Abstract
Attempts to develop a vaccine to prevent genital herpes simplex virus 2 (HSV-2) disease have been only marginally successful, suggesting that novel strategies are needed. Immunization with HSV-2 glycoprotein C (gC-2) and gD-2 was evaluated in mice and guinea pigs to determine whether adding gC-2 to a gD-2 subunit vaccine would improve protection by producing antibodies that block gC-2 immune evasion from complement. Antibodies produced by gC-2 immunization blocked the interaction between gC-2 and complement C3b, and passive transfer of gC-2 antibody protected complement-intact mice but not C3 knockout mice against HSV-2 challenge, indicating that gC-2 antibody is effective, at least in part, because it prevents HSV-2 evasion from complement. Immunization with gC-2 also produced neutralizing antibodies that were active in the absence of complement; however, the neutralizing titers were higher when complement was present, with the highest titers in animals immunized with both antigens. Animals immunized with the gC-2-plus-gD-2 combination had robust CD4+ T-cell responses to each immunogen. Multiple disease parameters were evaluated in mice and guinea pigs immunized with gC-2 alone, gD-2 alone, or both antigens. In general, gD-2 outperformed gC-2; however, the gC-2-plus-gD-2 combination outperformed gD-2 alone, particularly in protecting dorsal root ganglia in mice and reducing recurrent vaginal shedding of HSV-2 DNA in guinea pigs. Therefore, the gC-2 subunit antigen enhances a gD-2 subunit vaccine by stimulating a CD4+ T-cell response, by producing neutralizing antibodies that are effective in the absence and presence of complement, and by blocking immune evasion domains that inhibit complement activation.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) infection is the most common cause of genital ulcer disease (21). HSV-2 causes primary and recurrent infections that are often asymptomatic, yet HSV-2 increases the risk of acquiring HIV-1 by approximately 3-fold (52, 53, 63). Antiviral therapy reduces the duration of HSV-2 symptomatic infection, and daily suppressive therapy decreases symptomatic recurrences and asymptomatic viral shedding (4). Nevertheless, protection is incomplete because antiviral therapy does not eradicate latency (54, 55). HSV-2 is an important target for vaccine development to prevent genital ulcer disease, based in part on the association between HSV-2 infection and HIV-1 acquisition.
Two large clinical trials were performed to evaluate HSV-2 subunit antigen vaccines. One trial included HSV-2 glycoprotein B (gB-2) and gD-2, and another used a different adjuvant and involved gD-2 alone (12, 57). HSV-2 infection and reactivation rates were similar for the vaccine and placebo groups in the combined gB-2-plus-gD-2 vaccine trial, indicating poor efficacy of the candidate vaccine (12). The gD-2 subunit vaccine trial reported no significant difference in genital lesions between vaccine and placebo groups; however, in a subgroup analysis, the vaccine was found to be effective in women who were seronegative for both HSV-1 and HSV-2 prior to vaccination but not in men, regardless of their prior exposure to HSV (57). A follow-up trial in HSV-1- and HSV-2-seronegative women was conducted recently to further evaluate this unexpected finding. The results of this trial have not yet been published; however, the National Institute of Allergy and Infectious Diseases and GlaxoSmithKline reported in a press release that the gD-2 subunit vaccine failed to protect seronegative women against HSV-2 (11). Therefore, novel strategies are needed to develop an effective HSV-2 vaccine.
A possible explanation for the difficulty in developing an effective HSV-2 vaccine is that the virus has evolved mechanisms to escape immunity. HSV-1 and HSV-2 are human pathogens and are more adept at evading immune responses in humans than in mice or guinea pigs (24). Therefore, the impact of immune evasion strategies on vaccine efficacy may be underestimated in laboratory animal models.
Our approach to developing an HSV-2 subunit vaccine was to combine a potent immunogen, gD-2, with an immune evasion protein, gC-2, that was added to prevent the virus from evading innate and acquired immune responses mediated by complement (25, 56). Targeting of gC-2 to block immune evasion is possible because the glycoprotein is expressed on the viral envelope and at the infected cell surface. Antibodies directed against gC-2 can potentially bind to the glycoprotein and block its ability to inactivate complement, thereby allowing complement to participate more effectively in host defense against the virus, as previously demonstrated for gC-1 (1, 29).
Complement activation occurs by the classical, lectin, and alternative pathways to initiate innate and adaptive immune responses to viral infection (35). The classical pathway is activated when C1q binds to the Fc domain of natural antibody or virus-specific antibody (22). The lectin and alternative pathways are antibody independent. Complement activation leads to virus neutralization, lysis of infected cells, and enhancement of B- and T-cell responses (7, 8, 16, 34, 35, 58). Many viruses, including vaccinia virus, West Nile virus, influenza virus, pseudorabies virus, human cytomegalovirus, varicella zoster virus, HSV-1, and HSV-2, express IgG Fc binding proteins and regulators of complement activation that may inhibit complement activation (10, 17, 19, 20, 30, 31, 37, 38, 43, 50, 62, 65). HSV-1 infection in mice or humans produces only low titers of antibodies capable of blocking the interaction between gC-1 and complement component C3b (9). In contrast, immunization with gC-1 produces much higher titers of blocking antibodies (9). Adding gC-1 to a gD-1 subunit vaccine improves the ability of gD-1 antibody to neutralize HSV-1 in the presence of human complement and enhances the efficacy of a gD-1 subunit vaccine in mice (1).
In this study, immunization with gC-2 and gD-2 was evaluated in mice and guinea pigs. Immunization with gC-2 produced antibodies that blocked gC-2-mediated immune evasion, induced neutralizing antibodies, stimulated a potent CD4+ T-cell response, and improved the protection provided by a gD-2 subunit immunogen.
MATERIALS AND METHODS
Viruses, antigens, and antibodies.
Wild-type HSV-2 strains 2.12 and MS and HSV-2 gC deletion strain HSV-2 gCnull were grown in Vero cells and purified on sucrose gradients (22). The baculovirus-expressed gC-2 protein bac-gC-2(426t) extends from amino acid 27 to amino acid 426, where amino acid 27 is the first amino acid after the signal peptide (59). The baculovirus-expressed gD-2 protein bac-gD-2(306t) extends from amino acid 1 to amino acid 306, where amino acid 1 is the first amino acid in the protein (6, 60). The methods used to construct bac-gC-2(426t) (referred to as gC-2 antigen) and bac-gD-2(306t) (referred to as gD-2 antigen) resulted in an aspartic acid and a proline being added at the N terminus. Polyclonal anti-gC-2 and anti-gD-2 antibodies were prepared in female BALB/c mice (NCI) by immunizing mice intramuscularly (i.m.) in a calf muscle three times at 2-week intervals with 5 μg of gC-2 antigen or 250 ng of gD-2 antigen mixed with adjuvant containing 50 μg of CpG oligonucleotide (TCCATGACGTTCCTGACGTT; Coley Pharmaceutical) and 25 μg of alum per μg protein in a final volume of 50 μl (Alhydragel; Accurate Chemical and Scientific Corp.) (64). Nonimmune murine IgG was purchased from Sigma Chemical Co. (St. Louis, MO). Polyclonal anti-gC-2 and anti-gD-2 antibodies were prepared in female guinea pigs as described for mice, but with 10 μg of bac-gC-2(426t) or 5 μg bac-gD-2(306t) and with 100 μg of CpG oligonucleotide (TCGTCGTTGTCGTTTTGTCGTT; Trilink Inc.) and 20 μg of alum per μg protein.
Animal studies.
Laboratory animals were handled in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania. Mice were anesthetized before shaving, depilating, intraperitoneal passive antibody immunizations, and flank infection.
(i) Mouse strains, immunizations, and challenge experiments.
Immunization studies were performed in female BALB/c mice that were 8 to 9 weeks old at the time of the first immunization. The gC-2 or gD-2 antigen was incubated with various concentrations of CpG and alum at room temperature for 2 h. Mice were inoculated i.m. in the calf muscle three times at 2-week intervals (1). For immunizations involving both gC-2 and gD-2 antigens, each protein was incubated separately with adjuvant and combined just prior to i.m. immunization. Mock immunizations were performed using CpG and alum without HSV-2 subunit antigens.
C3 knockout mice were bred as previously described (40). Passive antibody immunization was performed in C3 knockout or C57BL/6 mice at 3 to 4 months of age. Animals were immunized passively intraperitoneally with 200 μg of murine anti-gC-2 IgG or nonimmune murine IgG, followed 24 h later by flank infection with 5 × 105 PFU of HSV-2 strain 2.12 (1).
