Abstract
The interferon-inducible antiviral factor BST-2 prevents several enveloped viruses, including HIV, from escaping infected cells. The HIV protein Vpu antagonizes this host defense. Little is known about the expression of BST-2 during HIV infection in vivo and whether it can be modulated to the host's advantage. We studied the expression of BST-2 on blood cells from HIV-infected patients during the acute and chronic phases of disease as well as after antiretroviral treatment (ART). The expression of BST-2 was increased on mononuclear leukocytes, including CD4-positive T lymphocytes from HIV-positive patients, compared to that on cells of uninfected controls. The expression of BST-2 was highest during acute infection and decreased to levels similar to those of uninfected individuals after ART. Treatment of primary blood mononuclear cells in vitro with alpha interferon or with Toll-like receptor (TLR) agonists increased the expression of BST-2 to levels similar to those found during infection in vivo. The interferon-induced levels were sufficient to overcome the Vpu protein in vitro, reducing the release of wild-type HIV. These data show that BST-2 is upregulated during HIV infection, consistent with its role as an interferon-stimulated gene. The data further suggest that this upregulation is sufficient to saturate the activity of Vpu and inhibit wild-type HIV.
INTRODUCTION
The interferon-inducible transmembrane protein BST-2 (HM1.24/CD317/tetherin) potently inhibits the release of viral particles and is counteracted by HIV-1 Vpu, which induces its downregulation from the cell surface (27, 42). This was demonstrated in numerous reports using various expression and cell line systems (reviewed by Tokarev et al. [40]). However, it is not yet clear to what extent BST-2 is expressed on primary cells in vivo and whether this innate restriction factor plays a role during human immunodeficiency virus (HIV) replication in infected persons. Similarly, the role of Vpu in vivo is not resolved. Nonhuman primate/simian immunodeficiency virus (SIV) models are hampered due to the lack of Vpu in most SIVs. However, pathogenic simian-human immunodeficiency viruses (SHIVs) encoding HIV-1 Vpu and Env are markedly less virulent when Vpu is not expressed (37). Whether this effect is due to Vpu's activity as a BST-2 antagonist or to other functions such as the downregulation of CD4 or NTB-A is unclear (33, 34). On the other hand, primary HIV isolates lacking a functional Vpu due to a start codon mutation have been described (11, 31), suggesting that Vpu is not absolutely essential for viral replication in vivo, although such isolates seem rare. A loss of Vpu function might reduce virus release and infection by cell-free virus but does not necessarily prevent viral spread, which can still occur through cell-to-cell transmission (reviewed by Andrew and Strebel [2]). These issues have kept the roles of BST-2 and Vpu in the pathogenesis of HIV infection and in the innate immune response to HIV unresolved.
The innate immune system constitutes the first line of defense against invading pathogens. Macrophages and dendritic cells (DCs) express pattern recognition receptors (PRRs), which recognize conserved pathogen-associated structures (PAMPs) and initiate the first response, including the production of type I interferons (IFNs) and proinflammatory cytokines (4). These mechanisms and a cellular antiviral state are induced upon HIV infection, although the PRRs that recognize HIV-1 are not fully elucidated. IFN and proinflammatory cytokines are released during acute HIV infection, and viremia is reduced; however, the innate immune response is not capable of eliminating the virus completely.
Type I interferons are a major component of the innate immune response and are secreted to limit systemic dissemination of viruses and to drive adaptive responses required for viral clearance (38). BST-2 is an interferon-induced protein, and the bst-2 gene promoter contains response elements suggesting that inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) might also induce its expression (28). Alpha interferon (IFN-α) binds to the IFN receptor on infected and uninfected bystander cells and induces the transcription of the interferon-stimulated genes (ISGs) through the Jak-STAT pathway to inhibit viral replication (41).
A prominent effect of IFN-α on HIV-1 (14, 20, 30) is the inhibition of the release of progeny virions from infected cells by BST-2 (26, 27, 42). HIV-1 particles are retained at the plasma membrane by this type II transmembrane protein. BST-2 is expressed on plasmacytoid DCs (pDCs), macrophages, and plasma cells, and it is induced on most cell types by type I IFNs (6). However, the expression on primary T cells, at least from healthy persons, seems to be relatively low (25). On the other hand, the levels of BST-2 induced in vivo by viral infections, including HIV-1, are currently unknown. Although the antiviral mechanisms of IFN-induced proteins are not fully elucidated, clinical studies have demonstrated that IFN-α inhibits HIV-1 replication in vivo (21, 35). IFN-α increases transiently during acute HIV-1 infection in vivo (13, 44), but it subsequently decreases, reportedly due to a refractory state of pDCs, the main producers of IFN (22). Plasmacytoid DC numbers are also reduced in the blood of chronically infected patients. This loss of pDCs correlates with higher viral loads, decreased numbers of CD4+ T cells, and the onset of opportunistic infections.
HIV-1 particles can stimulate release of IFN-α from dendritic cells through engagement with Toll-like receptors (TLRs); viral genomic single-stranded RNA (ssRNA) is recognized by TLR 7 and 8 from endocytosed virions (5), (15). Moreover, TLR 3 can sense double-stranded RNA (dsRNA) during HIV infection (7), and TRIM5 can recognize the viral capsid (29). As a result of TLR 3, 7, and 8 stimulation, IFN is released and HIV-1-infected individuals often have high levels of plasma type I IFN, which is induced during the acute phase of HIV-1 infection as early as 1 week after exposure (36). Therefore, we determined whether BST-2 expression is increased on target cells during different stages of HIV infection in vivo and whether inflammatory cytokines (IFN-α, IL-6, TNF-α) of the innate immune response or TLR agonists could induce increased levels of BST-2 on those cells in vitro. We found enhanced expression of BST-2 on leukocyte subtypes, including CD4+ T cells, most prominently during acute HIV-1 infection. Comparable expression levels of BST-2 were achieved in vitro by treatment of HIV target cells with IFN-α, and these levels were able to overcome Vpu and impair the release of wild-type HIV virions.
