Abstract
In the present study, we assessed the cooperative roles of C-terminal Src kinase (Csk) binding protein (Cbp) and Caveolin-1 (Cav-1) in the mechanism of Src family tyrosine kinase (SFK) inhibition by Csk. SFKs are inactivated by phosphorylation of their C-terminal tyrosine by Csk. Whereas SFKs are membrane-associated, Csk is a cytoplasmic protein and therefore requires membrane adaptors such as Cbp or Cav-1 for recruitment to the plasma membrane to mediate SFK inhibition. To determine the specific role of Cav-1 and Cbp in SFK inhibition, we measured c-Src activity in the absence of each membrane adaptor. It is noteworthy that in lungs and fibroblasts from Cav-1(−/−) mice, we observed increased expression of Cbp compared with wild-type (WT) controls. However, both c-Src activity and Csk localization at the membrane were similar between Cav-1(−/−) fibroblasts and WT cells. Likewise, Cbp depletion by small interfering RNA (siRNA) treatment of WT cells had no effect on basal c-Src activity, but it increased the phosphorylation state of Cav-1. Immunoprecipitation then confirmed increased association of Csk with phosphomimicking Cav-1. Knockdown of Cbp by siRNA in Cav-1(−/−) cells revealed increased basal c-Src activity, and re-expression of WT Cav-1 in the same cells reduced basal c-Src activity. Taken together, these results indicate that Cav-1 and Cbp cooperatively regulate c-Src activity by recruiting Csk to the membrane where it phosphorylates c-Src inhibitory tyrosine 529. Furthermore, when either Cav-1 or Cbp expression is reduced or absent, there is a compensatory increase in the phosphorylation state or expression level of the other membrane-associated Csk adaptor to maintain SFK inhibition.
Introduction
Src family tyrosine kinases (SFKs) are involved in many of the signaling mechanisms associated with G-protein-coupled receptors, integrins, receptor tyrosine kinases, T-cell receptors, and others (Thomas and Brugge, 1997). Of the eight family members, c-Src, Yes, and Fyn are expressed ubiquitously, with the other members being expressed primarily in lymphocytes (Chow and Veillette, 1995). SFKs all share a common general structural organization: an N-terminal membrane association domain, a unique domain, a Src homology (SH) 3 domain, an SH2 domain, a catalytic domain, and a C-terminal regulatory domain. The catalytic domain contains an autophosphorylated tyrosine (418 in c-Src), which is phosphorylated when the enzyme is active. SH2 domains bind phosphotyrosine motifs, and SH3 domains bind polyproline motifs. In the inhibited state, the SH2 domain of c-Src is involved in an intramolecular interaction with a C-terminal regulatory domain phosphotyrosine, tyrosine 529 (Xu et al., 1997), locking the enzyme in an inactive or closed state. Oncogenic activation in the case of v-Src results from the loss of this C-terminal regulatory domain (Martin, 2001).
Phosphorylation of the C-terminal regulatory tyrosine on SFKs is catalyzed by C-terminal Src kinase (Csk) (Okada et al., 1991). Csk is required for normal development, because Csk knockout mice die at embryonic day 9 or 10 (Imamoto and Soriano, 1993). The architecture of Csk is similar to SFKs with one SH2 domain, one SH3 domain, and a kinase domain (Nada et al., 1991). It is noteworthy that Csk lacks a regulatory C-terminal tyrosine, N-terminal myristoylation, and membrane association domain (Ogawa et al., 2002). So, although SFKs are membrane-associated and regulated by phosphorylation, Csk is intrinsically cytoplasmic (Howell and Cooper, 1994) and requires membrane adaptors to inhibit membrane-associated SFKs.
Studies have identified and characterized a Csk adaptor in T-cells (Brdicka et al., 2000) and rat brain (Kawabuchi et al., 2000) known as Csk binding protein (Cbp), or phosphoprotein associated with glycosphingolipid-enriched microdomains. Phosphorylation of Cbp by SFKs on tyrosine 314 facilitates the binding of Csk through its SH2 domain. It was also demonstrated that Csk activity increases approximately 3-fold when bound to a phospho-Cbp peptide (Takeuchi et al., 2000). Therefore, the interaction of Csk with Cbp helps to recruit the enzyme to the membrane and increase its activity, creating a feedback inhibition loop to terminate SFK signaling. In contrast to Csk knockouts, Cbp knockout mice are viable. It is noteworthy that Csk is recruited to the membrane in cells derived from Cbp(−/−) mice (Dobenecker et al., 2005), suggesting that there are additional membrane adaptors for Csk.
Caveolin-1 (Cav-1) has also been implicated as a Csk adapter. It is phosphorylated on tyrosine 14 (Tyr14) by v-Src (Li et al., 1996) and by c-Src (Aoki et al., 1999; Lee et al., 2000), which is believed to play a key role in the initiation of caveolae-mediated endocytosis (Minshall et al., 2000; Shajahan et al., 2004; Sverdlov et al., 2007, 2009). Cav-1 also serves as an important membrane-associated scaffolding protein for endothelial nitric-oxide synthase, G proteins, Ras, and SFKs (Okamoto et al., 1998; Minshall et al., 2000). This scaffolding function also includes the binding of Csk, as shown by a yeast two-hybrid screening (Cao et al., 2002) and association of phospho-Cav-1 with Csk in the negative regulation of protease activated receptor-1 signaling (Lu et al., 2006).
Increased SFK activity has been described in colon cancer. In a mouse model of colon cancer, Csk overexpression was associated with suppression of metastasis and invasiveness in vivo (Nakagawa et al., 2000), and in human colon cancer cells, Csk overexpression reduced invasiveness and SFK activity (Rengifo-Cam et al., 2004). On the other hand, dominant-negative Csk increased invasiveness and migration of these cells. Furthermore, it has been shown that reduced localization of Csk at the membrane may be an underlying cause of the pathologic increase in c-Src activity in human colon cancer (Sirvent et al., 2010). It is noteworthy that when the reduction of Csk adapter protein expression (both Cbp and Cav-1) in the highly metastatic cells was rescued by increasing Cbp expression, c-Src activity decreased, and invasiveness was reduced (Sirvent et al., 2010).