The mouse flank model was also used to infect BALB/c mice (1). Four groups of 10 mice each were challenged with 4 × 105 PFU HSV-2 strain 2.12 in a 10-μl volume (>2,000 50% lethal doses [LD50]). Five mice from each group were scored for severity of disease, on a scale of 0 to 4, at the inoculation and zosteriform sites (3). Dorsal root ganglia (DRG) were isolated from the remaining 5 mice per group for measurement of viral titers and determination of HSV-2 DNA copy numbers by quantitative PCR (qPCR). Intravaginal challenge with HSV-2 was performed in 80 mice. Prior to challenge, mice were inoculated subcutaneously with 2 mg of medroxyprogesterone (Sicor Pharmaceuticals, Inc.) (3). The vagina was cleared using a sterile swab moistened with phosphate-buffered saline (PBS), followed by inoculation of 5 μl containing 2 × 105 PFU of HSV-2 strain 2.12 (>2,000 LD50) or 5 × 104 PFU of HSV-2 strain MS (>2,000 LD50). The LD50 values were determined in prior experiments (results not shown). Mice were observed for survival, and viral titers were obtained by swabbing the vagina at the indicated times postinfection. The severity of vaginal disease was scored on a scale of 0 to 4 by assigning 1 point each for erythema, exudate, hair loss, and necrosis.
Cytokine induction in CD4+ and CD8+ splenocyte T cells following cell surface and intracellular cytokine staining by flow cytometry.
Mice were mock immunized with CpG and alum or immunized three times at 2-week intervals with combined gC-2 (5 μg) and gD-2 (2 μg) antigens mixed with CpG and alum. Five days after the third immunization, spleens were dissected and homogenized in RPMI 1640 by passing the cells through a 50-μm strainer. The red blood cells were lysed with hypotonic 0.2× PBS. The splenocytes were washed three times, and 106 splenocytes were incubated in 96-well plates with RPMI medium supplemented with 10% fetal bovine serum and stimulated with 2 μg gC-2 or 2 μg gD-2 at 37°C. After 1 h, brefeldin A (10 μg/ml) (BFA in Golgistop; BD Pharmingen) was added to the cells and incubated for an additional 11 h at 37°C.
The cytokine induction in CD4+ and CD8+ splenocyte T cells was quantified using an 18-color LSR II flow cytometer. Splenocytes were transferred from 96-well plates to 5-ml polystyrene fluorescence-activated cell sorter (FACS) tubes (BD Pharmingen). The cells were washed with PBS and stained with aqua blue to distinguish live from dead cells (Invitrogen) and with Pacific blue-conjugated anti-CD8 mouse monoclonal antibody (MAb) (Biolegend) and R-phycoerythrin–cyanine 5.5 (PE-Cy5.5) tandem-conjugated anti-CD4 mouse MAb (BD Pharmingen). Cells were washed with PBS and FACS buffer, fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen), and stained with an Alexa Fluor 700-conjugated anti-gamma interferon (anti-IFN-γ) mouse MAb, PE-Cy7 tandem-conjugated anti-tumor necrosis factor alpha (anti-TNF-α) mouse MAb, and allophycocyanin (APC)-Cy7 tandem-conjugated anti-CD3 mouse MAb. Splenocytes were fixed with 1% paraformaldehyde and analyzed by FACS (2, 14). The number of events included in each dot plot was 10,000 cells. FlowJo flow cytometry analytic software (version 9.3) was used to analyzed the data and to create graphs and charts.
(ii) Guinea pig immunizations and challenge studies.
Thirty female Hartley strain guinea pigs weighing 175 to 225 g (Charles River) were immunized i.m. in the right hind calf muscle three times at 2-week intervals. Animals were mock immunized with CpG oligonucleotide (TCGTCGTTGTCGTTTTGTCGTT; Trilink Inc.) and alum or immunized with 10 μg gC-2 antigen, 5 μg gD-2 antigen, or the combination of 10 μg gC-2 and 5 μg gD-2 with CpG and alum. CpG was used at 100 μg/guinea pig, and alum was used at 20 μg/μg protein in 50 μl per immunization. Guinea pigs were bled from the saphenous vein to collect serum prior to immunization and challenge. Animals were infected intravaginally with 5 × 105 PFU of HSV-2 strain MS (>1,000 LD50) and scored for acute disease on a scale of 0 to 4, where 0 reflects no disease, 1 redness, 2 a single lesion, 3 coalesced lesions, and 4 ulcerated lesions (23). Urinary retention and hind leg paralysis were recorded in addition to the vaginal disease. Guinea pigs were swabbed daily for vaginal viral titers at 1 to 6 days postinfection (dpi). Animals were observed daily from 15 to 60 dpi for recurrent lesions and were assigned a score of 1 point for each day that a lesion was present. Vaginal swabs were obtained at 28 to 48 dpi for HSV-2 DNA detection by qPCR.
Antibodies that inhibit C3b binding.
Mice were immunized three times at 2-week intervals with 5 μg of gC-2 antigen or 250 ng gD-2 antigen mixed with CpG and alum. Serum was collected 2 weeks after the third immunization, and IgG was purified on a protein G column (Amersham Biosciences). Wells of an enzyme-linked immunosorbent assay (ELISA) plate (Nalge Nunc International) were coated with 200 ng C3b. Serial 2-fold dilutions of anti-gC-2 or anti-gD-2 IgG were incubated with 50 ng gC-2 for 60 min at 37°C and added to the C3b-coated wells. Bound gC-2 or gD-2 was detected by ELISA at 405 nm, using rabbit anti-gC-2 or anti-gD-2 IgG and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (9).
ELISA and neutralizing antibody responses.
ELISA was used to detect antibodies to gC-2 or gD-2. Wells were coated with 50 ng of purified gC-2 antigen or gD-2 antigen, and serum obtained after three immunizations was serially diluted 2-fold and tested in duplicate. HRP-conjugated secondary antibodies were added, and the optical density (OD) was measured at 405 nm. The endpoint titer was considered the highest dilution of serum resulting in an OD of ≥0.1 that was at least 2-fold higher than the OD of sera obtained from mock-immunized animals at that dilution. Results are presented as log10 endpoint titers. Neutralizing titers were measured by heating serum to 56°C for 30 min to inactivate murine or guinea pig complement and then incubating serial dilutions of serum with HSV-2 at 37°C for 1 h. To evaluate the effects of complement, 2.5% human serum was obtained from an HSV-1- and HSV-2-seronegative donor and added to serum prior to incubation with virus. Virus titers were determined by plaque assay on Vero cells. The neutralizing titer was defined as the highest serum dilution that reduced plaques by ≥50% compared with those seen with sera from mock-immunized controls.
Real-time qPCR to quantify viral DNA.
For mouse experiments, the DRG that provide sensory innervation to the vaginal tissues were harvested, homogenized, and split into two equal aliquots. One aliquot was used to measure viral titers by plaque assay on Vero cells. The limit of detection of the plaque assay is 2 PFU. The other aliquot was processed for qPCR. DNA was isolated from guinea pig vaginal swab or mouse DRG samples by use of a DNeasy blood and tissue kit (Qiagen). Mouse DRG samples were analyzed using duplex qPCR to amplify the HSV-2 Us9 gene and the mouse adipsin gene. Standard curve samples were prepared using purified HSV-2 DNA (Advanced Biotechnologies) and mouse lung genomic DNA as the source of the adipsin gene (BioChain Institute). The standard curve samples were run in triplicate wells at 50,000, 5,000, 500, 50, and 5 copies of DNA. HSV-2 DNA PCR-positive samples containing fewer than 5 copies per reaction mix were extrapolated from the standard curve. Based on the standard curve, the limit of quantitation (LOQ) for the DRG qPCR assay was 5 copies of HSV-2 DNA. The DRG HSV-2 DNA copy number was expressed as log10 DNA copies per 104 adipsin genes. For vaginal swab samples, a cutoff of >150 copies of HSV-2 DNA per sample was considered positive, which we based in part on criteria established for shedding of HSV-2 DNA in human vaginal samples and in part by analyzing the ability of the assay to accurately discriminate between positive and negative samples at DNA levels of <150 copies (42, 51). DRG and vaginal swab samples that did not yield a positive signal in duplicate wells by 40 cycles were considered negative. Primers for mouse adipsin were as follows: forward, 5′-GCAGTCGAAGGTGTGGTTACG-3′; and reverse, 5′-GGTATAGACGCCCGGCTTTT-3′. The probe for adipsin, with reporter dye, was 5′-VIC-CTGTGGCAATGGC-MGBNFQ-3′ (MGBNFQ, minor groove binder nonfluorescent quencher). Primers for HSV-2 Us9 amplified from mouse and guinea pig samples were as follows: forward, 5′-GGCAGAAGCCTACTACTCGGAAA-3′; and reverse, 5′-CCATGCGCACGAGGAAGT-3′. The probe for Us9, with reporter dye, was 5′-FAM-CGAGGCCGCCAAC-MGBNFQ-3′ (FAM, 6-carboxyfluorescein). Reactions were performed on 5 μl of DNA in 25-μl reaction mixtures, using TaqMan gene expression master mix (Applied Biosystems) and an ABI 7500 Fast machine.
