Abstract
Xenotropic murine leukemia virus (MLV)-related virus (XMRV) has been amplified from human prostate cancer and chronic fatigue syndrome (CFS) patient samples. Other studies failed to replicate these findings and suggested PCR contamination with a prostate cancer cell line, 22Rv1, as a likely source. MLV-like sequences have also been detected in CFS patients in longitudinal samples 15 years apart. Here, we tested whether sequence data from these samples are consistent with viral evolution. Our phylogenetic analyses strongly reject a model of within-patient evolution and demonstrate that the sequences from the first and second time points represent distinct endogenous murine retroviruses, suggesting contamination.
TEXT
Detection of murine leukemia virus (MLV) DNA in patient samples has suggested that the human population may be infected with murine gammaretroviruses (4). A particular xenotropic MLV named xenotropic murine leukemia virus-related virus (XMRV) has been cloned from human prostate cancer tumors as well as blood samples from individuals suffering from chronic fatigue syndrome (CFS) (1, 18, 29, 39). XMRV detection in a small percentage of samples from healthy controls suggested widespread infection (7, 18, 29). Controversy has since surrounded XMRV detection, partly because many laboratories have been unable to detect XMRV in patient samples (2, 5, 6, 8–11, 14, 16, 19–21, 28, 30, 35, 37, 40) and partly because an almost identical virus has been found infecting a common prostate cancer cell line called 22Rv1 (12, 15, 23). These data strongly suggest that XMRV in patient material is the result of DNA contamination from laboratory cell lines or mouse DNA. Importantly, a recent study demonstrated that XMRV arose by recombination during the experiments in which the 22Rv1 cell line was developed by xenografting prostate tumors in mice (22). This observation confirms a date for XMRV genesis in the cell line at between 1990 and 1996 and rules out any human XMRV infection before this time. These observations have raised concerns that previous XMRV detection in humans is likely to be artifactual (3).
An important study in support of MLV infection in humans is that by Lo, Alter, and colleagues (17). These authors suggested that they could confirm human infection of MLV by PCR amplifying a variety of MLV sequences from the blood of CFS patients as well as healthy controls. A PCR test for mouse mitochondrial DNA was used to control for contamination with mouse DNA and found to be negative, but recently, more sensitive intracisternal type A particle (IAP)-based PCR tests for murine contamination reveal that in some cases mouse contamination is not detected by amplification of mitochondrial DNA (26). Surprisingly, Lo et al.'s study did not find XMRV but found a set of MLV sequences almost identical to known endogenous nonecotropic gammaretroviruses of mice. These MLV sequences were characterized as type 1 (18 patients), type 2 (2 patients), and type 3 (1 patient), based on their gag gene sequences. Importantly, the authors suggested that evolution of patient viruses could be demonstrated by the accumulation of significant sequence variation over time. Longitudinal samples were taken from eight individuals apparently infected with type 1 viruses 15 years after the first sampling. Seven of these had detectable MLV gag at the second time point (28). The sequences derived from six of these longitudinal samples have been deposited in GenBank under accession numbers HQ601957 to HQ601962. Here, we used phylogenetic analyses to consider whether MLV sequences described in this study are consistent with viral infective evolution, a conservative test of whether they are likely to represent genuine human MLV infections.