Here, we show that Cbp and Cav-1 cooperatively regulate basal c-Src activity through recruitment of Csk. We observed a compensatory increase in Cbp expression in the absence of Cav-1 and an increase in Cav-1 phosphorylation upon Cbp knockdown. Furthermore, reduction of Cbp in Cav-1(−/−) cells increased basal c-Src activity, whereas re-expression of wild-type (WT) Cav-1, but not the phospho-defective Cav-1 mutant, lowered basal c-Src activity. Thus, cooperation between Csk adapters Cbp and Cav-1 safeguard cells against the harmful effects of sustained c-Src activation.
Materials and Methods
Cells and Reagents.
WT and Cav-1(−/−) mouse lung fibroblasts (MFs) were isolated from 4- to 6-week-old C57BL6 mice (The Jackson Laboratory, Bar Harbor, ME). Lung lobes were perfused with RPMI medium and homogenized in RIPA buffer supplemented as described below for Western blot analysis or digested in collagenase type 1 solution (both from Sigma-Aldrich, St. Louis, MO) for MF isolation. Cells were collected and cultured in 10% fetal bovine serum containing Dulbecco's modified Eagle's medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Subcultures with fibroblast-like morphology were selected and grown routinely in the laboratory. All cells were maintained in 5% CO2/95% room air in a water-jacketed 37°C incubator. RIPA buffer (Boston Bioproducts, Ashland, MA) was supplemented with protease inhibitor cocktail, 200 mM PMSF, 1 mM EDTA, 1 mM NaF, and 1 mM Na3VO4 (all from Sigma-Aldrich). n-Octylglucoside buffer (ODG, 2%) (Research Products International, Mt. Prospect, IL) was supplemented the same as RIPA buffer. Total protein concentration was determined using a BCA protein assay kit (Pierce, Rockford, IL). Antibodies for c-Src (polyclonal), Csk, and glyceraldehyde-3-phosphate dehydrogenase were from Santa Cruz Biotechnology (Santa Cruz, CA); β-actin, Cav-1, and p-Cav-1 Tyr14 antibodies were from BD Biosciences (San Jose, CA); c-Src (monoclonal), p-Src Tyr418, and p-Src Tyr529 were from Cell Signaling Technology (Danvers, MA); and Cbp and Na+/K+ ATPase-α1 were from Abcam Inc. (Cambridge, MA).
Western Blotting.
Cells were lysed in RIPA or ODG buffer supplemented as described above, sonicated briefly, and cleared for 5 min at 13,200 rpm at 4°C in an Eppendorf 5415R microcentrifuge (Eppendorf North America, New York, NY). Lysates were boiled in lysis buffer plus 6× Laemmli sample buffer (Boston Bioproducts) and dithiothreitol (final concentration, 30 mM; Sigma-Aldrich) for 5 min before SDS-PAGE with equal quantities of protein loaded in each lane. Protein concentration of lysates was determined by a BCA protein assay kit (Pierce). After SDS-PAGE separation, proteins were blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA) and blocked with 5% blotting grade nonfat dry milk (Bio-Rad) in Tris-buffered saline with 0.05% Tween 20 (Sigma-Aldrich) (TBST) for 1 h. The membranes were then probed with primary antibodies in blocking buffer rocking overnight at 4°C. After three washes in TBST, secondary species-specific horseradish peroxidase-conjugated antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD) were then incubated for 1 h at room temperature in blocking buffer. After three more washes with TBST, enhanced chemiluminescence substrate (Pierce) was then used to visualize the bands on HyBlot CL film (Denville, South Plainfield, NJ).
Fluorescent Imaging.
Cells were seeded on glass coverslips and serum-deprived for at least 2 h before fixation for 20 min with 4% paraformaldehyde in Hanks' balanced salt solution with Ca2+ and Mg2+ (HBSS+/+; Invitrogen). Cells were then permeabilized for 30 min with buffer containing 5% goat serum, 0.2% bovine serum albumin, 0.01% NaN3, and 0.1% Triton X-100 (all from Sigma-Aldrich), washed briefly with HBSS+/+, and incubated overnight at 4°C with appropriate antibodies in the same buffer used for permeabilization. After three more washes with HBSS+/+, Alexa 488-labeled secondary goat anti rabbit IgG antibodies (Invitrogen) were then added and incubated for 2 h at room temperature. Coverslips were washed again three times with HBSS+/+ and mounted to glass slides with ProLong Gold antifade mounting reagent with 4,6-diamidino-2-phenylindole (Invitrogen). Images of cells were captured on a Zeiss LSM 510 META confocal microscope (Carl Zeiss Inc., Thornwood, NY), as described previously (Minshall et al., 2000).
Cytosol and Membrane Fractionation.
Cells were serum-deprived for 2 h and then scraped in 50 mM Tris-HCl, pH 7.5, supplemented with protease inhibitor cocktail (Sigma-Aldrich), 1 mM Na3VO4, 1 mM NaF, 1 mM EDTA, and 1 mM PMSF. The scraped cells were then centrifuged at 100,000g at 4°C for 1 h. The supernatant (cytosolic fraction) was collected, and the pellet (membranous fraction) was resuspended in RIPA buffer and sonicated. Protein concentration was measured by BCA assay (Pierce), and an equal amount of protein was loaded per lane onto 10% SDS-PAGE gels. Sodium/potassium ATPase-α1 subunit was used as a positive control marker and loading control for the membrane fraction.
Immunoprecipitation.