Explant cocultures of murine sacral DRG for reactivation of latent HSV-2 infection.
Thirty-four days after infection, sacral DRG were harvested, minced with scissors, and placed on a monolayer of Vero cells grown in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum supplemented with 25 μg/ml vancomycin (3, 61). Cultures were observed for 20 days for HSV-2 reactivation.
Statistics.
The area under the curve was calculated, followed by one-way analysis of variance (ANOVA), to evaluate the significance of neutralizing antibody titers. Repeated-measures ANOVA followed by the Bonferroni correction for multiple comparisons was performed to determine the significance of vaginal titers during acute infection. Nonparametric ANOVA followed by Tukey's posttest analysis was performed for multiple pairwise comparisons of DRG viral titers and DRG HSV-2 DNA copy numbers. The significance of survival data was calculated using the log rank (Mantel-Cox) test. Fisher's exact test was performed to compare the numbers of animals in each group that developed disease and the numbers of recurrent lesion days in guinea pigs.
RESULTS
Antibody responses in mice immunized with gC-2 and gD-2 antigens.
Mouse antibody responses were measured 10 to 14 days after three immunizations with 5 μg gC-2 antigen, 250 ng gD-2 antigen, or both antigens. ELISA titers showed robust anti-gC-2 and anti-gD-2 responses when mice were immunized with either antigen alone or in combination (Fig. 1A and C).
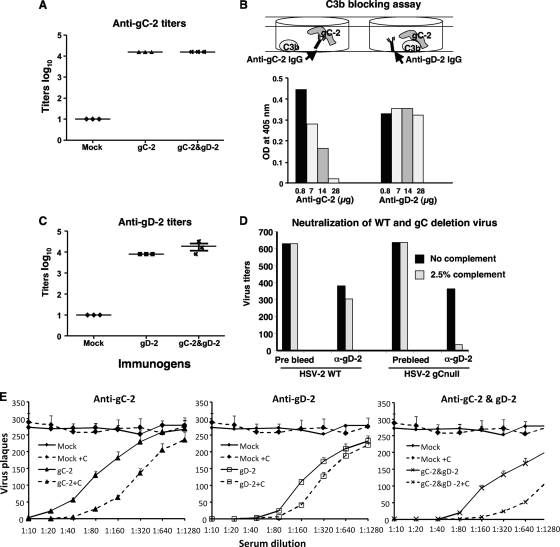
Fig. 1.
ELISA titers, neutralizing antibody titers, and C3b blocking antibody titers. Mice were mock immunized with CpG and alum or immunized with 5 μg of gC-2 antigen, 250 ng of gD-2 antigen, or 5 μg of gC-2 plus 250 ng of gD-2 antigen. Serum was collected 10 days after the third immunization and examined by ELISA for antibody to gC-2 (A) or gD-2 (C). Each symbol represents the serum from a single mouse tested in duplicate and is plotted as the mean log10 titer. The error bars in panel C represent standard deviations. (B) IgG was purified from sera of mice immunized with gC-2 or gD-2 antigen and evaluated in a C3b blocking assay. The top panel is a cartoon illustrating anti-gC-2 IgG blocking gC-2 binding to C3b (left figure), while anti-gD-2 IgG has no effect (right figure). Increasing concentrations of anti-gC-2 IgG resulted in decreased binding of gC-2 to C3b, while anti-gD-2 IgG had no effect on gC-2 binding. An IgG level of 28 μg represents the amount of IgG in an approximately 1:160 dilution of mouse serum. Data plotted are representative of three separate experiments. (D) HSV-2 or HSV-2 gCnull was incubated with PBS or anti-gD-2 IgG in the presence or absence of 2.5% human complement. Results are averages for duplicate wells and are representative of three separate experiments. (E) Serial dilutions of sera from gC-2 (left)-, gD-2 (middle)-, and gC-2-plus-gD-2 (right)-immunized animals were evaluated for neutralizing antibody to HSV-2 strain 2.12, without complement (solid lines) or with 2.5% human serum as the source of complement (dashed lines). Sera from mock-immunized animals were used as a control, and their data are plotted in each panel. Each data point is the mean ± standard deviation for sera from 5 mice tested in duplicate wells.
Antibody to gC-2 was evaluated for inhibition of C3b binding to gC-2. Anti-gD-2 IgG was used as a control. Increasing concentrations of anti-gC-2 IgG blocked the binding of C3b to gC-2, while anti-gD-2 IgG had no effect (Fig. 1B). Therefore, immunization with gC-2 antigen produces high titers of gC-2 antibody, including those that block C3b binding.
To assess the role of gC-2 protein expression on the virus envelope in preventing complement-mediated neutralization, the neutralizing activity of anti-gD-2 IgG (28 μg/ml) and 2.5% human complement was evaluated against 650 PFU of HSV-2 or HSV-2 gCnull, which is a mutant strain that fails to express gC-2 protein (Fig. 1D). In the absence of antibody, 2.5% complement alone had no neutralizing activity (result not shown) (22). Complement had little effect on anti-gD-2 IgG neutralization of wild-type virus; however, complement had a marked effect on anti-gD-2 IgG neutralization of HSV-2 gCnull (Fig. 1D). These results indicate that HSV-2 gC inhibits complement enhancement of antibody neutralization mediated by anti-gD-2 IgG.
The neutralizing activity in mouse serum was determined after three i.m. immunizations with 5 μg gC-2 antigen, 250 ng gD-2 antigen, or both immunogens, given with CpG and alum (Fig. 1E). Sera from gC-2-immunized mice neutralized HSV-2 at a titer of 1:80 in the absence of complement, which increased 4-fold in the presence of 2.5% human complement (left panel). The neutralizing titer of sera from gD-2-immunized mice was 1:160 without complement and increased 2-fold with complement (middle panel). The neutralizing titer of sera from gC-2-plus-gD-2-immunized mice was 1:320 without complement and increased to ≥1:1,280 in the presence of complement (right panel) (P < 0.001 for comparing gC-2-plus-gD-2 group titers in the presence of complement with those for either antigen alone in the presence of complement). Therefore, sera from gC-2-immunized mice have neutralizing activity when used alone, and sera from mice immunized with the combination of gC-2 plus gD-2 significantly enhance the neutralizing activity seen with either antigen alone in the presence of complement.
Anti-gC-2 IgG protects complement-intact but not C3 knockout mice from HSV-2 infection.
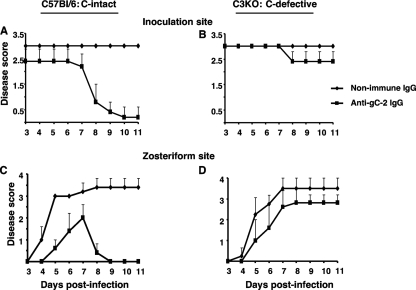
Complement-intact or C3 knockout mice were passively immunized with 200 μg of IgG obtained from mice immunized with 5 μg gC-2 antigen. Passive rather than active immunization was performed in this experiment because C3 knockout mice exhibit deficiencies in B- and T-cell responses to viral antigens (8). We postulated that anti-gC-2 IgG would protect complement-intact mice but have little effect in C3 knockout mice if the main effect of the antibody was to prevent the virus from evading complement-mediated immunity. Mice were challenged by flank inoculation with 5 × 105 PFU HSV-2 strain 2.12 1 day after passive immunization. Compared with nonimmune murine IgG, anti-gC-2 IgG significantly reduced inoculation site (P < 0.05) and zosteriform (P < 0.01) disease in complement-intact mice (Fig. 2A and C). In contrast, anti-gC-2 IgG was less effective in C3 knockout mice (Fig. 2B and D). Therefore, protection provided by anti-gC-2 IgG is at least partially complement dependent.
Fig. 2.
Passive transfer of anti-gC-2 IgG protects complement-intact mice from inoculation site and zosteriform disease. Inoculation site (A and B) and zosteriform (C and D) disease scores are shown for C57BL/6 and C3 knockout (C3KO) mice passively immunized intraperitoneally with 200 μg murine anti-gC-2 IgG or 200 μg murine nonimmune IgG 24 h before epidermal HSV-2 challenge with 5 × 105 PFU of HSV-2 strain 2.12. Results shown are the means ± standard deviations for 5 mice per group.
Immunization with combined gC-2 and gD-2 antigens protects mice from epidermal and vaginal disease.