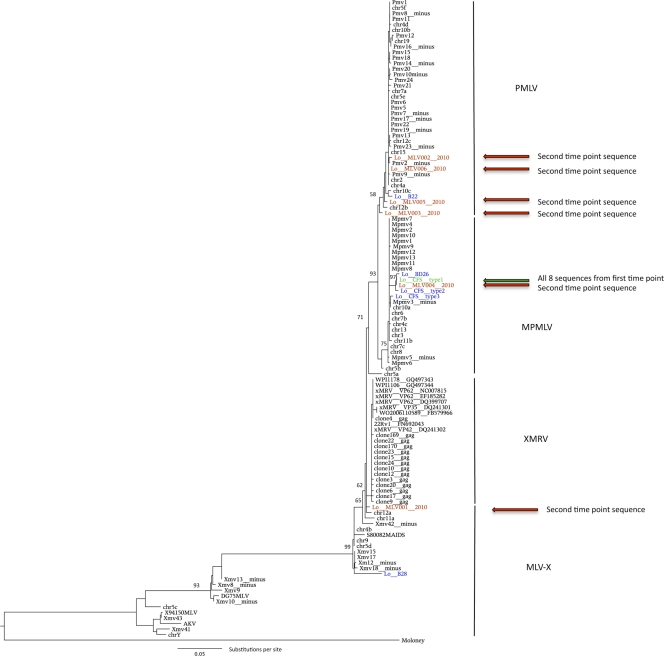
The shape of a phylogenetic tree reflects the evolutionary processes under which it has grown. The expectation for longitudinally sampled retroviral sequences from the same patient, or from a population of infected patients, is that they cluster with the initial sequences and to the exclusion of all other sequences in the data set. Phylogenetic analysis of the human-derived MLV sequences as well as a variety of known MLV sequences (see the supplemental material for details) indicates that the three patient-derived sequence types, CFS types 1 to 3, fall within the modified polytropic MLV clade. The overall shape of the phylogenetic tree, including the three main groupings (polytropic, modified polytopic, and xenotropic) and the relationships between them, is consistent with previous studies based on full-length proviruses (Fig. 1) (12, 13). Sequences described in Lo et al.'s original report are colored blue and green. While the type 3 sequence is clearly separated from types 1 and 2, the latter sequences form a strongly supported monophyletic cluster (97% bootstrap support) together with the sequence BD26, derived from a healthy donor, and the sequence MLV004_2010, a longitudinal CFS patient sample (Fig. 1). All the resampled patients originally yielded identical polytropic MLV CFS type 1 sequences (S. C. Lo, personal communication); in other words, all eight sequences derived from patients at the first time point are represented by a single branch in the phylogeny. Under a model of within-patient viral evolution, we would expect all of the 2010 daughter sequences to branch from the parental CFS type 1 sequence with longer but approximately equidistant branches. This is true for all other longitudinally sequenced viruses (reviewed in reference 24).
Fig. 1.
Maximum likelihood phylogeny of XMRV, xenotropic MLV (MLV-X), polytropic MLV (PMLV; Pmv), and modified polytropic MLV (MPMLV; Mpmv) gag gene sequences (710 nt). The initial chronic fatigue syndrome patient-derived XMRV sequences from Lo et al. (17) are indicated in blue. The eight sequences taken from the first time point are represented by a single branch, colored green. XMRV sequences sampled 15 years later from the same patients (GenBank accession numbers HQ601957 to HQ601962) are colored in red, while other sequences that do not have corresponding second-time-point sequences from Lo et al. are colored blue. The tree is rooted with Moloney MLV (GenBank accession number AF033811). Bootstrap scores of >50% are indicated on the corresponding branches. The scale bar represents the number of nucleotide substitutions per site.
We assessed the fit of the data to this model by inspection of the phylogenetic tree and by maximum likelihood-based model testing. The phylogenetic placement of the longitudinal sequences does not fit this expected model. When the sequences from the second time point were examined, we found that 5 of 6 are phylogenetically distinct from the parental CFS type 1 sequence and from each other. The more recent longitudinally sampled sequences are shown in red in Fig. 1. While the originating CFS type 1 sequences belong to the modified polytropic clade, longitudinally sampled sequences from the same CFS type 1-infected patients are derived from three strongly supported and distinct regions of the tree, namely, the polytropic, modified polytropic, and xenotropic clades. Surprisingly, 3 of the new sequences (MLV002_2010, MLV005_2010, and MLV006_2010) do not even form sister taxa within the polytropic clade. In fact, the two most distantly related sequences from these longitudinal patient samples are about as different from each other as the biggest distance possible within the polytropic clade. Another 2010 (MLV001_2010) sequence is placed within the xenotropic clade, at the base of the XMRV cluster. It also contains a large deletion in gag, which would be expected to inactivate the virus. Although this sequence is basal, aside from the deletion, it is very similar to other XMRV sequences, differing at only a single nucleotide position across its 330-nucleotide (nt) length from the prostate cancer patient sequence VP62 (39). It is therefore substantially more similar to XMRV than to either the polytropic or modified polytropic MLV sequences previously reported by Lo et al. (17). There is no evidence for hypermutation mediated by APOBEC proteins in the patient-derived sequences as might be expected during an infection, given the susceptibility of MLV to mutation by these proteins (23). Thus, these new patient-derived MLV sequences show tremendous variation from the parental CFS type 1 sequence and as such are extremely unlikely to have evolved from the CFS type 1 sequence.