MFs were seeded at 125,000 cells/well in a six-well plate and serum-deprived the following day for 2 h. Cells were treated (or not) before lysis in ODG buffer without sonication. Lysates (two wells per treatment) were then added to magnetic sheep anti-mouse-IgG coated Dynabeads (Invitrogen) that were preincubated for 30 min at 4°C with nonspecific mouse IgG (as a negative control) or anti-Cav-1 monoclonal antibodies (both from BD Biosciences). After a 1-h rotating incubation at 4°C, the lysates were placed on a magnetic particle concentrator (Invitrogen) and washed with cold (4°C) phosphate-buffered saline without Ca2+ and Mg2+ [PBS(−/−)] supplemented with protease inhibitor cocktail and NaVO4 as described above. After three washes, 6× Laemmli sample buffer was added with ODG lysis buffer and dithiothreitol (final concentration, 30 mM) and boiled for 5 min before loading onto SDS-PAGE gels for Western blotting.
Cbp siRNA.
MFs were seeded at 125,000 cells/well in a six-well plate and treated the following day with varying concentrations of scrambled (S) or Cbp siRNA. The siRNA was delivered using 6 to 8 μl of Dharmafect-1 per well according to the manufacturer's protocol. Both siRNA duplexes and Dharmafect-1 were from Dharmacon RNA Technologies (Lafayette, CO). The amount of scrambled control siRNA transfected was equal to the highest concentration of specific siRNA targeting mouse Cbp that was used in each experiment. Two or three days after transfection, cells were serum-deprived (2–4 h) and subsequently lysed in RIPA buffer on ice, sonicated, and cleared for 5 min at 13,200 rpm at 4°C for Western blotting. The siRNA sequences were as follows: 5′-AAGCCATACAGACTCTAAACA-3′ targeting mouse and rat Cbp, and 5′-AAGCGATACAGACTCTCAACA-3′ targeting human Cbp, which was used as the scrambled control in the mouse cells as described previously (Jiang et al., 2006).
In Vitro Kinase Assay.
The tyrosine kinase assay was performed according to the manufacturers' instructions (Millipore, Billerica, MA). In brief, c-Src was immunoprecipitated in RIPA buffer lysates from 2-h serum-deprived 10-cm dishes using goat anti-mouse Dynabeads (Invitrogen) coated with c-Src monoclonal antibodies (37.5 μg/IP; Cell Signaling Technology) for 1 h at 4°C, washed with Tris-buffered saline, and then incubated with reaction buffer and biotinylated substrate peptide for 1 h at 37°C. The reaction was stopped by heating to 95°C for 5 min and added to a well of a streptavidin-coated ELISA plate. Phosphorylation of the substrate peptide was detected using a horseradish peroxidase-conjugated anti-phosphotyrosine (4G10) antibody and TMB substrate, read at 450 nm. Phosphorylated control peptide was used to generate the standard curve for each ELISA assay, and phosphorylated substrate peptide (in nanograms per hour) was determined using the extrapolated linear equation of the standard curve. Units (U) of immunoprecipitated c-Src were determined by densitometry of Western blots of each well on the ELISA plate. The highest density bands were considered 1 U, and the other lanes were normalized to this value.
Densitometry and Statistics.
Densitometry of protein bands was performed with ImageJ software (http://rsbweb.nih.gov/ij/). Statistical significance was determined by Student's t test, with P < 0.05 considered significant.
Results
Cbp Expression Is Increased in Cav-1(−/−) Fibroblasts and Lungs.
We hypothesized that Cav-1 functions as a negative regulator of c-Src and that Cav-1(−/−) mice should have elevated c-Src activity compared with WT controls. To examine this, lungs from Cav-1(−/−) mice and WT age- and strain-matched control mice were excised from 4- to 6-week old mice, perfused, and digested with collagenase type I. MFs were cultured as described under Materials and Methods. Whole lungs from these mice were also excised, perfused, and homogenized in RIPA buffer for whole lung protein analysis. We were surprised to find that despite total eradication of Cav-1 protein in MFs, c-Src activity remained equal to WT control MFs under serum-deprived conditions, and there was no difference in total c-Src expression (Fig. 1, A and B). Src activity was measured by examining the phosphorylation state of c-Src Tyr418, of which we observed little difference, if any, between the two genotypes (Fig. 1B). The phosphorylation state of the Tyr529 residue of c-Src was also examined and we found no difference between the two cell lines as well (Fig. 1B). These results were validated by using immunoprecipitated c-Src from WT and Cav-1(−/−) MFs in an in vitro kinase reaction. Using standard curves of phosphorylated control peptide to determine the phosphorylated substrate (in nanograms), and normalization of differences in immunoprecipitated c-Src in the reactions to determine the units (nanograms per unit; see Materials and Methods), we found basal c-Src activity to be 0.343 ± 0.06 ng/U/h in the WT MFs and 0.285 ± 0.14 ng/U/h in the Cav-1(−/−) MFs. These results were not statistically different and confirmed that the measure of Tyr418 phosphorylation by Western blotting indeed reflects c-Src activity in our studies.
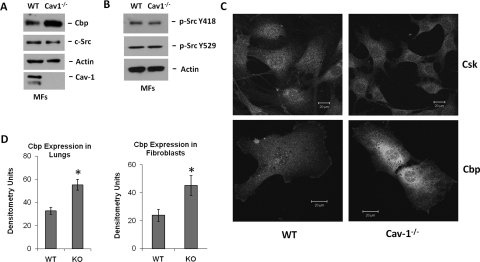
Fig. 1.