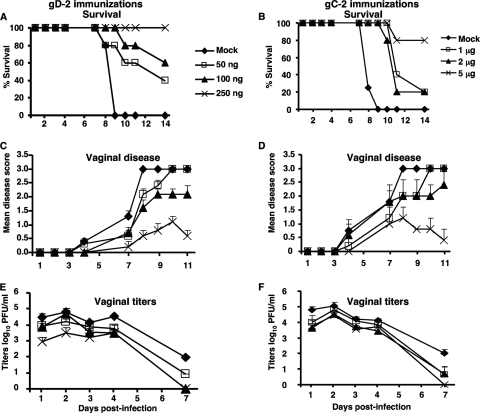
Experiments were performed to define a gD-2 antigen dose that provides partial protection against vaginal disease and then to determine whether gC-2 antigen combined with gD-2 antigen offers additional protection. Mice were immunized i.m. three times every 2 weeks with 50 ng, 100 ng, or 250 ng gD-2 antigen administered with CpG and alum. Two weeks after the third immunization, mice were treated with 2 mg of medroxyprogesterone, and they were challenged intravaginally 5 days later with 2 × 105 PFU HSV-2 strain 2.12. Survival, vaginal disease scores, and vaginal titers indicated that the 250-ng dose provided the best, though not complete, protection against vaginal disease. Vaginal titers were significantly reduced in the 250-ng group compared with mock-infected animals on days 1, 2, and 7 postinfection (P < 0.05) (Fig. 3A, C, and E). A separate group of mice were immunized with 1, 2, or 5 μg of gC-2 antigen given i.m. three times at 2-week intervals. Survival and protection against vaginal disease were best with 5 μg of gC-2 antigen (Fig. 3B, D, and F).
Fig. 3.
Dose escalation of gD-2 and gC-2 antigens in the mouse vaginal model. BALB/c mice were mock immunized with CpG and alum or immunized with 50, 100, or 250 ng of gD-2 antigen or with 1, 2, or 5 μg of gC-2 antigen, given with CpG and alum. Mice were challenged intravaginally with 2 × 105 PFU of HSV-2 strain 2.12. Animals were observed for survival (A and B), vaginal disease (C and D), and vaginal titers (E and F). Results shown are means ± standard deviations for 5 mice per group.
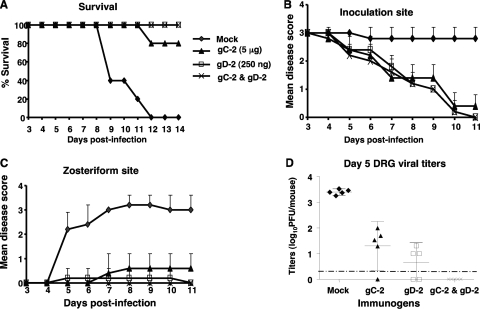
The mouse flank model was used to assess epidermal disease as a surrogate for genital disease in males. Mice were mock immunized or immunized with 5 μg gC-2 antigen, 250 ng gD-2 antigen, or both antigens in combination and then challenged by flank scarification with 4 × 105 PFU of HSV-2 strain 2.12. All mock-immunized mice died, while survival in the gC-2 group was 80% and that in the gD-2 or gC-2-plus-gD-2 group was 100% (Fig. 4A) (P < 0.001 for comparing mock-immunized group to gC-2, gD-2, or gC-2-plus-gD-2 group).
Fig. 4.
Combined immunization with gC-2 and gD-2 antigens protects DRG after flank infection. BALB/c mice were mock immunized with CpG and alum or immunized with 5 μg of gC-2 antigen, 250 ng of gD-2 antigen, or 5 μg of gC-2 plus 250 ng of gD-2, with CpG and alum (n = 10 per group). Mice were challenged by flank inoculation with 4 × 105 PFU of HSV-2 strain 2.12. Five animals in each group were observed for survival (A) and scored for severity of disease at the inoculation site (B) and zosteriform site (C). (D) The other 5 animals were euthanized on day 5 to measure DRG viral titers. The dotted line represents the limit of detection of the assay, which is <2 PFU per mouse. Results are plotted as means ± standard deviations.
Inoculation site disease scores were reduced in mice immunized with gC-2 antigen, gD-2 antigen, or the combined gC-2 and gD-2 antigens compared with mock-immunized mice (P < 0.001 for each comparison) (Fig. 4B), although no significant difference was detected between the groups receiving gC-2, gD-2, or both immunogens. Zosteriform disease was reduced in the gC-2 or gD-2 antigen group, while no zosteriform disease was observed in the gC-2-plus-gD-2 immunization group (P < 0.001 for comparing the mock-immunized group to each treatment group, while the gC-2 or gD-2 group was not significantly different from the gC-2-plus-gD-2 group) (Fig. 4C).
DRG viral titers were measured at 5 days postinfection (Fig. 4D). Virus was isolated from all 5 mock-immunized mice, at titers of approximately 3.5 log10 PFU. Virus titers were 1 to 2 log10 in 4 of 5 mice immunized with gC-2 antigen and slightly lower than that in 3 of 5 mice immunized with gD-2 antigen. In contrast, no virus was detected in the combined immunization group (P < 0.001 for comparing mock-immunized group to gD-2, gC-2, or combined group; differences were not significant for comparing the gD-2 group with the combined group).
The same antigens were used alone or in combination to evaluate protection against vaginal challenge with 2 × 105 PFU HSV-2 strain 2.12. All mock-immunized mice died, while survival in the gC-2 group was 80% and that in the gD-2 and gC-2-plus-gD-2 groups was 100% (P < 0.001 for comparing mock-immunized group to gC-2, gD-2, or gC-2-plus-gD-2 group) (results not shown). Mock-immunized mice showed extensive vaginal disease, while the groups receiving gC-2 alone, gD-2 alone, or combined gC-2 and gD-2 were significantly protected (P < 0.001 for comparing mock-immunized group to each treatment group; the gC-2 or gD-2 group was not significantly different from the gC-2-plus-gD-2 group) (Fig. 5A and B). In the combined group, 2 of 5 mice developed hair loss around the base of the tail that first appeared at 8 days postinfection. Animals with hair loss were assigned a score of 1, although the pathogenesis of this event is unclear and the animals appeared healthy. Mean vaginal titers in mock-immunized mice were between 5 and 6 log10 on day 1 and gradually declined but were still positive on day 6 (Fig. 5C). Titers were 2 log10 lower in the gC-2- or gD-2-immunized mice on day 1 and were negative by day 6. The combined gC-2-plus-gD-2 group had the lowest viral titers on day 1, which were 3 log10 lower than those of the mock-immunized group, and this group cleared the virus by day 5 (P < 0.001 for each day for comparing titers of the combined group and the mock group; P < 0.05 for comparing the combined group with the gD-2 group on days 1 and 5).
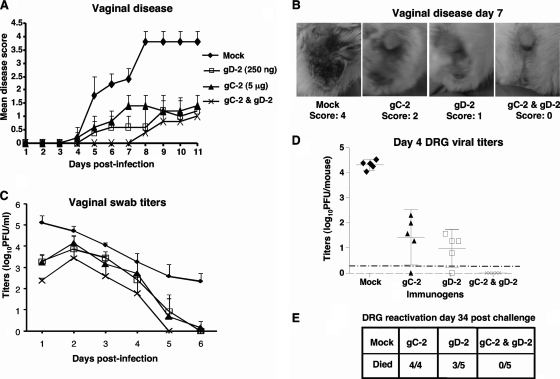
Fig. 5.
Combined immunization with gC-2 and gD-2 antigens protects DRG better after vaginal challenge than immunization with gD-2 antigen alone. BALB/c mice were immunized as described in the legend to Fig. 3. Mice were challenged with 2 × 105 PFU of HSV-2 strain 2.12 by vaginal inoculation. Five mice from each group were monitored for survival and disease, and the other five were evaluated for DRG infection. (A) Severity of vaginal disease. (B) Representative pictures of vaginal disease. The mock-immunized mouse had a score of 4 based on erythema, exudate, hair loss, and necrosis; the gC-2-immunized mouse had a score of 2 based on erythema and hair loss; the gD-2-immunized mouse had a score of 1 based on hair loss; and the gC-2-plus-gD-2-immunized mouse had no disease. (C) Vaginal swab titers. (D) DRG viral titers measured at 4 days postinfection. The dotted line represents the limit of detection of the assay, which is <2 PFU per mouse. (E) HSV-2 reactivation from sacral DRG. Data plotted are means ± standard deviations.
DRG titers were measured at 4 days postinfection (Fig. 5D). Virus was detected in all mock-immunized mice, with titers of approximately 4 log10 PFU. Virus was isolated from four of five mice immunized with gD-2 or gC-2 antigen, with titers that were 3 log10 lower than those in mock-immunized mice. Importantly, no virus was detected in the combined immunization group (P < 0.001 for comparing the combined group with mock- or gC-2-immunized mice; P = 0.025 for comparing the combined group with gD-2-immunized mice). DRG were harvested at 34 days postinfection and evaluated for reactivation by coculture with Vero cells (Fig. 5E). HSV-2 was recovered from 4 of 4 DRG taken from mice immunized with gC-2, 3 of 5 DRG from gD-2-immunized mice, and 0 of 5 DRG from gC-2-plus-gD-2-immunized mice (P < 0.001 for comparing mock-immunized group with gC-2, gD-2, or combined group; the P value was not significant for comparing the gD-2 group with the combined group).