MATERIALS AND METHODS
Patient population.
Blinded samples were obtained from the San Diego Acute and Early Infection cohort by D. Smith, D. Richman, and S. Little. All patients were white males having sex with men as their HIV risk factor. Frozen peripheral blood mononuclear cells (PBMCs) from different stages of HIV infection were used for flow cytometric studies, and matching plasma samples were analyzed by enzyme-linked immunosorbent assay (ELISA) for cytokine production.
Longitudinal samples were chosen based on the estimated time of infection and viral load. “Acute infection” samples showed levels of plasma virus of >25,000 RNA copies/ml and were obtained <4 weeks after the estimated date of infection. “Chronic” samples with similar levels of plasma virus were obtained >1 year after infection; these patients were not on antiretroviral treatment. Samples of patients who initiated antiretroviral therapy (ART) (efavirenz, tenofovir, and emtricitabine) had <50 copies of virus RNA/ml plasma. Uninfected, frozen PBMCs from 7 healthy donors were used as controls. All participants gave written, informed consent, in accordance with the requirements of the local institutional review board.
PBMC isolation and treatment.
Blood from healthy donors was collected by venipuncture into syringes containing sodium heparin according to institutional review board-approved protocols. Blood was separated by centrifugation, and plasma was stored in aliquots at −80°C. PBMCs were isolated using Ficoll gradients and either frozen in 10% dimethyl sulfoxide (DMSO)/fetal bovine serum (FBS) for comparison with patient samples or stimulated for 3 days with phytohemagglutinin (PHA)/IL-2 (recombinant human IL-2 [NIH AIDS Research and Reference Program], PHA [Sigma-Aldrich]) for in vitro studies.
CD4+ T cell isolation.
CD4+ T cells were isolated from buffy coats by negative selection with the RosetteSep Human CD4+T cell enrichment kit (StemCell Technologies, Vancouver, British Columbia, Canada) as described by Terry et al. (39). Cells were activated for 3 days with PHA/IL-2 followed by stimulation with IFN-α and TLR agonists.
TLR agonists.
Poly(I·C) (high molecular weight [HMW] average size, 1.5 to 8 kb; low molecular weight [LMW] average size, 0.2 to 1 kb) is a TLR 3 and retinoic acid-inducible gene I (RIG-I)/melanoma differentiation-associated gene 5 (MDA-5) agonist. Poly(A·U) is a synthetic double-stranded RNA molecule that signals only through TLR 3. ssRNA-LyoVec is a TLR 7 agonist. Poly(U) is a TLR 8 agonist. ODN2216 and 2006 are CpG DNAs and stimulate TLR 9. All were purchased from Invivogen, San Diego, CA.
Recombinant human IFN-α2a was purchased from PBL Interferon Source, Piscataway, NJ. Recombinant human IL-6 and human TNF-α were from R&D Systems, Minneapolis, MN.
Flow cytometry staining to analyze BST-2 expression on the cell surface of leukocyte subsets.
Antibodies CD3 ACP-Cy7, CD8 Pacific Blue, CD4 PE, CD14 fluorescein isothiocyanate (FITC), and CD19 PerCP-Cy5.5 were purchased from BD Biosciences, San Diego, CA. The BST-2 antibody (clone HM1.24) was a kind gift from Chugai Pharmaceuticals, Japan; the matching IgG control and secondary anti-mouse APC-conjugated antibody were purchased from BioLegend, San Diego, CA.
PBMCs from infected donors were quick-thawed at 37°C and washed with phosphate-buffered saline (PBS). Cells were resuspended in 1 ml PBS and stained with the Live/Dead fixable dead cell stain kit (Aqua) (Molecular Probes, Eugene, OR) for 30 min in the dark at room temperature (RT). TruStain (5 μl/100 μl; BioLegend, San Diego, CA) was used to block nonspecific binding to Fc receptors prior to staining with specific antibodies. Cells were stained for BST-2 (1 μg/100 μl) or equivalent isotype control on ice for 30 min, followed by staining with the secondary, APC-conjugated antibody (1 μg/100 μl). After blocking with normal mouse serum (10%), PBMCs were resuspended in 100 μl PBS and fluorophore-conjugated antibodies against subset markers were added and incubated for 30 min on ice. Cells were washed and fixed in 2% paraformaldehyde for 2 h before analysis on a BD FACS Canto II using BD Diva software.
Plasma IFN-α, IL-6, and TNF-α levels.
Frozen plasma samples of patients during acute (n = 3) and chronic (n = 6) infection and after ART treatment (n = 5) were used to determine the cytokine levels by ELISA using the human IFN-α serum sample ELISA kit from PBL Interferon Source, Piscataway, NJ, and the Legend Max human IL-6 and TNF-α ELISA kit from BioLegend, San Diego, CA, according to the manufacturers' protocols.
Virus stocks and PBMC infection.
The macrophage-tropic variant of NL4.3, 51-9, is a recombinant infectious molecular clone containing the StuI-NheI fragment of gp120 (C2 and V3 region) from a primary HIV isolate ligated into the NL4.3 clone (9, 10) and was used for infection of PBMCs. The Vpu-deleted version of p51-9 was generated by exchanging the EcoRI-NheI fragment of pNL4-3 Δvpu, originally named Udel-1 and originated by Klaus Strebel, to create p51-9 Δvpu.
Viruses used for infection were generated in 293T cells, concentrated, and normalized to equal nanograms of p24 capsid antigen. Wild-type and Δvpu viruses were tested for infectivity using a HeLa P4R5-based indicator assay; the viruses had similar infectivity per nanogram of p24.