The probability that the data are consistent with a model of within-patient evolution can be explicitly tested by comparing the maximum likelihood phylogenetic tree directly derived from the data with a tree in which sequences from the second time point are constrained to cluster with sequences from the first time point. The difference in likelihood of these two topologies was determined by using the Shimodaira-Hasegawa test (33), the approximately unbiased test (31), and expected likelihood weights (34) as implemented in CONSEL (32). We can reject the hypothesis of clustering of sequences from the second time point with those from the first (Table 1). In order to explore the robustness of this test to the possibility that any one individual sequence is consistent with within-patient evolution, and that the P value may be unduly influenced by any of the other sequences, we also explored the relative fit of each sequence from the second time point to a model consistent with within-patient evolution (i.e., constrained to cluster with sequences from the first time point). We compared the likelihood of five additional trees, where each patient sample was constrained in turn to cluster with the CFS type 1 sequence while the other patients were allowed to assume the most likely position within the tree. For each patient individually, the tree that is consistent with within-patient evolution was rejected (Table 1). The use of these likelihood-based tests is robust to the short sequences used in the alignment, which account for the low bootstrap support scores for many nodes in the phylogenetic tree.
Table 1.
Comparison of maximum likelihood phylogenetic tree with hypotheses consistent with within-patient viral evolutiona
| Constraint | Log likelihood | Decrease in likelihood | Standard deviation |
P value |
Expected likelihood wt | |
|---|---|---|---|---|---|---|
| SH | AU | |||||
| Best tree | −3,658.76 | 0.00 | NA | NA | NA | 0.99 |
| All 5 MLV 2010 | −3,721.22 | 62.46 | 14.92 | <0.001 | <0.0001 | <0.0001 |
| MLV001 2010 | −3,689.93 | 31.17 | 9.62 | 0.001 | <0.0001 | <0.0001 |
| MLV002 2010 | −3,696.66 | 37.90 | 10.80 | 0.002 | <0.0001 | <0.0001 |
| MLV003 2010 | −3,679.73 | 20.96 | 9.31 | 0.022 | 0.002 | 0.0086 |
| MLV005 2010 | −3,687.62 | 28.86 | 10.15 | 0.004 | 0.0003 | 0.0004 |
| MLV006 2010 | −3,701.63 | 42.87 | 12.90 | 0.002 | <0.0001 | <0.0001 |
The most stringent constraint involved all 5 MLV sequences from the second time point, while each of the 5-s time point sequences was also constrained individually to cluster with the CFS type 1 sequence from the first time point. The trees were compared using pairwise Shimodaira-Hasegawa (SH) tests, the approximately unbiased (AU) test, and expected likelihood weights. All of the constrained trees were significantly worse than the maximum likelihood tree. NA, not applicable.
To calculate the chance of a modified polytropic virus evolving into a polytropic virus, we reconstructed the common ancestors of the polytropic and modified polytropic clades by taking a consensus of each sequence set. We then estimated the probability of a virus making the specific changes required to evolve from one clade to the other by running 10,000 simulations in Seq-Gen and counting the number of times the mutations arose (25). The hypothesis that the characteristic mutations could have arisen by chance, given that a number of mutations equivalent to the distance between these groups had occurred, can be rejected with a P value of <0.0001. Indeed, the fact that 3 of the newly sampled viruses appear to have independently made these specific changes underlines the fact that these sequences represent different viruses rather than CFS type 1 descendants. The last of the longitudinal sequences is 100% identical to the CFS type 1 sequence identified in 18 patients at the initial time point, 15 years earlier. This observation indicates that in this case, there has been no viral evolution throughout 15 years of infection. Some slowly evolving retroviruses (e.g., simian foamy virus) can remain virtually identical over many years (36); however, this patient sample contrasts markedly with all the other samples from the second time point. In summary, in some patients, viral diversity is as vast as the diversity that the whole set of known nonecotropic MLVs allows, and yet from another patient, an identical sequence was amplified 15 years later. The repeat samples could not have been derived from the initial samples via a process of viral evolution. In fact, they represent different endogenous murine viruses. It is theoretically possible that the 6 patients were originally coinfected with diverse viruses, but this possibility is rather undermined by the fact that the original samples each gave rise to the same identical type 1 sequence from 18 independent patients. An alternative possibility is that all but one of the patients that retested positive for MLV were superinfected with a distinct MLV prior to the samples being taken at the second time point and that in each of these patients, the viral infection from the first time point was cleared.