In the experiments described above, gD-2 antigen was used at a concentration that provided partial protection against disease to make it easier to detect an added benefit conferred by gC-2 antigen. In the next series of experiments, mice were immunized with gD-2 antigen, but using an 8-fold higher concentration of gD-2 (2 μg instead of 250 ng). Mice were challenged with 5 × 104 PFU of HSV-2 strain MS. No mock (CpG and alum)-immunized mice survived, while 80% of mice immunized with gC-2 alone survived and 100% of mice immunized with gD-2 alone or gC-2 plus gD-2 survived (P < 0.01 for comparing mock-immunized group with gC-2, gD-2, or gC-2-plus-gD-2 group) (results not shown). Vaginal disease scores of gC-2-immunized mice were significantly lower than those of mock-immunized mice (P < 0.01), while gD-2-immunized mice and mice in the combined group had significantly less disease than gC-2-immunized mice (P < 0.01 for comparing gC-2 group with gD-2 or gC-2-plus-gD-2 group; the gC-2-plus-gD-2 group did not differ from the gD-2 group) (Fig. 6A). The vaginal titers of the gD-2 and gC-2-plus-gD-2 groups were significantly lower than those of the mock and gC-2 groups (P < 0.01). Vaginal titers were significantly different between gD-2-immunized mice and the combined group on days 1 and 5 (P < 0.05) (Fig. 6B).
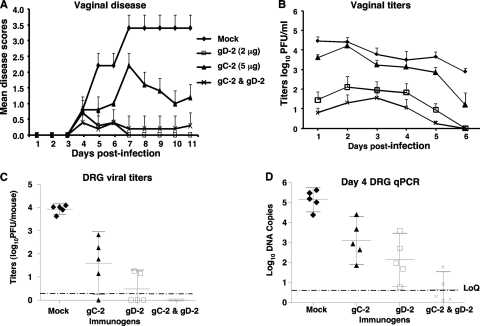
Fig. 6.
Combined immunization with gC-2 and gD-2 antigens protects DRG better than that with gD-2 alone at a higher gD-2 antigen dose. BALB/c mice were mock immunized with CpG and alum or immunized with 5 μg of gC-2 antigen, 2 μg of gD-2 antigen, or 5 μg of gC-2 plus 2 μg of gD-2 antigen, with CpG and alum (n = 10 per group), and were challenged with 5 × 104 PFU of HSV-2 strain MS by vaginal inoculation. Five mice in each group were monitored for survival, disease, and vaginal titers, and the other five were monitored for DRG infection. (A) Vaginal disease; (B) vaginal titers; (C) DRG viral titers. Results plotted are means ± standard deviations. (D) Real-time qPCR was performed on DNAs isolated from sacral DRG at 4 dpi. LoQ represents the lower limit of accurate quantitation of the qPCR (5 DNA copies).
Importantly, DRG titers were lowest in the combined immunization group. At the higher gD-2 antigen dose, virus was isolated from two of five mice, and the mean titer was <1 log10 (P < 0.001 for comparing viral titers of mock-immunized group with those of gC-2, gD-2, or combined gC-2-plus-gD-2 group) (Fig. 6C). Sacral DRG infection on day 4 was evaluated by real-time qPCR (Fig. 6D). Mock-immunized animals had mean DNA copy numbers of >5 log10 copies per 104 copies of mouse adipsin DNA, the gC-2 group had approximately 3 log10 copies, the gD-2 group had approximately 2 log10 copies, and the combined group had <1 log10 copy (P < 0.05 for comparing combined group with gD-2 group).
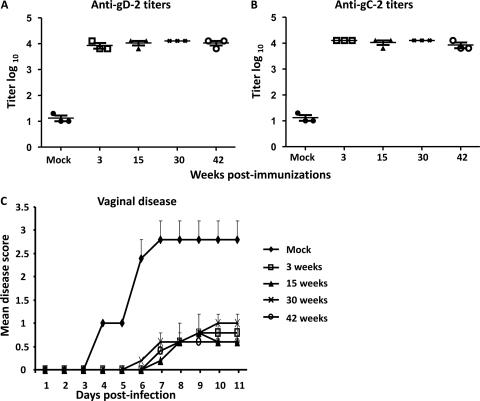
To assess the duration of immunity, mice were immunized i.m. three times with 250 ng gD-2 combined with 5 μg gC-2 antigen. Sera collected 3, 15, 30, and 42 weeks after the third immunization demonstrated that antibody titers to gD-2 and gC-2 were stable over time (Fig. 7A and B). Mice were challenged intravaginally with 2 × 105 PFU of HSV-2 strain 2.12 at 3, 15, 30, and 42 weeks postimmunization. Persistent protection from vaginal disease was noted for up to 42 weeks postimmunization (P < 0.001 for comparing mock-immunized group with gC-2-plus-gD-2 group for each time point) (Fig. 7C).
Fig. 7.
Persistence of antibody responses and protection. BALB/c mice were mock immunized with CpG and alum or immunized with the combination of 5 μg gC-2 plus 250 ng gD-2 antigen, given with CpG and alum. ELISA titers are given for antibodies to gD-2 (A) and gC-2 (B) 3, 15, 30, and 42 weeks after the third immunization. Results plotted are mean log10 titers ± standard deviations. (C) Mice were challenged with 2 × 105 PFU of HSV-2 strain 2.12 by vaginal inoculation 3, 15, 30, or 42 weeks after the third immunization. Challenge study results are means ± standard deviations for 5 mice per group.
gC-2 and gD-2 CD4+ and CD8+ T-cell responses in mice.
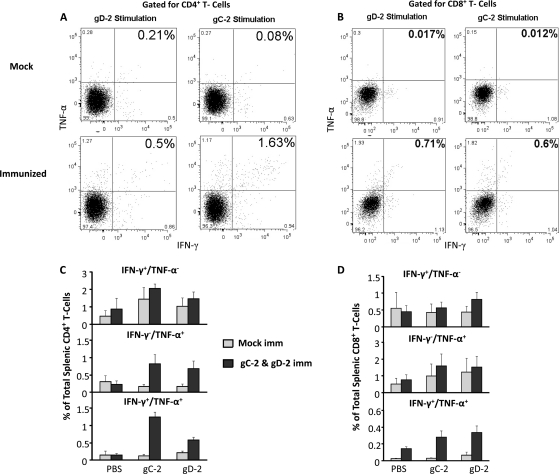
The gC-2- and gD-2-specific IFN-γ and TNF-α responses of CD4+ T cells were measured in mouse spleens (Fig. 8). Mice were mock immunized with CpG and alum or immunized with 5 μg gC-2 combined with 2 μg gD-2, with CpG and alum. Spleens were harvested 5 days after the third immunization, and splenocytes from mock-immunized and immunized mice were restimulated with 2 μg gC-2 antigen, 2 μg gD-2 antigen, or PBS. Dot plots representative of individual mice are shown in Fig. 8A and B, showing the numbers of CD4+ and CD8+ T cells producing IFN-γ, TNF-α, or both cytokines in mock-immunized or gC-2-plus-gD-2-immunized mice. The mean CD4+ T-cell responses and standard errors for nine mice immunized with gC-2 plus gD-2 or four mock-immunized mice are also shown (Fig. 8C). A modest (not statistically significant) increase in IFN-γ+ TNF-α− CD4+ T cells was detected in immunized mice after stimulation with gC-2 or gD-2 antigen (Fig. 8C, top panel); however, significant increases were detected in IFN-γ− TNF-α+ CD4+ T cells (Fig. 8C, middle panel) (P < 0.001 for gC-2 and P < 0.01 for gD-2 stimulation compared with mock controls) and IFN-γ+ TNF-α+ CD4+ T cells (Fig. 8C, bottom panel) (P < 0.001 for gC-2 and P < 0.01 for gD-2 stimulation compared with mock controls). Greater stimulation of IFN-γ+ TNF-α+ CD4+ T cells was noted in gC-2- than in gD-2-stimulated cells (P < 0.01). CD8+ T-cell responses were measured from the same 4 mock-immunized and 9 gC-2-plus-gD-2-immunized mice (Fig. 8D). Slight increases in IFN-γ+ TNF-α− CD8+ T cells and IFN-γ− TNF-α+ CD8+ T cells were detected by comparing mock with gC-2-plus-gD-2 immunization (Fig. 8D, top and middle panels, respectively); however, larger increases in IFN-γ+ TNF-α+ CD8+ T cells were detected in gC-2-plus-gD-2-immunized mice (Fig. 8D, bottom panel) (P < 0.001 for gC-2 and P < 0.05 for gD-2 stimulation, comparing immunized with mock groups).