Freshly isolated PBMCs were activated for 3 days with PHA and IL-2 and then infected with 51-9 virus (1 to 2 μg p24 for 4 h) at 37°C. The cells were washed to remove the viral inocula and then incubated in RPMI (plus 10% FBS, PenStrep, and IL-2) at 37°C. Cells were treated with IFN-α after the virus was washed out (about 5 h after the initiation of the infection). Cells and supernatant were analyzed on days 1 and 2 after infection: cell surface BST-2 by flow cytometry and secreted p24 by ELISA (Alliance HIV-1 p24 kit; Perkin Elmer, Waltham, MA). Cell culture supernatants were analyzed by ELISA after centrifugation through a 20% sucrose cushion to remove non-virion-associated p24.
Statistical analysis.
Results are shown as medians with ranges or means ± standard deviations (SD). Groups were compared using the Mann-Whitney test and the Wilcoxon matched-pair rank test (repeated measures for chronic versus ART-treated samples). Differences were considered significant when P ≤ 0.05. The software used was GraphPad Prism.
RESULTS
BST-2 expression levels on uninfected PBMCs.
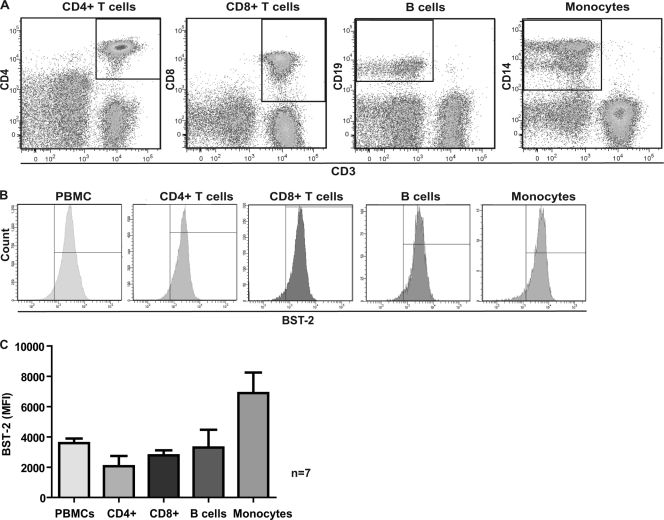
To determine the expression levels of BST-2 on different leukocyte subsets, including CD4+ and CD8+ T cells, B cells, and monocytes, we established a flow cytometric staining assay that allowed us to analyze BST-2 expression on the cell surface, the location at which BST-2 acts to restrict virion release. The cells were first analyzed with a live/dead stain, which allowed us to subsequently measure BST-2 only on the surface of viable cells with intact membranes. CD4+ T cells were identified as CD3 and CD4 positive, CD8+ cells as CD3 and CD8 positive, B cells as CD3 negative and CD19 positive, and monocytes as CD3 negative and CD14 positive, as shown in Fig. 1 A. Each of these cellular subsets showed detectable but varying expression of surface BST-2 (Fig. 1B and C). The most prominent expression of BST-2 on uninfected PBMCs was found on monocytes, whereas CD4+ T cells, the most relevant cell population for HIV replication, displayed lower but detectable amounts of BST-2. These data are in line with a report by Kawai et al. (19), in which the number of BST-2/HM1.24 molecules was estimated based on a quantitative flow cytometric assay: monocytes displayed 1 × 104/cell, and lymphocytes displayed 2 × 103 to 3 × 103/cell in healthy individuals.
Fig. 1.
BST-2 expression on uninfected PBMCs. (A) Identification of cellular subtypes of PBMCs by flow cytometry. (B) BST-2 expression on uninfected mononuclear leukocyte subtypes. (C) Median values of BST-2 expression (mean fluorescence intensity [MFI]) on frozen, uninfected cell subtypes (n = 7 donors).
BST-2 expression on PBMCs during different stages of HIV infection in vivo.
We used the established flow cytometric method to analyze the levels of BST-2 expression on subsets of mononuclear leukocytes from the peripheral blood of HIV-infected individuals at different stages of disease. We anticipated, due to an innate immune response in vivo, to detect increased BST-2 levels on HIV target cells during acute infection and possibly during chronic infection as well.
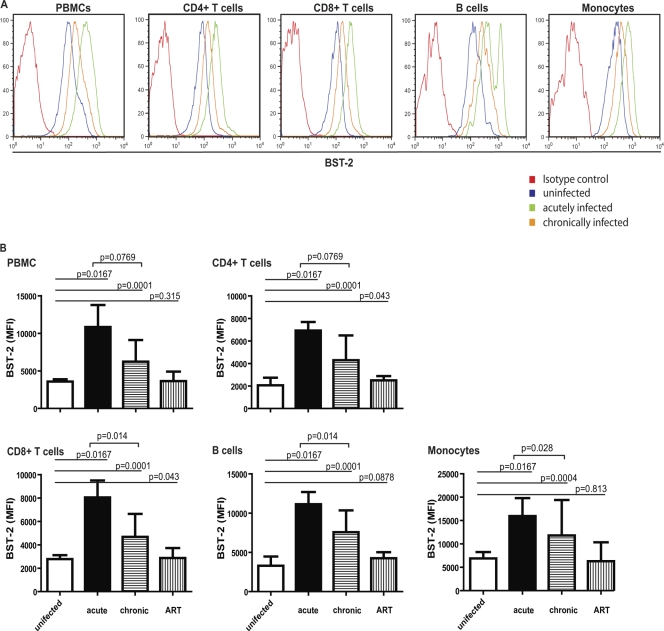
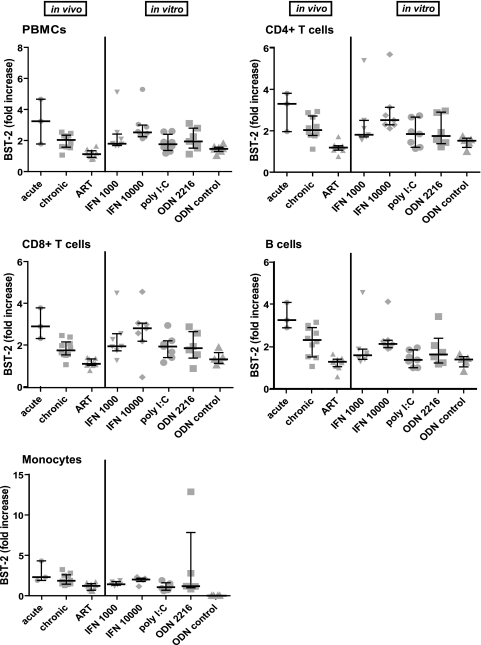
The levels of BST-2 expression were increased during HIV infection, with the greatest increase (compared to levels in uninfected controls) of about 2- to 3-fold during acute infection (Fig. 2 A). These relative increases were seen on all cell subsets, but they were greatest (over 3-fold) on CD4-positive T cells, the major host cell for HIV infection (Fig. 2B). The biphasic peak for BST-2 in the histogram seen on B cells with acute infection (Fig. 2A) was not consistent and present only for this donor.