Examination of the viral sequences amplified reveals that they are almost identical to known sequences in mouse genomic DNA. The numbers of differences between the patient amplified samples and their nearest relatives in the published mouse C57BL/6J genome are shown in Table 2 . For example, MLV002 is 1 nucleotide out of 339 nucleotides different from polytropic MLV on the C57BL/6J mouse chromosomes 4, 2, and 15, most likely due to a single nucleotide polymorphism within the mouse population. The only realistic explanation that could account for these observations is that all of the MLV-positive patient samples, or the PCRs performed using patient DNA as template, were contaminated with mouse DNA. This would act as a source for amplification of the diverse viruses found and could perhaps have occurred as a result of repeated handling of patient-derived samples (see reference 41 for a discussion). There is no credible hypothesis that could explain these observations in the absence of PCR contamination. It appears to be extremely difficult to do mouse-free PCR, and we note that other studies in which contamination has been demonstrated have also amplified a diverse range of MLVs (27, 38). We propose that the detection of murine virus in human samples be more rigorously controlled using IAP PCR (26) to rule out murine DNA contamination and robust phylogenetic analysis to rule out random amplification of endogenous proviruses (12), which can exist at a high copy number in the genomes of mice or in cell lines that become infected with mouse viruses during routine experimentation.
Table 2.
Comparison of patient-derived MLV sequences and known MLV sequences within the mouse genomea
| Sequence | GenBank accession no. | Sampling yr(s) | Closest relative in the C57BL/6J mouse genome (July 2007 assembly) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Strand | Span (nt) | Start (nt) | End (nt) | % identity | Changesb | Gaps (length in nt) | |||
| BD22 | HM630560 | Mid-1990s | 10 | − | 697 | 8269377 | 8270073 | 99.30 | 5/697 | 0 |
| 10 | − | 696 | 50145707 | 50146402 | 99.30 | 5/696 | 0 | |||
| X | + | 696 | 15052167 | 15052862 | 99.30 | 5/696 | 0 | |||
| BD26 | HM630561 | Mid-1990s | 6 | − | 339 | 73242571 | 73242909 | 98.30 | 6/339 | 0 |
| 2 | − | 339 | 15949590 | 15949928 | 98.30 | 6/339 | 0 | |||
| 10 | − | 339 | 4627251 | 4627589 | 98.30 | 6/339 | 0 | |||
| BD28 | HM630557 | Mid-1990s | 9 | + | 331 | 62288048 | 62288378 | 98.10 | 6/318 | 1 (21) |
| 5 | + | 331 | 23722164 | 23722494 | 98.10 | 6/318 | 1 (21) | |||
| 4 | + | 331 | 133716363 | 133716693 | 98.10 | 6/318 | 1 (21) | |||
| CFS type 1 | HM630562 | Mid-1990s | 8 | + | 697 | 125689652 | 125690348 | 99.30 | 5/697 | 0 |
| 11 | + | 697 | 102946013 | 102946709 | 99.30 | 5/697 | 0 | |||
| 6 | − | 696 | 73242413 | 73243108 | 98.90 | 5/696 | 0 | |||
| CFS type 2 | HM630558 | Mid-1990s | 8 | + | 698 | 125689652 | 125690349 | 99.00 | 7/698 | 1 (1) |
| 11 | + | 698 | 102946013 | 102946710 | 99.00 | 7/698 | 1 (1) | |||
| 6 | − | 697 | 73242412 | 73243108 | 98.60 | 7/697 | 1 (1) | |||
| CFS type 3 | HM630559 | Mid-1990s | 2 | − | 697 | 15949431 | 15950127 | 99.90 | 1/697 | 0 |
| 13 | + | 697 | 21905315 | 21906011 | 99.80 | 2/697 | 0 | |||
| 6 | − | 696 | 73242413 | 73243108 | 99.30 | 1/696 | 1 (1) | |||
| MLV001 | HQ601957 | 2010 | 12 | + | 340 | 19250254 | 19250593 | 99.70 | 1/276 | 2 (63; 1) |
| 9 | + | 339 | 62288048 | 62288386 | 99.00 | 2/276 | 1 (63) | |||
| 5 | + | 339 | 23722164 | 23722502 | 99.00 | 3/276 | 1 (63) | |||
| MLV002 | HQ601958 | 2010 | 4 | − | 339 | 107826090 | 107826428 | 99.80 | 1/339 | 0 |
| 2 | − | 339 | 57074273 | 57074611 | 99.80 | 1/339 | 0 | |||
| 15 | − | 339 | 76395902 | 76396240 | 99.80 | 1/339 | 0 | |||
| MLV003 | HQ601959 | 2010 | 6 | − | 339 | 73242571 | 73242909 | 99.20 | 3/339 | 0 |
| 4 | − | 339 | 107826090 | 107826428 | 99.20 | 3/339 | 0 | |||
| 2 | − | 339 | 57074273 | 57074611 | 99.20 | 3/339 | 0 | |||
| MLV004 | HQ601960 | 2010 | 6 | − | 339 | 73242571 | 73242909 | 98.60 | 5/339 | 0 |
| 2 | − | 339 | 15949590 | 15949928 | 98.60 | 5/339 | 0 | |||
| 10 | − | 339 | 4627251 | 4627589 | 98.60 | 5/339 | 0 | |||
| MLV005 | HQ601961 | 2010 | 4 | − | 339 | 107826090 | 107826428 | 99.50 | 2/339 | 0 |
