Abstract
Binding of the agonist GABA to the GABAA receptor causes channel gating, whereas competitive antagonists that bind at the same site do not. The details of ligand binding are not well understood, including which residues interact directly with ligands, maintain the structure of the binding pocket, or transduce the action of binding into opening of the ion channel gate. Recent work suggests that the amine group of the GABA molecule may form a cation-π bond with residues in a highly conserved “aromatic box” within the binding pocket. Although interactions with the carboxyl group of GABA remain unknown, three positively charged arginines (α1Arg67, α1Arg132, and β2Arg207) just outside of the aromatic box are likely candidates. To explore their roles in ligand binding, we individually mutated these arginines to alanine and measured the effects on microscopic ligand binding/unbinding rates and channel gating. The mutations α1R67A or β2R207A slowed agonist binding and sped unbinding with little effect on gating, demonstrating that these arginines are critical for both formation and stability of the agonist-bound complex. In addition, α1R67A sped binding of the antagonist 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)pyridazinium bromide (SR-95531), indicating that this arginine poses a barrier to formation of the antagonist-bound complex. In contrast, β2R207A and α1R132A sped antagonist unbinding, indicating that these arginines stabilize the antagonist-bound state. α1R132A also conferred a new long-lived open state, indicating that this arginine influences the channel gate. Thus, each of these arginines plays a unique role in determining interactions with agonists versus antagonists and with the channel gate.
Introduction
Activation of the GABAA receptor involves formation of an agonist-receptor complex (binding) followed by conformational rearrangements that open an integral chloride channel (gating). The ligand binding pocket is formed by the interface between β and α subunits (Sigel et al., 1992; Amin and Weiss, 1993; Smith and Olsen, 1994). Many candidate GABA-binding residues have been identified by observing that their mutation right-shifts the GABA dose-response curve, or that modification of substituted cysteines alters the ability of GABA to activate the channel (Boileau et al., 1999, 2002; Westh-Hansen et al., 1999; Wagner and Czajkowski, 2001; 2002; Newell and Czajkowski, 2003; Holden and Czajkowski, 2002). Recent work has highlighted aromatic residues that are highly conserved among cysteine-loop receptors, including GABAA, nicotinic acetylcholine (nACh), glycine, and 5-hydroxytryptamine3 receptors (Lummis, 2009). Unnatural amino acid substitution of these aromatics demonstrates that the electronegativity of their π-electron orbitals correlates strongly with apparent affinity for agonists, leading to the proposal that the ligand amine group forms a direct cation-π bond with specific aromatic residues. The other end of the GABA molecule, however, is a carboxylate group whose binding partners remain unknown.
The above-mentioned studies used macroscopic measures (e.g., EC50) that are composites of multiple microscopic binding and gating transitions, and thus were not able to distinguish whether a residue is specifically involved in binding, gating, or both. Here, we used submillisecond ligand application and kinetic modeling to estimate the microscopic binding/unbinding rates of agonists and a competitive antagonist. By taking advantage of the competition between agonist and antagonist to occupy the agonist binding site, we can determine the agonist binding rate apart from any gating processes (Clements et al., 1992; Jones et al., 1998, 2001; Wagner et al., 2004).
The substituted cysteine accessibility method (Boileau et al., 1999; Wagner and Czajkowski, 2001) and homology modeling (Cromer et al., 2002) have identified three arginines (α1Arg67, α1Arg132, and β2Arg207) at the β/α intersubunit ligand-binding interface, in positions in which they could serve as binding partners for the carboxylate group of GABA (Fig. 1A). We showed previously that in the rat α1β2 GABAA receptor, mutating β2Arg207 to cysteine slowed GABA binding and sped unbinding, with no effect on gating (Wagner et al., 2004), raising the possibility that β2Arg207 could directly interact with GABA. Here, we compare the roles of all three arginines in the human receptor by mutating them individually to alanine, which more closely approximates the removal of the native side chain than mutation to cysteine.