Fig. 2.
BST-2 expression on PBMCs during different stages of HIV infection. (A) Example of one patient followed longitudinally, representative of n ≥ 3 donors. Cell surface BST-2 was stained with antibody clone to HM1.24 (BST-2) on cellular subtypes of frozen PBMCs from patients during acute and chronic infection and compared to uninfected, frozen PBMCs. (B) Quantification of cell surface BST-2 of all donors during acute (n = 3) and chronic (n = 10) HIV infection and after initiation of antiretroviral therapy (n = 10) (n = 7 for uninfected). BST-2 expression is shown as median values of the mean fluorescence intensity (MFI).
These samples were obtained within 4 weeks after the estimated time of infection, so they might underestimate the maximal induction of BST-2 that could have occurred more acutely. Consistent with this notion, IFN-α, IL-6, and TNF-α levels had already decreased to undetectable levels in the plasma of most patients. We detected a clearly elevated plasma level of IFN-α in only one acutely infected patient (418.5 pg/ml, approximately 100 U/ml); IFN-α levels were close to the detection limit of the assay for the other two plasma samples from acutely infected patients (range of IFN-α ELISA, 12.5 to 500 pg/ml). This is in line with previous studies reporting a peak of IFN-α levels in the plasma around day 10 after infection in humans and in African green monkey and rhesus macaques (17, 36). Similar results were seen for plasma IL-6: only one acutely infected patient showed a slightly increased level of 35.4 pg/ml (minimum detectable concentration by ELISA, 1.6 pg/ml). No elevated levels of plasma TNF-α were found, and with one exception, no increase in any of the above cytokines was seen during chronic infection or after ART treatment (n = 5/6; data not shown).
The levels of BST-2 observed in chronically infected patients were also significantly increased compared to those in uninfected controls, although this increase was not as great as that observed during acute infection. This increase was again seen on all cell subsets; most importantly, a 2-fold increase persisted on CD4+ T cells.
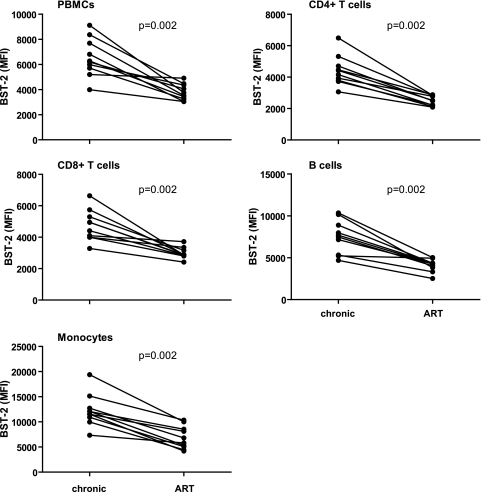
Strikingly, upon antiretroviral treatment (ART), the expression levels of BST-2 decreased significantly, reaching levels similar to those on cells from uninfected donors (Fig. 2A and B and Fig. 3, paired sample analysis of cells from chronically infected patients before and after initiation of ART).
Fig. 3.
Paired sample comparison of cells from chronically infected patients versus cells from the same patients followed longitudinally after initiation of ART (n = 10). Shown are single values of BST-2 expression (mean fluorescence intensity [MFI]) for each donor. The Wilcoxon matched-pair rank test was used to calculate P values.
These data show that BST-2 is upregulated during HIV infection in vivo, most prominently during acute infection. The data also show that antiviral therapy, which reduced plasma viremia to undetectable levels, was associated with a decrease in the expression of BST-2. The latter observation is consistent with the notions that BST-2 is an interferon-stimulated gene (ISG) and that the innate immune response is being driven by the presence of the virus.
Induction of BST-2 on PBMCs and CD4+ T cells by cytokines, IFN-α, and TLR agonists in vitro.
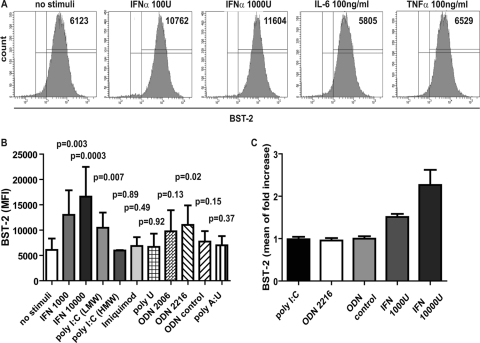
Interferons and cytokines of the innate immune response induce increased expression of BST-2 on several cell types and thereby reduce HIV virion release in vitro (12, 27, 42). To test if levels comparable or even higher than those found in vivo can be induced on uninfected cells in vitro, we stimulated activated PBMCs with IFN-α, IL-6, and TNF-α for 24 h. The activation of the cells with PHA/IL-2 for 3 days did not have a significant effect on BST-2 expression (see Fig. S1 in the supplemental material). IFN-α induced a dose-dependent increase of BST-2 expression on PBMCs (Fig. 4 A), and maximum BST-2 levels were achieved with 10,000 U of IFN-α (Fig. 4B). The maximal fold increases obtained in vitro were similar to those seen on mononuclear leukocytes of acute HIV-infected patients (Fig. 2B; about 3-fold). In contrast, IL-6 and TNF-α did not induce BST-2 expression on these cells in vitro (Fig. 4A; only the highest concentrations used are shown).