| 2 | − | 339 | 57074273 | 57074611 | 99.50 | 2/339 | 0 | |||
| 15 | − | 339 | 76395902 | 76396240 | 99.50 | 2/339 | 0 | |||
| MLV006 | HQ601962 | 2010 | 4 | − | 339 | 107826090 | 107826428 | 100.00 | 0/339 | 0 |
| 2 | − | 339 | 57074273 | 57074611 | 100.00 | 0/339 | 0 | |||
| 15 | − | 339 | 76395902 | 76396240 | 100.00 | 0/339 | 0 | |||
Due to the short lengths of the patient-derived sequences, several mouse sequences are equally similar. Thus, three sequences are shown. The span column refers to the total length of the best match in the murine chromosome (Chr.).
Number of nucleotides different/total number of nucleotides.
Supplementary Material
Acknowledgments
This work was funded by a fellowship from the Royal Society to A.K., the European Community's Seventh Framework Programme (FP7/2007-2013) under the project Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN), grant agreement no. 223131, Wellcome Trust Sanger Institute, the National Institute of Health Research UCL/UCLH Comprehensive Biomedical Research Centre, Wellcome Trust Senior Fellowship WT090940 to G.J.T., and the Medical Research Council.
We thank Jonathan Stoye for sharing unpublished observations.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Arnold R. S., et al. 2010. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology 75:755–761 [DOI] [PubMed] [Google Scholar]
- 2. Barnes E., et al. 2010. Failure to detect xenotropic murine leukemia virus-related virus in blood of individuals at high risk of blood-borne viral infections. J. Infect. Dis. 202:1482–1485 [DOI] [PubMed] [Google Scholar]
- 3. Callaway E. 2011. Virology: fighting for a cause. Nature 471:282–285 [DOI] [PubMed] [Google Scholar]
- 4. Coffin J. M., Stoye J. P. 2009. A new virus for old diseases? Science 326:530–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornelissen M., et al. 2010. Lack of detection of XMRV in seminal plasma from HIV-1 infected men in The Netherlands. PLoS One 5:e12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erlwein O., et al. 2010. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS One 5:e8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer N., et al. 2010. Xenotropic murine leukemia virus-related gammaretrovirus in respiratory tract. Emerg. Infect. Dis. 16:1000–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groom H. C., et al. 2010. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henrich T. J., et al. 2010. Xenotropic murine leukemia virus-related virus prevalence in patients with chronic fatigue syndrome or chronic immunomodulatory conditions. J. Infect. Dis. 202:1478–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hohn O., et al. 2009. Lack of evidence for xenotropic murine leukemia virus-related virus (XMRV) in German prostate cancer patients. Retrovirology 6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong P., Li J., Li Y. 2010. Failure to detect Xenotropic murine leukaemia virus-related virus in Chinese patients with chronic fatigue syndrome. Virol. J. 7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hué S., et al. 2010. Disease-associated XMRV sequences are consistent with laboratory contamination. Retrovirology 7:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jern P., Stoye J. P., Coffin J. M. 2007. Role of APOBEC3 in genetic diversity among endogenous murine leukemia viruses. PLoS Genet. 3:2014–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeziorski E., et al. 2010. No evidence for XMRV association in pediatric idiopathic diseases in France. Retrovirology 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knouf E. C., et al. 2009. Multiple integrated copies and high-level production of the human retrovirus XMRV (xenotropic murine leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J. Virol. 83:7353–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunstman K. J., Bhattacharya T., Flaherty J., Phair J. P., Wolinsky S. M. 2010. Absence of xenotropic murine leukemia virus-related virus in blood cells of men at risk for and infected with HIV. AIDS 24:1784–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lo S. C., et al. 2010. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc. Natl. Acad. Sci. U. S. A. 107:15874–15879 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Lombardi V. C., et al. 2009. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 326:585–589 [DOI] [PubMed] [Google Scholar]