Cbp expression is increased, but c-Src activity and Cbp localization are unaffected. A and B, Cav-1(−/−) and WT MFs were grown to confluence and serum-deprived for 2 h before lysis as described under Materials and Methods. Cav-1 was absent, but Cbp was elevated in the Cav-1(−/−) MFs. Expression of c-Src and phosphorylation of Tyr418 and Tyr529 of c-Src were equal. C, the same cells used in A and B were immunostained for Csk and Cbp and imaged by confocal microscopy. The confocal images confirm increased expression of Cbp in Cav-1(−/−) MFs and Cbp localization in membrane-associated structures in both cell types. Csk expression and localization were similar in the two cell types. Scale bar, 20 μm. D, whole lung homogenates were prepared as described under Materials and Methods, and Cbp expression was determined by Western blot. Quantification by densitometry of three independent experiments in lung and MF lysates are shown (mean ± S.E.). Cbp expression increased 2-fold in Cav-1(−/−) MFs and lungs. *, P < 0.05 versus WT (n = 3).
The primary difference observed in our study was that the level of expression of Cbp in the Cav-1(−/−) MFs and lungs was approximately two times higher than in WT MFs and lungs (Fig. 1, A, C, and D). This increase in Cbp expression was further confirmed by immunofluorescent staining of Cbp in the MFs. Confocal micrographs captured using identical detector settings confirmed increased Cbp expression in Cav-1(−/−) MFs. These micrographs also revealed that both Cbp and Csk were similarly localized to the plasma membrane in WT and Cav-1(−/−) MFs (Fig. 1 C). These results demonstrate that Cbp expression is 2-fold higher in Cav-1(−/−) mouse lungs and MFs compared with WT litter mates and that Cbp and Csk are localized correctly. Furthermore, c-Src activity in Cav-1(−/−) MFs was the same as that in WT control cells, suggesting that the increase in Cbp expression compensates for the absence of Cav-1 to negatively regulate c-Src activity.
Csk Expression and Subcellular Localization Is Similar in Cav-1(−/−) and WT MFs.
Given the 2-fold increase in expression of Cbp but equal c-Src activity in Cav-1(−/−) MFs, we assessed Csk expression level and localization. Csk expression level was found to be the same in WT and Cav-1(−/−) MFs, and subcellular fractionation revealed that the same amount of Csk was present in both the membrane and cytosolic fractions from serum-deprived cells (Fig. 2 A). Na+/K+ ATPase-α1 was used as a loading control for the membrane fraction, and Cbp localization was shown to be restricted to this compartment as well. These results demonstrate that Csk is still localized to the membrane to hold c-Src at its baseline level of activity in the absence of Cav-1, and that Csk expression was not affected by the absence of Cav-1. Furthermore, Cbp and Cav-1 may be the only membrane-associated Csk adapter proteins expressed in MFs, because the increase in Cbp expression in the absence of Cav-1 served to compensate for the lack of Cav-1 and to recruit Csk to the membrane to the same extent as that observed in WT MFs.
Fig. 2.
Csk negatively regulates basal c-Src activity, and Csk localization is not perturbed in Cav-1(−/−) MFs. A, WT and Cav-1(−/−) MFs were grown to confluence and serum-deprived for 3 h. Cytosol and membrane fractions, prepared as described under Materials and Methods, contained equivalent amounts of Csk despite total loss of Cav-1 expression. Cbp levels were greater in the Cav-1(−/−) MFs and present in the membrane fraction only. Na/K ATPase α1 subunit was used as a membrane loading control. B, WT MFs were treated with 60 nM Csk siRNA for 48 h, serum-deprived for 2.5 h, and lysed. S, 60 nM scrambled nontargeting siRNA. Csk level was reduced by approximately 30%, which increased c-Src Tyr418 phosphorylation (approximately 2-fold) and decreased c-Src Tyr529 phosphorylation. C, bar graph represents three independent experiments (mean ± S.E.) quantified using densitometry. *, P < 0.05 versus S (n = 3). The ratio of c-Src p-Tyr418/p-Tyr529 is displayed from a representative experiment.
Csk siRNA Increases Basal c-Src Tyr418 Activity.
As described previously, Csk negatively regulates SFKs by phosphorylating the negative regulatory tyrosine in their C termini (Okada et al., 1991; Imamoto and Soriano, 1993; Howell and Cooper, 1994; Brdicka et al., 2000; Takeuchi et al., 2000; Khanna et al., 2007). To confirm that Csk inhibits c-Src to its basal state in our experimental system, we performed knockdown experiments using Csk siRNA in WT MFs. Csk levels were reduced by 30% compared with scrambled siRNA-treated cells levels after 48 h. Basal activity of c-Src, as measured by Tyr418 phosphorylation levels after serum deprivation, was approximately 2-fold higher in the Csk siRNA-treated cells compared with the scrambled control siRNA-treated cells (Fig. 2, B and C). In addition, c-Src Tyr529 phosphorylation was diminished in MFs treated with Csk siRNA (Fig. 2B). These data indicate that inhibition of c-Src activity to basal levels after serum removal is dependent on Csk.
Cbp Depletion Increases Cav-1 Tyr14 Phosphorylation.
To specifically explore the role of Cav-1 in the coordination of Csk, we reduced Cbp expression by siRNA transfection in WT MFs and measured c-Src activity. Knockdown of Cbp in WT MFs (using a specific siRNA that targets the mouse isoform at residues 1160–1180), compared with scrambled siRNA targeting the human isoform (residues 1163–1183), was very efficient and specific as described previously (Jiang et al., 2006). We achieved Cbp knockdown of more than 80% in these cells (Fig. 3, A and F). However, to our surprise, we observed no increase in phosphorylation of c-Src Tyr418 under basal, serum-deprived conditions (Fig. 3A). We did however, detect a 65% increase in the phosphorylation state of Cav-1 Tyr14, a known c-Src substrate (Li et al., 1996), under the same conditions without effecting total Cav-1 expression (Fig. 3, C and F). It should be noted that this is also the tyrosine that Csk is reported to bind to on Cav-1 (Cao et al., 2002; Lu et al., 2006). This increase was statistically significant and detected both in WT MFs and rat lung microvascular endothelial cells (data not shown).