Fig. 4.
Induction of BST-2 on PBMCs and CD4+ T cells by cytokines, IFN-α and TLR agonists. (A and B) Fresh PHA/IL-2 activated PBMCs were treated with the indicated concentrations of cytokines (IFN-α, IL-6, and TNF-α) (A) or with Toll-like receptor (TLR) agonists [poly(I·C) LMW (TLR 3 agonist), poly(I·C) HMW (TLR 3 agonist), imiquimod (TLR 7 agonist), poly(U) (TLR 8 agonist), and poly(A·U) (TLR 3-specific agonist) (all at 10 μg/ml), ODN2006 and ODN2216 (CpG DNA, TLR 9 agonists, including ODN control, 5 μM), and IFN-α (1000 and 10,000 U/ml) (B) for 24 h before analysis of BST-2 surface levels by flow cytometry. Shown are mean values ± SD of mean fluorescence intensities (MFI) from n ≥ 2 experiments. The Mann-Whitney test was used to calculate P values. (C) Treatment of isolated CD4+ T cells (on average 98.3% CD4 positive) with IFN-α (1,000 and 10,000 U/ml) and TLR agonists poly(I·C) LMW and ODN2216 (10 μg/ml and 5 μM, respectively, for 24 h) before staining with antibody to BST-2 for flow cytometry. Shown are mean values ± SD of results from n = 3 donors.
To identify further triggers of antiviral immune pathways which might increase BST-2 expression, we investigated the potential of Toll-like receptor (TLR) agonists to induce enhanced BST-2 expression on PBMCs. Freshly isolated, activated PBMCs from up to 8 donors were treated with TLR 3, 7, 8 and 9 agonists and in parallel with IFN-α for 20 to 24 h. As shown in Fig. 4B, the TLR 3 agonist poly(I·C) (low molecular weight [LMW]) and the TLR 9 agonist ODN2216 (type A CpG DNA) were able to induce a significant increase of cell surface BST-2 in comparison to untreated PBMCs, although these increases were not as great as those induced by IFN.
The TLR 7 agonist imiquimod, as well as the TLR 8 agonist poly(U) and the TLR 9 agonist ODN2006 (type B CpG DNA), did not induce BST-2 expression. Since ODN2216, a class A CpG DNA which stimulates pDCs to produce IFN-α, enhanced the expression of BST-2 but the class B CpG DNA ODN2006, which activates B and NK cells rather than triggering IFN secretion (43), did not, we thought to examine whether IFN-α is the necessary signal to induce BST-2 expression on CD4+ T cells. Moreover, since the detection of viruses by TLR 3, 7, and 9 within the endosomal compartment leads to the production of IFN-α but CD4+ T cells do not express TLR 7 or 9, we suspected that the upregulation of BST-2 on CD4+ T cells requires IFN-α secretion by other cells in the PBMC mixture in response to the TLR ligands. Therefore, we isolated CD4+ T cells by negative selection from PBMCs and assumed that this cell population does not contain pDCs, which express the relevant HIV-detecting TLR 7 and 9 (16) and are the major IFN-α-producing cell type in vivo. Stimulation of isolated CD4+ T cells with TLR 3 [poly(I·C) LMW] and 9 (OGN2216) agonists did not increase BST-2 levels; however, a 2- to 3-fold increase was achieved by treating CD4+ T cells with IFN-α (Fig. 4C). Since CD4+ T cells do not express TLR7 and 9, these results were expected; however, the stimulation of TLR 3, which is present in CD4+ T cells, might have been able to trigger BST-2 expression. These data showed that IFN-α can induce the expression of BST-2 on mononuclear leukocytes in vitro. The data also suggested that treatment of PBMCs with TLR agonists induces BST-2 on CD4+ T cells through triggering IFN-α secretion by pDCs and/or other IFN-α-producing cells.
The levels of BST-2 induced by HIV infection in vivo are similar to those achieved by treatment of PBMCs with IFN-α in vitro.
We directly compared the levels of BST-2 induced by HIV infection in vivo (relative to frozen PBMCs from uninfected persons) with the levels on uninfected PBMCs achieved by treatment with IFN-α or TLR agonists in vitro (stimulated relative to unstimulated fresh, unfrozen cells) (Fig. 5). We used fold enhancement rather than absolute values of fluorescent staining intensity for this comparison, because we observed that freeze-thawing decreased the staining intensity of BST-2 on all cell subsets (see Fig. S2 in the supplemental material). Samples derived from patients were available only in a frozen state, whereas our in vitro experiments used freshly isolated PBMCs that were not subjected to freeze-thawing. The induction of BST-2 by treatment with IFN-α was comparable to the increase observed during acute HIV infection in vivo: 2- to 3-fold on CD4+ and CD8+ T cells and monocytes. The agonists of TLR 3 and 9 [poly(I·C) LMW and ODN2216] induced an increase in BST-2 levels but to a lesser extent. These data suggested that the induction of BST-2 on CD4-positive T cells during acute infection in vivo is at least as great as the maximal induction achievable in vitro by high concentrations of IFN.
Fig. 5.
Cells stimulated in vitro with interferon or TLR agonists display comparable induction of BST-2 as is observed in vivo during HIV infection. Fold increase of BST-2 cell surface levels on frozen, infected PBMCs (in vivo) versus those on fresh, PHA/IL-2-activated PBMCs treated (in vitro) with the indicated IFN-α (1,000 and 10,000 U/ml) and TLR agonists [poly(I·C) LMW and ODN2216, n = 7 donors] for 24 h before analysis by flow cytometry. Shown are single values (symbols), median values (horizontal line) and the interquartile range of fold increase in BST-2 mean fluorescence intensity (MFI) relative to that of uninfected or unstimulated cells.