- 19. Luczkowiak J., Sierra O., Gonzalez-Martin J. J., Herrero-Beaumont G., Delgado R. 2011. No xenotropic murine leukemia virus-related virus detected in fibromyalgia patients. Emerg. Infect. Dis. 17:314–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maric R., et al. 2010. Absence of xenotropic murine leukaemia virus-related virus in Danish patients with multiple sclerosis. J. Clin. Virol. 49:227–228 [DOI] [PubMed] [Google Scholar]
- 21. McCormick A. L., Brown R. H., Jr., Cudkowicz M. E., Al-Chalabi A., Garson J. A. 2008. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology 70:278–283 [DOI] [PubMed] [Google Scholar]
- 22. Paprotka T., et al. 2011. Recombinant origin of the retrovirus XMRV. Science 333:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paprotka T., et al. 2010. Inhibition of xenotropic murine leukemia virus-related virus by APOBEC3 proteins and antiviral drugs. J. Virol. 84:5719–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pybus O. G., Rambaut A. 2009. Evolutionary analysis of the dynamics of viral infectious disease. Nat. Rev. Genet. 10:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rambaut A., Grassly N. C. 1997. Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic frees. Comput. Appl. Biosci. 13:235–238 [DOI] [PubMed] [Google Scholar]
- 26. Robinson M. J., et al. 2010. Mouse DNA contamination in human tissue tested for XMRV. Retrovirology 7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakuma T., et al. 2011. No evidence of XMRV in prostate cancer cohorts in the Midwestern United States. Retrovirology 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satterfield B. C., et al. 2011. Serologic and PCR testing of persons with chronic fatigue syndrome in the United States shows no association with xenotropic or polytropic murine leukemia virus-related viruses. Retrovirology 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schlaberg R., Choe D. J., Brown K. R., Thaker H. M., Singh I. R. 2009. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc. Natl. Acad. Sci. U. S. A. 106:16351–16356 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Sfanos K. S., et al. 2008. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate 68:306–320 [DOI] [PubMed] [Google Scholar]
- 31. Shimodaira H. 2002. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51:492–508 [DOI] [PubMed] [Google Scholar]
- 32. Shimodaira H., Hasegawa M. 2001. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17:1246–1247 [DOI] [PubMed] [Google Scholar]
- 33. Shimodaira H., Hasegawa M. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16:1114–1116 [Google Scholar]
- 34. Strimmer K., Rambaut A. 2002. Inferring confidence sets of possibly misspecified gene trees. Proc. Biol. Sci. 269:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Switzer W. M., et al. 2010. Absence of evidence of xenotropic murine leukemia virus-related virus infection in persons with chronic fatigue syndrome and healthy controls in the United States. Retrovirology 7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Switzer W. M., et al. 2005. Ancient co-speciation of simian foamy viruses and primates. Nature 434:376–380 [DOI] [PubMed] [Google Scholar]
- 37. Tang S., et al. 2011. Absence of detectable xenotropic murine leukemia virus-related virus in plasma or peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected blood donors or individuals in Africa. Transfusion 51:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tuke P. W., Tettmar K. I., Tamuri A., Stoye J. P., Tedder R. S. 2011. PCR master mixes harbour murine DNA sequences. Caveat emptor! PLoS One 6:e19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urisman A., et al. 2006. Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2:e25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. van Kuppeveld F. J., et al. 2010. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ 340:c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiss R. A. 2010. A cautionary tale of virus and disease. BMC Biol. 8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.