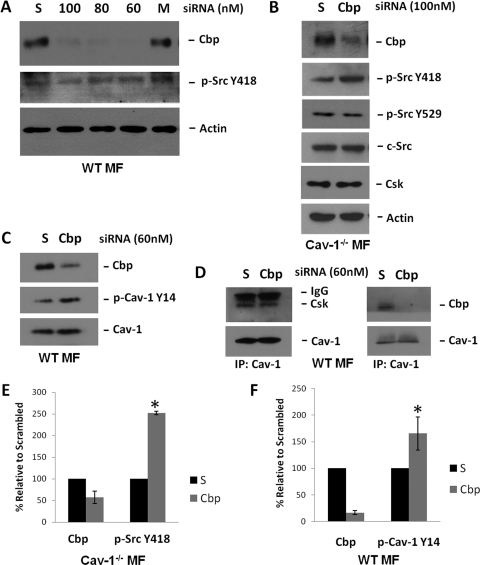
Fig. 3.
Cbp siRNA increases c-Src basal activity in Cav-1(−/−) MFs but not in WT MFs. A, WT MFs were treated with Cbp siRNA for 72 h as described under Materials and Methods. S represents scrambled siRNA (100 nM, directed against Human Cbp), and 100, 80, and 60 is the siRNA concentration (nanomolar) directed against rodent Cbp. Cells were serum deprived for 2 h before lysis. Activity of c-Src (p-Tyr418) remained constant under the same conditions as seen in reprobed blots of the original Cbp blots. B, Cav-1(−/−) MFs were treated with Cbp siRNA or scrambled siRNA (both 100 nM) for 48 h and then serum-deprived for 3 h before lysis. Cbp siRNA reduced Cbp expression by more than 40%, and c-Src activity increased 2.5-fold over scrambled siRNA-treated cells. C, Cbp reduction by siRNA in WT MFs did not alter total Cav-1 levels. D, Cav-1 immunoprecipitates from serum-deprived WT MFs treated with Cbp siRNA or scrambled siRNA as described in A displayed equivalent amounts of associated Csk, despite the loss of Cbp from the immunoprecipitated complex. E, bar graph displays summarized data of Cbp expression and Src Tyr418 phosphorylation (mean ± S.E.; n = 3), indicating that Cbp knockdown in Cav-1(−/−) cells significantly increases c-Src activity. F, bar graph displays four independent experiments (mean ± S.E.) performed on the WT MFs indicating that Cbp levels were decreased by approximately 85% with 60 nM Cbp siRNA, and Cav-1 Tyr14 phosphorylation increased 65% after Cbp knockdown. *, P < 0.05 versus S.
Increases in Cav-1 Tyr14 Phosphorylation Facilitate Csk Association after Cbp Knockdown.
Because Cbp knockdown in WT MFs increased Cav-1 Tyr14 phosphorylation levels without increasing c-Src Tyr418 phosphorylation, we tested the hypothesis that this increase was facilitating Csk binding and localization at the plasma membrane to inhibit c-Src. In Cav-1 immunoprecipitates of serum-deprived WT cells, we observed the presence of associated Csk and Cbp under normal conditions (Fig. 3D). This revealed a complex of Csk and the two adapters present at the plasma membrane under basal conditions. We then reduced Cbp expression in the WT MFs with Cbp siRNA in the same Cav-1 immunoprecipitation experiment, and we observed equivalent amounts of Csk associated with Cav-1 and no Cbp present in the complex (Fig. 3D). This further suggests that the elevated Cav-1 Tyr14 phosphorylation state indeed was allowing for an appropriate amount of Csk coordination at the plasma membrane to maintain low basal c-Src activity.
Knockdown of Cbp in Cav-1(−/−) Cells Increases c-Src Basal Activity.
Because c-Src activity did not increase in the absence of Cbp or Cav-1 alone, studies were carried out to investigate the baseline activity of c-Src during the reduction of both membrane adapters for Csk, Cbp, and Cav-1. Cbp siRNA specific for the mouse isoform was transfected into Cav-1(−/−) MFs for 48 h followed by 3 h of serum deprivation before lysis. As seen in Fig. 3 (B and E), Cbp expression was reduced by more than 40% in these cells, which was associated with a 2.5-fold increase in c-Src activity compared with control siRNA-treated cells. This treatment had no effect on total c-Src or Csk expressions levels (Fig. 3B). These data suggest that reduction of Cbp expression in cells lacking Cav-1 leads to an increase in basal c-Src activity because of reduced control by Csk (as demonstrated with Csk siRNA experiments above), which was not perturbed when one or both of the adapters were present at appropriate levels. This further demonstrates the requirement of a certain level of expression of the membrane adapters for Csk to inhibit c-Src.
Expression of a Phosphodefective Cav-1 Tyr14 Mutant Fails to Reduce c-Src Activity.
Because association of Csk with Cav-1 has been reported to follow phosphorylation of Cav-1 at tyrosine 14, we wanted to explore the importance of this residue for the basal control of c-Src activity. We expressed WT Cav-1, a phospho-defective (Y14F) Cav-1 mutant, or a phosphomimicking (Y14E) Cav-1 mutant in Cav-1(−/−) MFs and measured basal c-Src activity after 1 h of serum deprivation. Cells expressing the Y14F mutant failed to reduce basal c-Src activity below empty vector control levels. On the other hand, rescue with WT Cav-1 or Y14E Cav-1 expression reduced basal c-Src activity below control levels (Fig. 4, A and B). Total levels of Cbp, c-Src, and Csk were unaffected by the expression of these constructs (Fig. 4A). The transfection of WT and Y14E Cav-1 lowered basal c-Src activity by approximately 35% compared with the Y14F-expressing cells (Fig. 4B). This confirms the requirement of Cav-1 Tyr14 phosphorylation for control of c-Src basal activity and that this equilibrium can be modulated by increases or decreases in phosphorylation state of Cav-1 Tyr14 because the expression of Cbp, c-Src, and Csk remained constant.