IFN treatment overcomes the effect of Vpu.
We hypothesized that the levels of BST-2 induced on primary cells by IFN might be able to overcome counteraction by the viral protein Vpu. To test this, we exposed activated PBMCs to an R5-tropic variant of HIV-1 (NL4.3-51-9) and induced BST-2 by adding IFN-α 5 h later. We then measured virion release and BST-2 expression levels the next day (Fig. 6). By this design, we minimized potential effects of interferon during the early stages on the viral replication cycle. Instead, we expected to observe primarily late effects during the first cycle of viral replication, like the restriction of virion release by BST-2. We also measured virion release and BST-2 expression after 2 days, to detect more pleiotropic effects of IFN that would not be specific to Vpu.
Fig. 6.
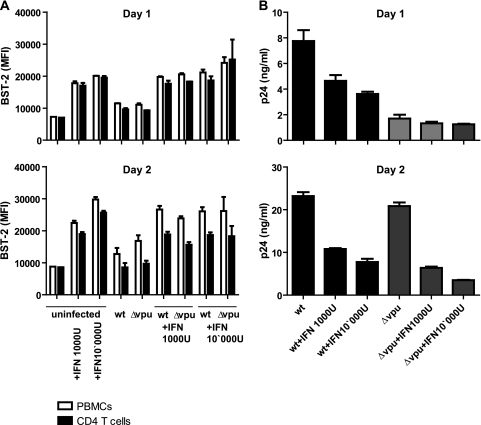
Release of wild-type HIV virions is impaired by IFN-induced BST-2. (A) Activated PBMCs were infected with R5-tropic HIV-1 for 4 h followed by removal of the virus and stimulation with IFN-α as indicated. On day 1 and 2 after infection, total PBMCs and CD4+ T cells were analyzed by flow cytometry for BST-2 surface expression (mean fluorescence intensity [MFI]). (B) In parallel, virus release was monitored by detection of secreted p24 capsid antigen by ELISA after centrifugation of culture supernatants through sucrose cushions. Shown are mean values ± SD of duplicates from one representative donor out of three.
The levels of BST-2 induced at day 1 after infection (Fig. 6A) were comparable to those found in vivo (compare PBMCs from acutely infected patients in Fig. 2B and in vitro treated PBMCs in Fig. 4B and 6A) and increased further by day 2. Notably, the infection itself (i.e., virus alone) did not upregulate BST-2, suggesting that these PMBCs do not sense the presence of HIV-1 and initiate an innate response. This lack of a virus-induced innate response is consistent with the recently reported action of the exonuclease TREX1, which degrades HIV DNA and prevents the detection of HIV-1 by cytoplasmic DNA sensors (45). The levels of BST-2 were not appreciably affected by the expression of Vpu (compare cells infected with the wild type and Δvpu). This was not unexpected, since only a fraction of the cells in the culture were expected to be productively infected at the nominal multiplicity of infection (MOI) used (about 0.06 infectious units per cell).
On day one, the release of wild-type HIV, which encodes Vpu, was greater than that of virus lacking Vpu (Δvpu), reflecting the ability of Vpu to antagonize the restrictive activity of BST-2 and enhance virus release (Fig. 6B). Moreover, IFN inhibited the release of wild-type virus, consistent with the induction of higher levels of BST-2 on the cell surface (Fig. 6A). These results suggest that the mechanism by which Vpu counteracts BST-2 is essentially saturated in infected T cells, such that further induction of BST-2 by IFN decreases virion release. In contrast, IFN did not reduce the release of HIV-1 lacking Vpu (Δvpu) on day one, suggesting that for virus lacking Vpu, the mechanism by which BST-2 restricts virion release is saturated and cannot be further enhanced by induction with IFN. By day two, the effect of IFN was no longer specific for wild-type virus. This likely reflects the pleiotropic effects of IFN and the actions of additional interferon-inducible host factors such as TRIM22 (3). Moreover, other less-well-characterized effects of IFN are likely to affect early events in the viral replication cycle and thus affect the second round of viral replication. With the exception of restriction of virion release by BST-2, none of these effects are likely to be antagonized by Vpu. The difference between wild-type and Δvpu viruses became minimal on day two, consistent with the enhanced cell-to-cell transmission reported to occur when the virus lacks Vpu in some in vitro systems (18).
Overall, these data indicate that IFN treatment and the associated induction of BST-2 specifically inhibits Vpu-expressing virus in a single round of infection in vitro. Given that these levels of BST-2 are similar to those observed during HIV infection in vivo, the vpu gene appears to be suboptimally adapted to antagonizing BST-2, rendering HIV-1 susceptible to high levels of BST-2 expression.
DISCUSSION
We have shown the detection of BST-2 by flow cytometry on the surface of subsets of peripheral blood leukocytes, including CD4-positive T cells from uninfected and HIV-infected individuals. BST-2 was upregulated by 2- to 3-fold on cells of acutely infected patients. These elevated levels decreased slightly during chronic HIV infection and were reduced to levels similar to those of uninfected persons by effective antiretroviral therapy.
The induction of surface BST-2 in vitro by IFN to levels comparable to those found during acute infection in vivo could overcome the action of Vpu and decrease the release of wild-type HIV from primary cultures of human mononuclear leukocytes. High levels of BST-2 might thus be able to restrict the release of HIV early after infection in vivo. It is possible, however, that this restriction of cell-free virus release is circumvented by cell-to-cell transmission of the virus, a process that can be paradoxically enhanced by BST-2 under certain circumstances (18).
IFN signaling seems necessary to induce BST-2 expression, since only treatment with IFN-α induced BST-2 on isolated CD4+ T cells. Apparently, other cells in the mixture comprising the PBMCs respond to certain TLR agonists (e.g., TLR 3 and 9), presumably to produce IFN. This hypothesis is supported by the finding that TLR 3 and 9 agonists inhibit HIV replication in lymphoid tissue (8), where the effect of TLR agonists may be even more pronounced due to the higher abundance of IFN-producing cells than are found in PBMCs.