Fig. 8.
IFN-γ+ and TNF-α+ CD4+ and CD8+ T cells in gC-2- and gD-2-immunized mice. Mice were immunized three times at 2-week intervals. Representative dot plots are shown for CD4+ T cells (A) and CD8+ T cells (B) from one mock- and one gC-2-plus-gD-2-immunized mouse. The means and standard errors for all animals evaluated for CD4+ T-cell (C) and CD8+ T-cell (D) responses are shown for the individual cytokines or both IFN-γ and TNF-α for mock- and gC-2-plus-gD-2-immunized mice (n = 4 for mock controls and n = 9 for CD4+ and CD8+ T-cell measurements).
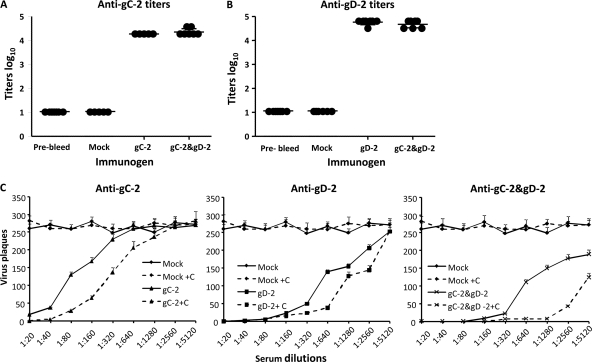
Antibody responses to gC-2 and gD-2 antigens in guinea pigs.
Antibody responses were evaluated prior to immunization and 2 weeks after the third immunization with 10 μg gC-2, 5 μg gD-2, or both antigens. ELISA titers showed >4-log10 antibody responses when guinea pigs were immunized with either antigen alone or in combination (Fig. 9A and B). Sera from guinea pigs immunized with gC-2 were evaluated for blocking of C3b binding to gC-2 at 1:10 and 1:20 dilutions. Sera from the gC-2-immunized animals blocked C3b binding, while sera from mock-immunized animals had no effect (n = 4 per group; results not shown). Neutralization titers were determined with and without complement. In the absence of complement, sera from gC-2-immunized guinea pigs neutralized virus at a titer of 1:80, which increased 4-fold in the presence of 2.5% human complement (Fig. 9C, left panel). The mean neutralizing titer in sera from gD-2-immunized guinea pigs was 1:640 without complement and increased 2-fold in the presence of complement (Fig. 9C, middle panel). Guinea pigs immunized with gC-2 and gD-2 had a mean neutralizing antibody titer of 1:640 in the absence of complement; however, the titers increased 8-fold in the presence of complement (Fig. 9C, right panel) (P < 0.001 for comparing gC-2-plus-gD-2 group with either antigen group in the presence of complement). Therefore, in guinea pigs, as in mice, the neutralizing activity of gD-2 antibody is enhanced by gC-2 antibody in the presence of human complement.
Fig. 9.
ELISA and neutralizing antibody titers. Guinea pigs were mock immunized with CpG and alum (n = 5) or immunized with 10 μg of gC-2 antigen (n = 5), 5 μg of gD-2 antigen (n = 10), or 10 μg of gC-2 plus 5 μg of gD-2 antigen (n = 10). Sera were collected 14 days after the third immunization and examined by ELISA for antibody against gC-2 (A) or gD-2 (B). Each symbol represents serum from a single guinea pig tested in duplicate. Results are plotted as mean log10 titers. (C) Serial dilutions of sera from gC-2 (left)-, gD-2 (middle)-, and gC-2-plus-gD-2 (right)-immunized animals were evaluated for neutralizing antibody to HSV-2 strain MS, without complement (solid lines) or with 2.5% human serum as the source of complement (dashed lines). Sera from mock-immunized animals were used as a control. Each data point represents the mean ± standard deviation for sera from 3 guinea pigs tested in duplicate wells.
Immunization with combined gC-2 and gD-2 antigens protects guinea pigs from acute vaginal disease and recurrent vaginal shedding of HSV-2 DNA.
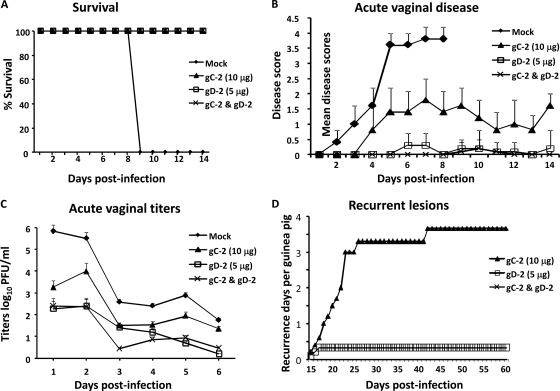
After three immunizations, guinea pigs were challenged intravaginally with 5 × 105 PFU HSV-2 strain MS. All mock-immunized guinea pigs died by 9 dpi, while animals immunized with gC-2, gD-2, or both immunogens all survived through day 14 (P < 0.001 for comparing mock-immunized group with gD-2, gC-2, or gD-2-plus-gC-2 group) (Fig. 10A), although 2 gC-2-immunized guinea pigs died at 23 dpi. Severe vaginal disease was observed in mock-immunized guinea pigs prior to their death on day 9, while animals immunized with gC-2 antigen had less severe disease than mock-immunized guinea pigs (P < 0.02) (Fig. 10B). The gD-2 antigen group and the combined gC-2-plus-gD-2 group also had significantly less disease than the mock-immunized animals (P < 0.001 for comparing gD-2 or combined group with mock group); however, the gC-2, gD-2, and combined immunization groups were not significantly different from each other. None of the animals in the gC-2, gD-2, or combined group had hind limb paralysis; however, 3 of 5 animals in the gC-2 antigen group had urinary retention, compared with none in the gD-2 and combined groups (P < 0.05 for comparing gC-2 group with gD-2 or combined group).
Fig. 10.
Protection provided by gC-2 and gD-2 immunization of guinea pigs. Guinea pigs were immunized as described in the legend to Fig. 9 and were challenged intravaginally with 5 × 105 PFU HSV-2 strain MS. (A) Survival; (B) vaginal disease; (C) vaginal titers. Results are means ± standard deviations for 5 animals per group for the mock- and gC-2-immunized animals and for 10 animals per group for the gD-2- and gC-2-plus-gD-2-immunized animals. (D) Average number of days with recurrent lesions per animal. All mock-immunized animals died during acute infection; therefore, recurrent disease is plotted only for the gC-2-, gD-2-, and gC-2-plus-gD-2-immunized animals.
Vaginal titers for the mock-immunized animals were approximately 6 log10 on day 1 (Fig. 10C). Day 1 vaginal titers for the gD-2 and combined immunization groups were approximately 4 log10 lower than those for the mock-immunized animals. Overall, viral titers for the gC-2, gD-2, or combined immunization group were significantly lower than those for the mock-immunized group (P < 0.001). The gD-2-immunized animals did not differ significantly from gC-2-immunized animals. The combined group titers were statistically different from those of the gC-2 group (P < 0.001) but not from those of the gD-2 group, except on day 3 (P < 0.02).
Recurrent infections were monitored from 15 to 60 dpi. All mock-immunized guinea pigs died by day 9; therefore, these animals could not be evaluated for recurrent infection. All five guinea pigs immunized with gC-2 antigen and 2 of 10 guinea pigs immunized with gD-2 antigen had recurrent lesions, compared with 0 of 10 guinea pigs in the combined group. The mean number of recurrences per animal in the gD-2 or combined group was significantly lower than that for the gC-2 group (P < 0.02 for comparing gC-2 group with gD-2 group and P < 0.01 for comparing gC-2 group with combined group), while differences between the combined group and the gD-2 group were not significant (Fig. 10D).
Vaginal viral shedding measured by qPCR during recurrent infections in guinea pigs.
