sarcoma-associated herpesvirus tegument protein ORF45. PLoS One 5:e10573. [DOI] [PMC free article] [PubMed] [Google Scholar]