The expression of BST-2 on murine bone marrow-derived DCs was shown to be induced by treatment with the TLR ligands LPS and CpG and after stimulation with IFN-α, IFN-β, and IFN-γ (6). In line with the results shown here, IL-6 and TNF-α did not induce enhanced BST-2 expression on those cells, despite the apparent IL-6-responsive elements in the promoter of human BST-2.
The fold increase of BST-2 levels during acute infection in vivo is similar to that observed in vitro in response to IFN, suggesting that maximal BST-2 expression might be occurring during acute (but apparently not chronic) infection. An induction of BST-2 mRNA expression in CD4+ T cells from the peripheral blood and lymph nodes was similarly demonstrated in a study of African green monkeys and rhesus macaques infected with SIV, and these animals had increased IFN-α plasma levels during acute infection (17). A potential exists, then, to increase the levels of BST-2 using IFN therapy to restrict virus release, at least during chronic infection. Restriction by BST-2 might account for at least part of the inhibitory effects of interferon in vivo. Indeed, the extent of BST-2 induction (as measured by mRNA levels) correlated with the reduction in HIV-1 viremia during treatment with pegylated IFN-α/ribavirin in HIV/hepatitis C virus (HCV)-coinfected patients (S. Pillai, UCSF, personal communication).
BST-2 appears to respond to the level of virus production in vivo, as supported by the relative reduction in BST-2 levels that occurs with successful antiviral therapy. Importantly, this observation is consistent with the characterization of BST-2 as an interferon-stimulated gene. It is also consistent with the notion that the virus itself is driving the innate immune response during HIV infection. Using the samples herein, however, the correlation between the levels of viremia and BST-2 was not statistically significant across all patients (data not shown), presumably because insufficient samples were available and because of variation between individuals. Moreover, factors in addition to virus production and IFN secretion might regulate the expression of BST-2 in vivo.
The levels of BST-2 expression on infected PBMCs in vitro increased upon IFN stimulation but did not increase in response to infection (i.e., the virus) itself. Furthermore, the stimulation with TLR agonists did not show a significant impact on virus release in this system (data not shown), indicating that the released amounts of IFN after viral triggering of TLRs are not enough to increase BST-2 to levels sufficient to overwhelm Vpu in cultures of PBMCs. This might be due to the low percentage of pDCs and other IFN-producing cells present in PBMC cultures, which secrete IFN and induce BST-2 in response to viral triggering of TLR 7 and 9.
The TLR 3 agonist poly(I·C) LMW, as well as TLR 9 agonist ODN2216, significantly increased BST-2 levels on PBMCs. These levels were similar to those detected during chronic infection in vivo. Poly(I·C) is a synthetic analog of double-stranded RNA (dsRNA), a molecular pattern associated with viral infection and sensed by cytoplasmic RNA helicases, including retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5), as well as by TLR 3 in endosomes (24). The size of the dsRNA strands varies, and poly(I·C) HMW may reflect long dsRNA, which triggers IFN secretion through MDA-5 signaling. This pathway did not induce BST-2 in our PBMC system, possibly due to the very low expression of MDA-5 and RIG-I in pDCs. TLR 3 is widely expressed in innate immune cells, however (except for pDCs [32]), and the stimulation of TLR 3 by poly(I·C) was shown to contribute to IFN and cytokine production by macrophages (1). The specific TLR 3 agonist poly(A·U) did not induce BST-2 expression in our PBMC system, suggesting that poly(I·C) LMW might signal through a different pathway than TLR 3 to induce increased BST-2 expression.
Stimulation with the agonists to TLR 7 and 8, two TLRs that have been implicated in the response to HIV ssRNA, did not increase the expression of BST-2 in freshly isolated PBMCs. This result was unexpected, since ssRNA derived from the HIV genome caused human PBMCs to produce IFN-α, IL-6, and TNF-α (15). As mentioned before, this might be due to the low abundance of pDCs in PBMCs and thereby to a low concentration of IFN produced in vitro under these conditions. The observation that virus alone was insufficient to induce BST-2 in PBMC cultures is consistent with this scenario. TLR 9 is highly expressed in pDCs, and the stimulation with CpG DNA leads to a strong IFN-α response in PBMCs (23), which might explain the increase of BST-2 expression induced by TLR 9 in our study of PBMCs. Why the stimulation of PBMCs with the TLR 9 agonist, but not with TLR 7 or 8 agonists, leads to the increased expression of BST-2 is unclear.
In summary, we demonstrated that the interferon-inducible restriction factor BST-2 is upregulated during HIV-1 infection in vivo. This upregulation occurs on CD4-positive T lymphocytes and appears to be part of an innate response to the virus itself. In vitro, during single-round infections designed to look at late events during the replication cycle, interferon specifically inhibits Vpu-expressing virus. These findings are consistent with a scenario in which the Vpu protein is essentially at the limit of its effectiveness as a BST-2 antagonist. The findings support the hypothesis that further induction of BST-2 in vivo would overcome Vpu and decrease viral replication.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients who participated in the San Diego Cohort Study, Nancy Keating of the CFAR Translational Virology Core for excellent processing and storage of the samples, and Valerie Terry and Celsa Spina, as well as Neal Sekiya and Judy Nordberg at the CFAR Flow Cytometry core. We thank Chugai Pharmaceutical Co. for the gift of anti-HM1.24 (BST-2) antibody.
This study was supported by NIH grants AI081668 and AI038201 to J.G., by grants AI69432, AI043638, MH62512, MH083552, AI077304, AI36214, AI047745, AI74621, and AI080353 from the NIH, by grants from the James B. Pendleton Charitable Trust to D.R. and D.S., and by the California AIDS Research Program grant RN07-SD-702 to S.L.