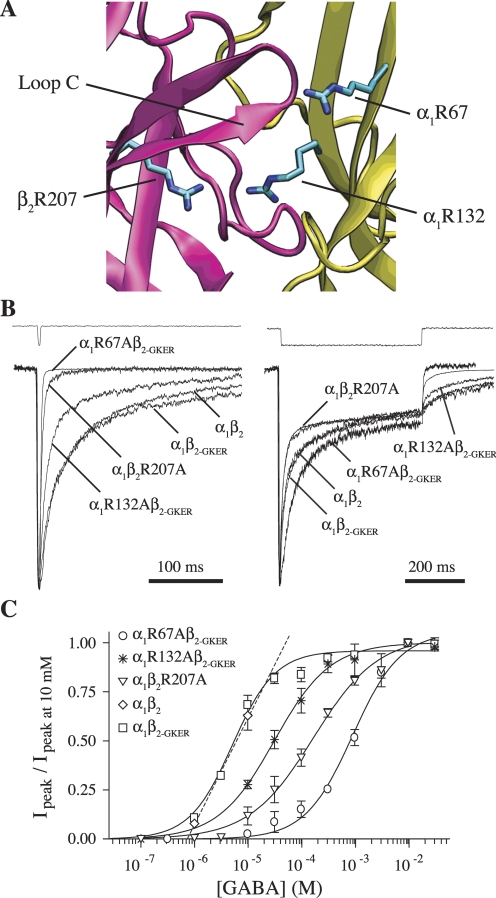
Fig. 1.
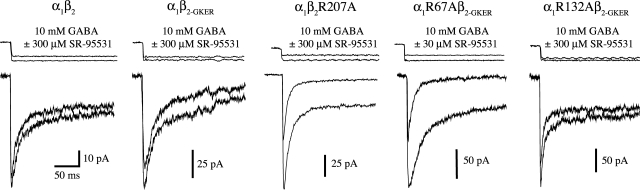
Mutation of β2Arg207, α1Arg67, or α1Arg132 to alanine increases the rate of deactivation and right-shifts concentration-response curves but does not affect desensitization. A, homology model of the GABAA receptor agonist binding site at the interface between β2 (pink) and α1 (yellow) subunits showing residues β2Arg207, α1Arg67, and α1Arg132 (O'Mara et al., 2005; with α1Arg67 rotated arbitrarily so as to protrude into the binding pocket). B, mutants and wild-type receptors were challenged with 2 to 4 ms (left) or 500 ms (right) pulses of 10 mM GABA (because of differences in EC50, we ensured saturation by using 30 mM GABA for R67A). All traces were normalized to their peak to ease comparison. Peak currents of traces shown varied between 60 and 300 pA. Each trace is the average of between 8 and 25 sweeps, recorded while the patches were held at −60 mV. The top traces in both are example recordings from open pipette tips at the end of an experiment to demonstrate solution exchange. C, GABA concentration-response curves of peak currents, fit with the equation IGABA/IGABA-max = Ymax/[(EC50/[GABA])N + 1], where N is the Hill coefficient (Prism 4). Only two data points are shown for α1β2, which were linearly interpolated to estimate EC50 (Table 2).
We find that α1Arg67 and β2Arg207 are critical for both rapid and stable binding of the agonists GABA and THIP but not for channel gating. In contrast, α1Arg67 hinders binding of the competitive antagonist 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)pyridazinium bromide (SR-95531), whereas β2Arg207 and α1Arg132 stabilize bound antagonist. Mutation of α1Arg132 also confers a new open state. Thus, α1Arg67 and β2Arg207 are good candidates for interacting directly with GABA, whereas β2Arg207 and α1Arg132 may interact with antagonist and α1Arg132 may also participate in transducing binding to gating.
Materials and Methods
Cell Culture and Transfection.
Human embryonic kidney (HEK-293) cells were cultured in minimum essential medium with Earle's salts (Mediatech, Inc., Herndon, VA) containing 10% bovine calf serum (Sigma-Aldrich, St. Louis, MO) in a 37°C incubator under a 5% CO2 atmosphere. Cells were transfected using either a calcium phosphate precipitation method (Jordan et al., 1996) or with the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) with the prescribed protocol, with 1 to 2 μg total of one α and one β construct in a 1:1 ratio, from α1, α1R67A, or α1R132A and β2, β2-GKER (see below), or β2R207A human cDNAs in vector pcDNA3.1 (Invitrogen). Recordings were performed 24 to 80 h after transfection. Wild-type constructs were obtained from Dr. Steven Petrou (Howard Florey Institute, Melbourne, Australia), and mutant constructs were made using recombinant polymerase chain reaction as described previously (Kucken et al., 2000). Each mutation was verified by double-stranded sequencing of the entire coding region to verify that no unwanted mutations had been introduced during the procedure.
Patch-Clamp Electrophysiology.