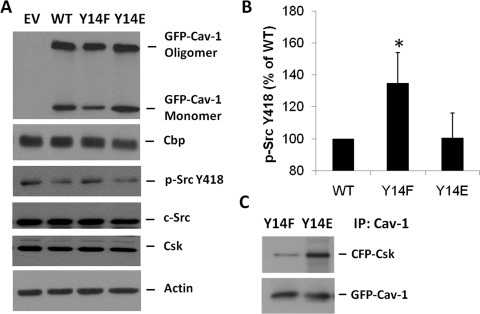
Fig. 4.
Importance of Cav-1 Tyr14 in Csk Recruitment and c-Src Inhibition. A, GFP-Cav-1 WT, GFP-Cav-1 Y14F (a phospho-defective mutant), or GFP-Cav-1 Y14E (a phosphomimicking mutant) were transiently transfected into Cav-1(−/−) MFs and then after 24 h were serum-deprived for 1 h and lysed as described under Materials and Methods. Expression of the Cav-1 Y14F mutant failed to significantly lower basal c-Src activity compared with empty vector (EV)-transfected cells, whereas Cav-1 WT and Cav-1 Y14E expression significantly lowered c-Src activity. Total c-Src, Cbp, and Csk expression remained unchanged. B, quantification of three independent experiments by densitometry normalized to loading controls (mean ± S.E.) indicates that the Cav-1 Y14F mutant failed to lower basal c-Src activity (*, P < 0.05 versus Cav-1 WT). C, CFP-Csk and GFP-Cav-1 Y14F or GFP-Cav-1 Y14E was transiently cotransfected into HEK cells, lysed after 24 h, and Cav-1 was immunoprecipitated. CFP-Csk was associated with the immunoprecipitated GFP-Cav-1 Y14E but not with GFP-Cav-1 Y14F.
Phosphorylation Dependence of the Csk and Cav-1 Interaction.
Other groups have reported that Cav-1 and Csk associate in a Csk SH2 domain-dependent manner when Cav-1 Tyr14 is phosphorylated (Cao et al., 2002; Lu et al., 2006). To confirm this interaction we expressed the Y14F and Y14E Cav-1 mutants, along with Csk-CFP, in HEK cells. After immunoprecipitation of Cav-1, we observed Csk binding to the Y14E mutant and not the Y14F mutant (Fig. 4 C). Although we did see a faint band in the Y14F lane, this is most likely due to the small amount of endogenous Cav-1 found in HEK cells oligomerizing with the expressed mutant and is therefore considered background signal.
Discussion
Constitutive activation of c-Src, as seen in v-Src, leads to cell proliferation, survival, cytoskeletal alteration, migration/invasiveness (Thomas and Brugge, 1997), and oncogenic transformation (Martin, 2001). Thus, tight control of SFKs by Csk is essential for normal cellular homeostasis. The importance of Csk as the central regulator of SFK activity is further evidenced by the fact that Csk knockout mice have developmental defects and die on day 9 or 10 of gestation (Imamoto and Soriano, 1993). The present study demonstrates that Csk membrane adapter proteins Cav-1 and Cbp play cooperative roles in the coordination of c-Src inhibition by Csk. When Cbp is reduced transiently, Cav-1 phosphorylation increases to localize Csk at the membrane and negatively regulate c-Src activity. On the other hand, when the Cav-1 gene is completely absent, Cbp expression increases to maintain Csk localization at the membrane and c-Src inhibition. This study is the first description of the cooperative role of Cav-1 and Cbp in the regulation of basal c-Src activity.
The current observations reveal that cells respond to the loss of Cav-1 by increasing the expression level of Cbp. The increase in expression of Cbp in the Cav-1(−/−) mice may be a compensation that occurred during the development of these mice, probably before day 9 or 10 of gestation, to control SFK activity. This is probably because this is the point at which Csk(−/−) mice arrest (Imamoto and Soriano, 1993). Another mechanism for regulating c-Src activity in the Cav-1(−/−) mice would be to increase Csk expression or decrease c-Src expression. However, this was not the case because no change in expression of either Csk or c-Src was observed between WT and Cav-1(−/−) MFs. Not only did the expression level of Csk remain constant, but Csk localization also remained unchanged in these cells. An equivalent amount of Csk was present in the membrane and cytosolic fractions of the Cav-1(−/−) cells compared with WT cells under basal conditions. Increased Cbp expression therefore enabled appropriate Csk recruitment to the membrane to regulate c-Src. Furthermore, reduction of Cbp in Cav-1(−/−) cells increased basal c-Src activity, revealing that these two proteins are the only membrane adapters for Csk in fibroblasts. This may explain why the single knockouts of Cav-1 (Murata et al., 2007) and Cbp (Dobenecker et al., 2005) are viable, whereas the Csk knockout mice are not (Imamoto and Soriano, 1993).
Like Cav-1(−/−) mice, Cbp(−/−) mice are viable as stated above and proceed normally through development (Dobenecker et al., 2005). The authors of this study concluded that Cbp is not essential for embryonic development or Csk compartmentalization and that there may be other membrane adapters for Csk. In addition, there was no significant increase in overall tyrosine phosphorylation in T-cells from these mice. This study also found normal Csk, phospho-Fyn, and phospho-Lyn subcellular distribution in cells derived from Cbp(−/−) mice, which is similar to that observed in Cav-1(−/−) cells. Compensation for the lack of Cbp was confirmed by us in the present in vitro study by demonstrating that Cav-1 Tyr14 phosphorylation increased after Cbp reduction by 80%, with no increase in c-Src activity. In addition, under these reduced Cbp conditions, immunoprecipitation experiments displayed equivalent amounts of Csk associated with Cav-1. The increase in Cav-1 phosphorylation therefore served to coordinate Csk to inhibit basal c-Src activity in the presence of reduced Cbp expression. It is noteworthy that reduction of both adapters by knocking down Cbp in Cav-1(−/−) MFs led to elevated basal c-Src activity. Thus, in the absence of both adapters, elevated c-Src activity was the result of an inability of Csk to access and phosphorylate c-Src C-terminal Tyr529, which was possible when only one adapter (either Cav-1 or Cbp) was present. This observation is consistent with the finding that Csk SH2 domain mutants that are unable to translocate to the plasma membrane do not inhibit c-Src (Howell and Cooper, 1994). Therefore, defective Csk targeting, whether by mutation or reduced expression of both adapters, leads to an inability to return c-Src to its baseline activity.