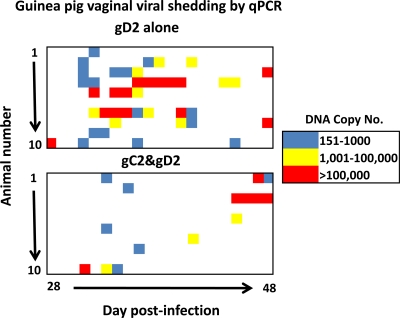
The extent of viral shedding during recurrent infection correlates with the DRG viral load; therefore, viral shedding provides useful information about vaccine efficacy in protecting DRG (36). Vaginal viral shedding in the gD-2 and combined immunization groups was measured by qPCR from 28 to 48 dpi. The DNA copy number for each animal was plotted in a heat chart (Fig. 11). Blue indicates low positive values (151 to 1,000 copies), yellow indicates intermediate positive values (1,001 to 100,000 copies), and red indicates high positive values (>100,000 DNA copies). In the gD-2 group, more DNA shedding occurred on more days than in the combined immunization group (Fig. 11, compare top and bottom panels). The heat chart results are summarized in Table 1. Overall, the gD-2 group had HSV-2 DNA detected on 41/210 days (19.1%), compared with 15/210 days (7.1%) for the combined group (P < 0.001). Significant differences between the two groups were noted for the numbers of days with low, intermediate, and high levels of HSV-2 DNA shedding (P < 0.01 or P < 0.001 at each level). The gD-2 group had 22 episodes of DNA shedding, compared with 10 episodes for the combined group (P < 0.01), where an episode is defined as one or more DNA-positive days preceded and followed by a day without shedding. Three episodes of >1-day shedding were noted in the gC-2-plus-gD-2 group, compared with 10 episodes in the gD-2 group (P < 0.01). Overall, the combined gC-2-plus-gD-2 immunization group had significantly fewer days of recurrent HSV-2 DNA vaginal shedding; however, on days that shedding was detected, the average DNA copy numbers were comparable in the two groups (4.1 log10 DNA copies in the gD-2 group and 4.0 log10 DNA copies in the combined group).
Fig. 11.
Vaginal shedding of HSV-2 DNA as measured by qPCR. The heat charts show vaginal viral shedding for 10 guinea pigs in the gD-2-immunized group (top) and 10 animals in the gC-2-plus-gD-2-immunized group (bottom), measured at 28 to 48 dpi.
Table 1.
Recurrent vaginal shedding of HSV-2 DNA in gD-2 or gC-2-plus-gD-2 groupa
| Group | Days of DNA shedding (% of days) |
No. of episodes (duration [days] if >1 day) |
|||||
|---|---|---|---|---|---|---|---|
| 151–1,000 copies | 1,001–100,000 copies | >100,000 copies | Total | 1 day | >1 day | Total | |
| gD2 | 16 (7.6) | 10 (4.8) | 15 (7.1) | 41 (19.1) | 12 | 10 (2–6) | 22 |
| gC2 plus gD2 | 6 (2.8)** | 3 (1.5)*** | 6 (2.8)** | 15 (7.1)*** | 7 | 3 (2–4)** | 10** |
**, P < 0.01; ***, P < 0.001.
DISCUSSION
Despite many years of effort in vaccine development, no HSV-1 or HSV-2 vaccine is currently available that prevents disease (12, 13, 32, 44, 47, 57). HSV-1 and HSV-2 carry numerous immune evasion molecules that likely reduce the effectiveness of innate and acquired immune responses in humans. Blocking of the activities of immune evasion molecules is gaining recognition as a novel approach for vaccine development for herpes simplex virus and other pathogens, such as Neisseria meningitidis (18, 29, 41, 49). Two clinical trials for vaccines against Neisseria meningitidis include a strategy of blocking complement evasion by factor H binding protein (binds complement factor H) and PorA (binds complement regulator C4BP) (26, 48). Many immune evasion molecules function within infected cells and are not accessible to blocking antibodies; however, gC-1 and gC-2 are ideal candidates for blocking evasion functions by immunization, since these glycoproteins are expressed on the viral envelope and the infected cell. The vaccine strategy pursued in this report is one that uses gD-2 antigen to induce potent immunity and gC-2 antigen to block complement-mediated immune evasion. We also determined that gC-2 antigen induces moderately high titers of neutralizing antibodies even in the absence of complement (1:80), as well as a robust CD4+ T-cell response, which likely enhances the protection provided by gC-2 combined with gD-2 antigen.
A concern about assessing vaccine responses in laboratory animals is that results may be misleading. HSV-1 and HSV-2 are less effective at evading antibody and complement responses of mice and guinea pigs than those of humans. For example, HSV-1 glycoprotein E (gE-1) binds the Fc domain of human IgG and blocks its activities, including complement activation and antibody-dependent cellular cytotoxicity (15, 39). However, gE-1 does not bind the Fc domain of murine or guinea pig IgG; therefore, activities mediated by the Fc domain of human IgG but not by that of mouse or guinea pig IgG are blocked (27, 46). HSV-1 and HSV-2 gC molecules bind complement component C3b, which reduces the effectiveness of complement in host defense. While gC-1 binds to human, mouse, and guinea pig C3b, it binds to human C3b with a higher affinity, suggesting that gC-1 may be more effective in immune evasion in humans than in the animal models used to study pathogenesis and vaccine efficacy (24). The increased binding affinity between gC-1 and human C3b also suggests that blocking antibodies may not be as effective in humans as in mice or guinea pigs. To address some of these concerns, the C3b blocking assays and neutralization studies reported here used human complement rather than mouse or guinea pig complement.
Comparative studies in C3 knockout and C57BL/6 mice demonstrated that gC-2 antibodies were effective in vivo because they blocked complement-mediated immunity. If the antibodies were effective because of virus neutralization independent of complement, we would expect the activities to be similar in C3 knockout and complement-intact mice, which was not the result obtained. In vitro neutralization assays using mouse and guinea pig sera from immunized animals provided additional support for the hypothesis that gC-2 antibodies block HSV-2 evasion from complement. The results demonstrated that neutralizing antibody titers in sera of animals immunized with gC-2 plus gD-2 increased up to 8-fold in the presence of human complement, while there was little increase in antibody titers of gC-2-plus-gD-2-immunized animals in the absence of human complement.
We previously reported that HSV-1 infection in humans and mice produces antibodies to gC-1; however, much higher gC-1 antibody titers are produced in mice after immunization than after infection, and antibodies produced by immunization block C3b binding to gC-1 at higher titers than those produced after infection (9). HSV-2-infected humans have gC-2 antibody titers that are comparable to those detected in HSV-2-infected mice and guinea pigs; however, gC-2 antibodies in human serum are not effective at blocking C3b binding to gC-2 (S. Awasthi et al., unpublished observation). These observations suggest that immunization with gC-2 antigen in humans may greatly increase gC-2 antibody titers that block C3b binding. The successful implementation of the vaccine strategy proposed in this report requires high titers of blocking antibodies that persist over time. The gC-2 antibody titers persisted beyond 42 weeks in mice, as did protection against vaginal challenge.
T-cell immunity is important for control of HSV infection. Both HSV-specific CD4+ and CD8+ T cells have been isolated from lesions of humans, and these cells are required for the clearance of virus from the genital epithelium (33, 45). Significant increases in IFN-γ- and TNF-α-producing CD4+ and CD8+ T cells occurred in response to gC-2-plus-gD-2 immunization. Interestingly, in gC-2-plus-gD-2-immunized mice, restimulation in vitro with gC-2 antigen produced a significantly greater frequency of IFN-γ+ TNF-α+ CD4+ T cells than did restimulation with gD-2 antigen. Interestingly, CD4+ T cells were shown to be critically important for virus clearance from sensory neurons of HSV-1-infected mice (28). To assess the activation of CD4+ and CD8+ T cells, we used purified gC-2 and gD-2 antigen. It is possible that CD4+ or CD8+ T-cell stimulation with peptide pools may have produced even more robust responses; nevertheless, significant increases in IFN-γ and TNF-α in CD4+ and CD8+ T cells were detected in immunized mice.
The main observation in this paper was that gC-2 antigen administered with gD-2 antigen enhanced the protection of DRG in mice during acute infection and decreased vaginal shedding in guinea pigs during recurrent infection compared with immunization with either subunit antigen alone. In these animal models, gD-2 antigen alone is a highly effective immunogen, which is not surprising, since various vaccine manufacturers decided to pursue this antigen as an HSV-2 vaccine candidate based in part on animal studies (5, 12, 57). Despite the potency of gD-2 immunization, enhanced protection was observed in the murine and guinea pig models when gC-2 antigen was coadministered with gD-2 antigen. Importantly, the combined immunization in mice demonstrated improved protection of DRG when gD-2 antigen was administered at a low (250 ng) or high (2 μg) dose and when challenge was by the flank or vaginal route, while in guinea pigs the combined immunization resulted in fewer episodes of HSV-2 DNA shedding.
Protecting the DRG from HSV-2 infection is an important marker of vaccine efficacy, since the DRG is the site of latency and the source of virus for recurrent infections (36). No infectious virus was recovered from the DRG of mice immunized with gC-2 plus gD-2, although low levels of HSV-2 DNA were detected by qPCR. Replication of virus in the vagina during acute infection indicated that the combined gC-2-plus-gD-2 vaccine did not provide sterilizing immunity in mice or guinea pigs. In addition, HSV-2 DNA titers of ≥105 DNA copies were detected 6 times in guinea pigs immunized with gC-2 plus gD-2 during surveillance for viral shedding, compared with 15 times in the gD-2 group. The high titers of HSV-2 DNA suggest that lesions were present, yet none were detected in the gC-2-plus-gD-2 group by inspection of the perineal region, indicating that the lesions were likely intravaginal.