Recordings from outside-out patches excised from HEK-293 cells were made using borosilicate glass pipettes filled with 140 mM KCl, 10 mM EGTA, 2 mM MgATP, 20 mM phosphocreatine, and 10 mM HEPES, pH 7.3, with osmolarity of 315 mOsM. Patches were voltage-clamped at −60 mV and placed in the stream of a multibarreled flow-pipe array (Vitrodynamics, Rockaway, NJ) mounted on a piezoelectric bimorph (Morgan Electro Ceramics Inc., Bedford, OH). A computer-controlled constant current source drove the bimorph to move solution interfaces over the patch with 10 to 90% exchange times of <200 μs, as measured by the liquid junction current at the open pipette tip after each experiment. Junction currents were generated by altering the ionic strength with an additional 5 mM NaCl or 1% H2O in solutions containing agonist or antagonist, respectively. GABA, THIP, and SR-95531 were dissolved in the perfusion solution, which usually contained 145 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 4 mM glucose, pH 7.4, with osmolarity of 320 mOsM adjusted with sucrose. For extracellular solutions that contained greater than 30 mM GABA or THIP, the concentration of NaCl was reduced, and the appropriate combination of sucrose and agonist was added to compensate for the reduced osmolarity. When using low NaCl extracellular solution, the concentration of KCl in the pipette solution was also reduced to maintain a constant Cl− driving force, and potassium gluconate was added to maintain the osmolarity. All chemicals were obtained from Sigma-Aldrich. Currents were low-pass-filtered at 2 to 5 kHz with a four-pole Bessel filter and digitized at a rate no less than twice the filter frequency. Data were collected using an Axopatch 200B amplifier and Digidata 1320A digitizer (Molecular Devices, Sunnyvale, CA), controlled by AxoGraph software (Axograph Scientific, Sydney, Australia) running on a Macintosh G4 (Apple Computer, Cupertino, CA). Curve-fitting was performed using either AxoGraph or Prism 4 (GraphPad Software, Inc., San Diego, CA) software. Deconvolution of antagonist unbinding experiments (Jones et al., 2001) was done using home-written routines in MATLAB 7 (The MathWorks, Natick, MA).
Statistical Analysis.
In all cases, significant differences were tested using one-way ANOVA with post hoc Tukey test at a significance level of p < 0.05 (Prism 4). Weighted time constants (τw) for biexponential fits to macroscopic kinetics [i.e., I(t) = ΣIaiexp(−t/τi)] were calculated as τw = Σiaiτi, where ai and τi are the fractional amplitude and time constant of the ith component, I is current, and t is time.
β2-Subunit GKER Mutation.
The GABAA receptor mutation α1R67A (human numbering, equivalent to Arg66 in the rat) has been shown previously to disrupt receptor assembly with the β2 subunit (Bollan et al., 2003). Consistent with these findings, we observed very little GABA-evoked current in outside-out patches from HEK-293 cells transfected with α1R67A and β2 subunits (3 of 50 patches had detectable current with a mean of 8 pA). However, it has also been reported that assembly in the presence of α1R67A can be rescued by replacing four amino acid residues in the β2 subunit with the aligned residues from the β3 subunit (Taylor et al., 1999; Bollan et al., 2003). We refer to this construct as β2-GKER representing the four mutations D171G, N173K, T179E, and K180R.
Single-Channel Records.
Single-channel currents were recorded in the presence of 30 mM GABA from patches held at −80 mV, sampled at 20 kHz, and filtered at 2 kHz. Openings and closures were defined by entry into specific amplitude windows using a 200-μs minimum event width, with home-written routines in MATLAB 7 (The MathWorks). Events that included multiple openings were discarded. Open time distributions were fitted by the maximum likelihood method, with corrections for missed events (Colquhoun and Sigworth, 1995).
Nonstationary Variance Analysis.
Nonstationary variance analysis (Sigworth, 1980) was performed on responses to repeated pulses of saturating GABA (10 mM), from which ensemble mean current (I) and variance (σ2) were calculated at each time point. The mean current was divided into 100 equally sized bins, and the variances in each bin were averaged. Plots of binned variance versus current were fit with the equation σ2 = iI − I2N−1, where i is the single channel current and N is the number of channels. Conductance was computed by dividing i by the holding potential of −60 mV. Variance resulting from slow drift (i.e., rundown or run-up) was corrected by local linear fitting of the drift, calculating the variance due to this trend at each point, and subtracting this drift variance (scaled by the square of normalized current amplitude) from the total variance before fitting. This method yields accurate estimates of i and N when tested on simulated data with drift.
Kinetic Modeling.