It has been suggested previously that the Cav-1 scaffolding domain (residues 82–101) (Okamoto et al., 1998) can sequester c-Src and thus affect its activation. Because no reduction in basal c-Src activity was observed when Y14F Cav-1 was expressed in Cav-1(−/−) cells, but c-Src activity was reduced upon WT Cav-1 and Y14E Cav-1 expression, Cav-1 Tyr14 phosphorylation seems to play the dominant role in the mechanism of c-Src inhibition by Cav-1. The Cav-1 scaffolding domain therefore may be necessary for correct localization of c-Src into caveolae but does not seem to affect c-Src inhibition without Tyr14 phosphorylation of Cav-1 for Csk binding. Thus, the direct comparison of phosphodefective Y14F Cav-1 with phospho-mimicking Y14E Cav-1 and WT Cav-1 conducted here illustrates that Cav-1 Tyr14 phosphorylation is critical for c-Src inhibition through Csk coordination.
In the present study, we examined the activity and phosphorylation state of c-Src, which is only one of the three ubiquitously expressed members of the SFKs. It is likely that Csk adapters Cbp and Cav-1 mediated the inactivation of the other SFK members in our study as well. There is a high degree of homology between SFK members in which the conserved C-terminal tyrosine is the target of the SFK-inactivating kinase Csk. It should also be noted that upon discovery of Csk, Okada et al. (1991) found that Csk was able to phosphorylate and repress the activity of the other ubiquitously expressed SFKs, Fyn and Lyn, and not just c-Src. Thus, Csk membrane-localized adapters Cav-1 and Cbp may also cooperatively participate in the negative regulation of c-Src, Fyn, and Lyn (Li et al., 1996; Brdicka et al., 2000; Kawabuchi et al., 2000).
Serum deprivation has been used throughout this report to assess basal c-Src activity (i.e., in the absence of growth factor activation of c-Src). Cells were grown in the presence of serum, and then the serum was removed for indicated times before lysis. This protocol would therefore allow basal c-Src activity to be reset by C-terminal c-Src Tyr529 phosphorylation by Csk. Increased basal c-Src activity was thus the result of an inability to inactivate c-Src through Csk-mediated C-terminal phosphorylation of c-Src Tyr529 after the removal of stimulus. Reduced expression of membrane Csk adaptors Cbp and Cav-1, as well as Csk per se, led to the sustained increase in basal c-Src activity that could not be reset, demonstrating that a certain level of either Cbp or Cav-1 (and Csk) is required for c-Src inhibition. As shown in our proposed model (Fig. 5), Cbp and Cav-1 recruit cytoplasmic Csk to the membrane where Csk mediates the inactivation of c-Src. If one adapter is absent or not functional, there is a compensatory increase in the expression or phosphorylation state of the other adapter to enable coordination of Csk and safeguard against sustained c-Src activation.
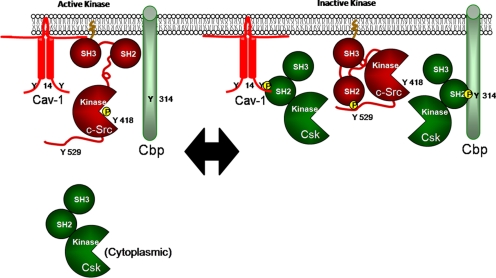
Fig. 5.
Proposed equilibrium model of c-Src activity regulation. Cbp and Cav-1 recruit cytoplasmic Csk to the membrane, where Csk mediates inactivation of c-Src. If one adapter is absent or not functional, there is a compensatory increase in the other to enable coordination of Csk and negative regulation of c-Src activity. This model also predicts that when both Csk adapters are absent or nonfunctional, Csk is unable to localize to the membrane, resulting in sustained c-Src activation.
The finding that expression of WT or Y14E Cav-1 was able to further reduce basal c-Src activity when expressed in Cav-1(−/−) MFs, whereas the phosphodefective Cav-1 mutant (Y14F) had no effect on c-Src activity and was unable to bind Csk, may be clinically important. This finding indicates that despite the approximately 2-fold increase in Cbp expression in these cells, the equilibrium of c-Src activation and inactivation can still be affected by both Csk expression level and Csk adapter protein expression level and/or phosphorylation state. Defective c-Src inactivation probably explains the increase in c-Src activity noted in colon cancer (Rengifo-Cam et al., 2004; Sirvent et al., 2010), in which a decrease in Csk membrane localization was associated with increased metastasis and invasiveness. These studies also showed reduced adapter protein expression for both Cav-1 and Cbp in metastatic cells and tumors. Furthermore, Sirvent et al. (2010) went on to demonstrate that rescued expression of Cbp in colorectal cancer cells reduced the invasiveness of these cells. Our study indicates that Cav-1 and Cbp are cooperative, and increases in their expression or phosphorylation favors repression of c-Src activity, suggesting that this may represent an important therapeutic mechanism or diagnostic tool for the treatment of colon cancer and other pathologies associated with sustained or elevated c-Src activation.