In multiple experiments, the combined gC-2 and gD-2 immunogens significantly outperformed gD-2 alone (Fig. 1E, 5C and D, 6B and D, and 9C; Table 1). In other experiments, the differences were not significant but the trend favored the combined immunization group, while in certain experiments no additional improvement was noted with the combined group, since gD-2 alone provided 100% protection. Overall, the combined gC-2 and gD-2 immunogens provided better protection than that by gD-2 alone; nevertheless, the combined immunogens did not completely protect against vaginal viral shedding in guinea pigs. Therefore, additional approaches, such as adding another subunit antigen or combining subunit antigens with inactivated whole virus or attenuated live virus vaccines, may be required to achieve the goal of total DRG protection.
ACKNOWLEDGMENTS
This work was supported by NIH grant HL28220 and by a grant from Merck and Co., Inc.
We thank Ryan King for help with breeding the C3 knockout mice, Gary Cohen and Roselyn Eisenberg for providing purified baculovirus immunogens, and Stuart Isaacs for advice on adjuvant administration. We also thank Sarah Ratcliffe (Center for AIDS Research Epidemiology and Biostatistics Core) and Farida Shaheen (Center for AIDS Research Viral and Molecular Core) for expert advice on statistics and quantitative PCR, respectively.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Awasthi S., Lubinski J. M., Friedman H. M. 2009. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine 27:6845–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Betts M. R., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brittle E. E., Wang F., Lubinski J. M., Bunte R. M., Friedman H. M. 2008. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J. Virol. 82:8431–8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bryson Y. J., et al. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N. Engl. J. Med. 308:916–921 [DOI] [PubMed] [Google Scholar]
- 5. Byars N. E., et al. 1994. Vaccinating guinea pigs with recombinant glycoprotein D of herpes simplex virus in an efficacious adjuvant formulation elicits protection against vaginal infection. Vaccine 12:200–209 [DOI] [PubMed] [Google Scholar]
- 6. Canziani G., et al. 1999. Exploring biomolecular recognition using optical biosensors. Methods 19:253–269 [DOI] [PubMed] [Google Scholar]
- 7. Carroll M. C. 2004. The complement system in B cell regulation. Mol. Immunol. 41:141–146 [DOI] [PubMed] [Google Scholar]
- 8. Carroll M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986 [DOI] [PubMed] [Google Scholar]
- 9. Chang Y. J., Jiang M., Lubinski J. M., King R. D., Friedman H. M. 2005. Implications for herpes simplex virus vaccine strategies based on antibodies produced to herpes simplex virus type 1 glycoprotein gC immune evasion domains. Vaccine 23:4658–4665 [DOI] [PubMed] [Google Scholar]
- 10. Chung K. M., et al. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. U. S. A. 103:19111–19116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen J. 2010. Painful failure of promising genital herpes vaccine. Science 330:304. [DOI] [PubMed] [Google Scholar]
- 12. Corey L., et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340 [DOI] [PubMed] [Google Scholar]
- 13. Da Costa X. J., Morrison L. A., Knipe D. M. 2001. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology 288:256–263 [DOI] [PubMed] [Google Scholar]
- 14. Darrah P. A., et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 15. Dubin G., Socolof E., Frank I., Friedman H. M. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang C., Miwa T., Shen H., Song W. C. 2007. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J. Immunol. 179:3178–3186 [DOI] [PubMed] [Google Scholar]
- 17. Frank I., Friedman H. M. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman H. M. 2000. Immunologic strategies for herpes vaccination. JAMA 283:746. [DOI] [PubMed] [Google Scholar]
- 19. Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633–635 [DOI] [PubMed] [Google Scholar]
- 20. Girgis N. M., et al. 2008. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral A56 protein and protects infected cells from complement attack. J. Virol. 82:4205–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta R., Warren T., Wald A. 2007. Genital herpes. Lancet 370:2127–2137 [DOI] [PubMed] [Google Scholar]
- 22. Hook L. M., Lubinski J. M., Jiang M., Pangburn M. K., Friedman H. M. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 80:4038–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoshino Y., et al. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 200:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huemer H. P., Larcher C., van Drunen Littel-van den Hurk S., Babiuk L. A. 1993. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch. Virol. 130:353–364 [DOI] [PubMed] [Google Scholar]
- 25. Hung S. L., et al. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299–312 [DOI] [PubMed] [Google Scholar]
- 26. Jarva H., Ram S., Vogel U., Blom A. M., Meri S. 2005. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J. Immunol. 174:6299–6307 [DOI] [PubMed] [Google Scholar]
- 27. Johansson P. J., Myhre E. B., Blomberg J. 1985. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J. Virol. 56:489–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson A. J., Chu C. F., Milligan G. N. 2008. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J. Virol. 82:9678–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Judson K. A., et al. 2003. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J. Virol. 77:12639–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kapadia S. B., Levine B., Speck S. H., Virgin H. W. T. 2002. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17:143–155 [DOI] [PubMed] [Google Scholar]
- 31. Kapadia S. B., Molina H., van Berkel V., Speck S. H., Virgin H. W. T. 1999. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 73:7658–7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koelle D. M., Corey L. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koelle D. M., et al. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Invest. 101:1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kopf M., Abel B., Gallimore A., Carroll M., Bachmann M. F. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373–378 [DOI] [PubMed] [Google Scholar]
- 35. Lachmann P. J., Davies A. 1997. Complement and immunity to viruses. Immunol. Rev. 159:69–77 [DOI] [PubMed] [Google Scholar]
- 36. Lekstrom-Himes J. A., Pesnicak L., Straus S. E. 1998. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J. Virol. 72:2760–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lilley B. N., Ploegh H. L., Tirabassi R. S. 2001. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J. Virol. 75:11218–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Litwin V., Jackson W., Grose C. 1992. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J. Virol. 66:3643–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lubinski J. M., Lazear H. M., Awasthi S., Wang F., Friedman H. M. 2011. The herpes simplex virus 1 IgG Fc receptor blocks antibody-mediated complement activation and antibody-dependent cellular cytotoxicity in vivo. J. Virol. 85:3239–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lubinski J. M., et al. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Madico G., et al. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magaret A. S., Wald A., Huang M. L., Selke S., Corey L. 2007. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J. Clin. Microbiol. 45:1618–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehlhop E., Diamond M. S. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meignier B., Martin B., Whitley R. J., Roizman B. 1990. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J. Infect. Dis. 162:313–321 [DOI] [PubMed] [Google Scholar]
- 45. Milligan G. N., Bernstein D. I. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481–489 [DOI] [PubMed] [Google Scholar]
- 46. Nagashunmugam T., et al. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nass P. H., Elkins K. L., Weir J. P. 2001. Protective immunity against herpes simplex virus generated by DNA vaccination compared to natural infection. Vaccine 19:1538–1546 [DOI] [PubMed] [Google Scholar]
- 48. Ngampasutadol J., et al. 2005. Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc. Natl. Acad. Sci. U. S. A. 102:17142–17147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ngampasutadol J., et al. 2008. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J. Immunol. 180:3426–3435 [DOI] [PubMed] [Google Scholar]
- 50. Para M. F., Goldstein L., Spear P. G. 1982. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J. Virol. 41:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phipps W., et al. 2011. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J. Infect. Dis. 203:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Renzi C., et al. 2003. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J. Infect. Dis. 187:19–25 [DOI] [PubMed] [Google Scholar]
- 53. Reynolds S. J., et al. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513–1521 [DOI] [PubMed] [Google Scholar]
- 54. Sawtell N. M., Bernstein D. I., Stanberry L. R. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J. Infect. Dis. 180:821–823 [DOI] [PubMed] [Google Scholar]
- 55. Sawtell N. M., Thompson R. L., Stanberry L. R., Bernstein D. I. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J. Infect. Dis. 184:964–971 [DOI] [PubMed] [Google Scholar]
- 56. Seidel-Dugan C., et al. 1988. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J. Virol. 62:4027–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stanberry L. R., et al. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 58. Strainic M. G., et al. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tal-Singer R., et al. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tengvall S., Lundqvist A., Eisenberg R. J., Cohen G. H., Harandi A. M. 2006. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 80:5283–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thompson R. L., Sawtell N. M. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van de Walle G. R., Favoreel H. W., Nauwynck H. J., Pensaert M. B. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protect pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84:939–947 [DOI] [PubMed] [Google Scholar]
- 63. Wald A., Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52 [DOI] [PubMed] [Google Scholar]
- 64. Xiao Y., et al. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang J., et al. 2009. Influenza A virus M1 blocks the classical complement pathway through interacting with C1qA. J. Gen. Virol. 90:2751–2758 [DOI] [PubMed] [Google Scholar]