Authorship contributions: J.G. and S.H. designed the experiments; S.H. performed all experiments and analyses. S.L., D.R., and D.S. provided the HIV patient samples of the San Diego cohort. D.S. provided help with statistical analyses and discussions. J.G. supervised the project, and S.H. and J.G. wrote the manuscript.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 17 August 2011.
REFERENCES
- 1. Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738 [DOI] [PubMed] [Google Scholar]
- 2. Andrew A., Strebel K. 2011. The interferon-inducible host factor bone marrow stromal antigen 2/tetherin restricts virion release, but is it actually a viral restriction factor? J. Interferon Cytokine Res. 31:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr S. D., Smiley J. R., Bushman F. D. 2008. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 4:e1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baum A., Garcia-Sastre A. 2010. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids 38:1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beignon A. S., et al. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasius A. L., et al. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260–3265 [DOI] [PubMed] [Google Scholar]
- 7. Breckpot K., et al. 2010. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J. Virol. 84:5627–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brichacek B., et al. 2010. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS One 5:e12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chesebro B., Wehrly K., Nishio J., Perryman S. 1992. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J. Virol. 66:6547–6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Day J. R., Van Damme N., Guatelli J. C. 2006. The effect of the membrane-proximal tyrosine-based sorting signal of HIV-1 gp41 on viral infectivity depends on sequences within gp120. Virology 354:316–327 [DOI] [PubMed] [Google Scholar]
- 11. Dejucq N., Clapham S. G. P. R. 2000. T-cell line adaptation of human immunodeficiency virus type 1 strain SF162: effects on envelope, vpu and macrophage-tropism. J. Gen. Virol. 81:2899–2904 [DOI] [PubMed] [Google Scholar]
- 12. Douglas J. L., et al. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaines H., et al. 1990. Immunological changes in primary HIV-1 infection. AIDS 4:995–999 [DOI] [PubMed] [Google Scholar]
- 14. Gottlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. U. S. A. 88:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heil F., et al. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529 [DOI] [PubMed] [Google Scholar]
- 16. Hornung V., et al. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 17. Jacquelin B., et al. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119:3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jolly C., Neil B. N. S. J. 2010. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol. 84:12185–12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawai S., et al. 2006. Construction of a conventional non-radioisotope method to quantify HM1.24 antigens: correlation of HM1.24 levels and ADCC activity of the humanized antibody against HM1.24. Leuk. Res. 30:949–956 [DOI] [PubMed] [Google Scholar]
- 20. Kornbluth R. S., Oh P. S., Munis J. R., Cleveland P. H., Richman D. D. 1989. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 169:1137–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy J. A., Scott I., Mackewicz C. 2003. Protection from HIV/AIDS: the importance of innate immunity. Clin. Immunol. 108:167–174 [DOI] [PubMed] [Google Scholar]
- 22. Martinelli E., et al. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 104:3396–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinson J. A., et al. 2007. Impact of class A, B and C CpG-oligodeoxynucleotides on in vitro activation of innate immune cells in human immunodeficiency virus-1 infected individuals. Immunology 120:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meylan E., et al. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172 [DOI] [PubMed] [Google Scholar]
- 25. Miyagi E., Andrew A. J., Kao S., Strebel K. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neil S. J., Sandrin V., Sundquist W. I., Bieniasz P. D. 2007. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neil S. J., Zang T., Bieniasz P. D. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 28. Ohtomo T., et al. 1999. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 19:583–591 [DOI] [PubMed] [Google Scholar]
- 29. Pertel T., et al. 2011. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poli G., Orenstein J. M., Kinter A., Folks T. M., Fauci A. S. 1989. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science 244:575–577 [DOI] [PubMed] [Google Scholar]
- 31. Richards K. H., Clapham P. R. 2007. Effects of vpu start-codon mutations on human immunodeficiency virus type 1 replication in macrophages. J. Gen. Virol. 88:2780–2792 [DOI] [PubMed] [Google Scholar]
- 32. Schroder M., Bowie A. G. 2005. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 26:462–468 [DOI] [PubMed] [Google Scholar]
- 33. Schubert U., et al. 1998. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol. 72:2280–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah A. H., et al. 2010. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siegal F. P., Fitzgerald-Bocarsly P., Holland B. K., Shodell M. 2001. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 15:1603–1612 [DOI] [PubMed] [Google Scholar]
- 36. Stacey A. R., et al. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83:3719–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephens E. B., et al. 2002. Deletion of the vpu sequences prior to the env in a simian-human immunodeficiency virus results in enhanced Env precursor synthesis but is less pathogenic for pig-tailed macaques. Virology 293:252–261 [DOI] [PubMed] [Google Scholar]
- 38. Stetson D. B., Medzhitov R. 2006. Type I interferons in host defense. Immunity 25:373–381 [DOI] [PubMed] [Google Scholar]
- 39. Terry V. H., Johnston I. C., Spina C. A. 2009. CD44 microbeads accelerate HIV-1 infection in T cells. Virology 388:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tokarev A., Fitzpatrick S. M. K., Guatelli J. 2009. Antiviral activity of the interferon-induced cellular protein BST-2/tetherin. AIDS Res. Hum. Retroviruses 25:1197–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Boxel-Dezaire A. H., Rani M. R., Stark G. R. 2006. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25:361–372 [DOI] [PubMed] [Google Scholar]
- 42. Van Damme N., et al. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verthelyi D., Ishii K. J., Gursel M., Takeshita F., Klinman D. M. 2001. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J. Immunol. 166:2372–2377 [DOI] [PubMed] [Google Scholar]
- 44. von Sydow M., Sonnerborg A., Gaines H., Strannegard O. 1991. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res. Hum. Retroviruses 7:375–380 [DOI] [PubMed] [Google Scholar]
- 45. Yan N., Regalado-Magdos A. D., Stiggelbout B., Lee-Kirsch M. A., Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.