Acknowledgments
We thank Dr. Richard E. Pagano at the Mayo Clinic and Foundation (Rochester, MN) for the Y14E Cav-1 mutant; Drs. Vasily Shinin and Nikolaos Maniatis for providing mouse fibroblasts; Maricela Castellon, Debra Salvi, and Dr. Tiffany Sharma for excellent technical assistance; and Drs. Masuko Ushio-Fukai and Kishore K. Wary for helpful comments. We also thank Drs. Masato Okada and Shigeyuki Nada at the Division of Oncogene Research, Research Institute for Microbial Diseases, Osaka University (Osaka, Japan) for providing us with the untagged rat Csk that we used for our Csk-CFP construct.
This research was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL71626, P01-HL60678]; and the American Heart Association Midwest Affiliate [Grant 0910026G].
This article is based on a thesis submitted in partial fulfillment of the requirements for a doctoral degree: Place AT (2011) Molecular mechanism of Src regulation by Csk, SHP-2, Cbp, and caveolin-1. Ph.D. thesis, Department of Pharmacology, University of Illinois, Chicago, IL.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073957.
- SFK
- Src family kinase
- Csk
- C-terminal Src kinase
- Cbp
- Csk-binding protein
- MF
- mouse fibroblasts
- SH
- Src homology
- WT
- wild type
- siRNA
- small interfering RNA
- PAGE
- polyacrylamide gel electrophoresis
- RIPA
- radioimmunoprecipitation assay
- PMSF
- phenylmethylsulfonyl fluoride
- ODG
- n-octylglucoside
- TBST
- Tris-buffered saline/Tween 20
- HBSS+/+
- Hanks' balanced salt solution with Ca2+ and Mg2+
- ELISA
- enzyme-linked immunosorbent assay
- HEK
- human embryonic kidney
- GFP
- green fluorescent protein
- S
- scrambled.
Authorship Contributions
Participated in research design: Place, O'Bryan, and Minshall.
Conducted experiments: Place, Chen, and Bakhshi.
Performed data analysis: Place, Chen, and Liu.
Wrote or contributed to the writing of the manuscript: Place, Minshall, Liu, Chen, Bakhshi, and O'Bryan.
References
- Aoki T, Nomura R, Fujimoto T. (1999) Tyrosine Phosphorylation of caveolin-1 in the endothelium. Exp Cell Res 253:629–636 [DOI] [PubMed] [Google Scholar]
- Brdicka T, Pavlistová D, Leo A, Bruyns E, Korínek V, Angelisová P, Scherer J, Shevchenko A, Hilgert I, Cerný J, et al. (2000) Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med 191:1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Courchesne WE, Mastick CC. (2002) A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem 277:8771–8774 [DOI] [PubMed] [Google Scholar]
- Chow LM, Veillette A. (1995) The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol 7:207–226 [DOI] [PubMed] [Google Scholar]
- Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. (2005) The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol 25:10533–10542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Cooper JA. (1994) Csk suppression of Src involves movement of Csk to sites of Src activity. Mol Cell Biol 14:5402–5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto A, Soriano P. (1993) Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73:1117–1124 [DOI] [PubMed] [Google Scholar]
- Jiang LQ, Feng X, Zhou W, Knyazev PG, Ullrich A, Chen Z. (2006) Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene 25:5495–5506 [DOI] [PubMed] [Google Scholar]
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. (2000) Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404:999–1003 [DOI] [PubMed] [Google Scholar]
- Khanna S, Roy S, Park HA, Sen CK. (2007) Regulation of c-Src activity in glutamate-induced neurodegeneration. J Biol Chem 282:23482–23490 [DOI] [PubMed] [Google Scholar]
- Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, et al. (2000) Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14:1750–1775 [DOI] [PubMed] [Google Scholar]
- Li S, Seitz R, Lisanti MP. (1996) Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271:3863–3868 [PubMed] [Google Scholar]
- Lu TL, Kuo FT, Lu TJ, Hsu CY, Fu HW. (2006) Negative regulation of protease-activated receptor 1-induced Src kinase activity by the association of phosphocaveolin-1 with Csk. Cell Signal 18:1977–1987 [DOI] [PubMed] [Google Scholar]
- Martin GS. (2001) The hunting of the Src. Nat Rev Mol Cell Biol 2:467–475 [DOI] [PubMed] [Google Scholar]
- Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. (2000) Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 150:1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204:2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. (1991) Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351:69–72 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tanaka S, Suzuki H, Takayanagi H, Miyazaki T, Nakamura K, Tsuruo T. (2000) Overexpression of the csk gene suppresses tumor metastasis in vivo. Int J Cancer 88:384–391 [PubMed] [Google Scholar]
- Ogawa A, Takayama Y, Sakai H, Chong KT, Takeuchi S, Nakagawa A, Nada S, Okada M, Tsukihara T. (2002) Structure of the carboxyl-terminal Src kinase, Csk. J Biol Chem 277:14351–14354 [DOI] [PubMed] [Google Scholar]
- Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. (1991) CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem 266:24249–24252 [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422 [DOI] [PubMed] [Google Scholar]
- Rengifo-Cam W, Konishi A, Morishita N, Matsuoka H, Yamori T, Nada S, Okada M. (2004) Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene 23:289–297 [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. (2004) Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem 279:48055–48062 [DOI] [PubMed] [Google Scholar]
- Sirvent A, Bénistant C, Pannequin J, Veracini L, Simon V, Bourgaux JF, Hollande F, Cruzalegui F, Roche S. (2010) Src family tyrosine kinases-driven colon cancer cell invasion is induced by Csk membrane delocalization. Oncogene 29:1303–1315 [DOI] [PubMed] [Google Scholar]
- Sverdlov M, Shajahan AN, Minshall RD. (2007) Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J Cell Mol Med 11:1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. (2009) Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20:4531–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Takayama Y, Ogawa A, Tamura K, Okada M. (2000) Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J Biol Chem 275:29183–29186 [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609 [DOI] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. (1997) Three-dimensional structure of the tyrosine kinase c-Src. Nature 385:595–602 [DOI] [PubMed] [Google Scholar]