Kinetic modeling was performed with home-written software using the Q-matrix method (Colquhoun and Hawkes, 1995). Before optimization of the model shown in Fig. 7A, the GABA binding rate constant kon was fixed to the value we determined experimentally (Table 2), and the maximal open probability (Po-max) was set to 0.44 based on nonstationary variance analysis (Fig. 6). The remaining unconstrained rate constants were optimized for individual patches expressing α1β2, α1β2R207A, or α1R67Aβ2-GKER receptors by fitting current responses to 2 to 4 ms and 500-ms pulses of 10 to 30 mM GABA (Fig. 7, B and C). Optimization used a Nelder-Mead simplex algorithm to minimize the amplitude-weighted sum of squared errors between actual and simulated currents. In all cases, significant differences in fitted parameters between constructs were tested using one-way ANOVA with post hoc Tukey's test, p < 0.05.
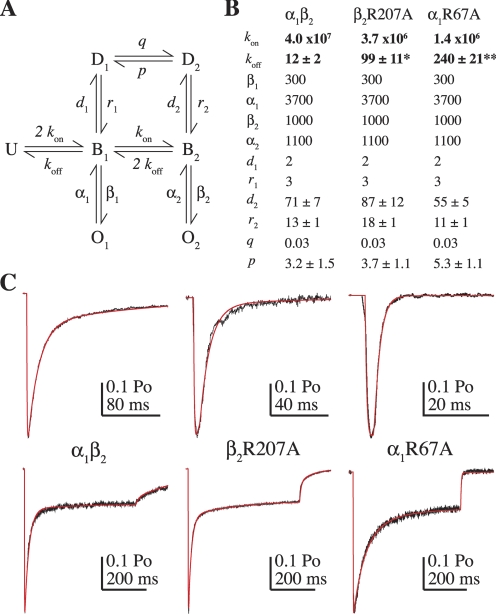
Fig. 7.
Kinetic modeling demonstrates that the kinetic effects of the mutations β2R207A and α1R67A can be explained by slower GABA binding and faster unbinding. A, the Markov model used to simulate GABA-evoked currents (U, unbound; B, bound; O, open; D, desensitized; previously described in Jones et al., 1998). B, rate constants used to simulate α1β2, R207A, and R67A receptors (units are in seconds−1 except for GABA binding steps kon and q, which are M−1 · s−1). The values of koff, d2, r2, and p are reported as mean ± S.E.M. because they were allowed to vary. The model was optimized to fit current responses to 2 to 4 ms and 500-ms pulses of 10 to 30 mM GABA from individual patches (see Materials and Methods). koff was the only unconstrained rate constant that significantly differed comparing mutant and wild-type models (one-way ANOVA with post hoc Tukey's test, *, p < 0.05, **, p < 0.01). C, current responses (black) evoked by 2 to 4 ms (top) and 500-ms (bottom) pulses of 10 to 30 mM GABA from individual patches containing α1β2 (left), α1β2R207A (middle) and α1R67Aβ2-GKER (right) receptors overlaid with simulated responses (red). Note the different time scales for the short pulses.
TABLE 2.
Summary of microscopic binding/unbinding rates and affinities for the competitive antagonist SR-95531 and binding rates and macroscopic affinities for the agonists GABA and THIP
Data are mean ± S.E.M.
| KD-SR | koff-SR | kon-SR | EC50-GABA | kon-GABA | kon-THIP | |
|---|---|---|---|---|---|---|
| nM | s−1 | M−1·s−1 | mM | M−1·s−1 | ||
| α1β2 | 124 | 10 ± 1 | (8.2 ± 0.7) × 107 | 6 | (2.2 ± 0.4) × 107 | (5.5 ± 0.7) × 106 |
| α1β2-GKER | 69 | 7 ± 1 | (9.7 ± 0.8) × 107 | 8 | (2.0 ± 0.2) × 107 | N.A. |
| α1β2R207A | 230 | 23 ± 2* | (9.9 ± 1.0) × 107 | 139 | (3.8 ± 0.4) × 106* | (1.9 ± 0.7) × 105* |
| α1R67Aβ2-GKER | 26 | 12 ± 2 | (4.5 ± 0.6) × 108* | 1100 | (1.4 ± 0.1) × 106* | (5.6 ± 1.7) × 105* |
| α1R132Aβ2-GKER | 542 | 63 ± 9* | (1.2 ± 0.2) × 108 | 31 | (1.7 ± 0.4) × 107 | N.A. |
N.A., not available.
Differences between mutants and their appropriate control (see Results) were calculated using one-way ANOVA with post-hoc Tukey test at p < 0.05 (Prism 4). GABA unbinding rates were also estimated using a kinetic model (see Fig. 7).
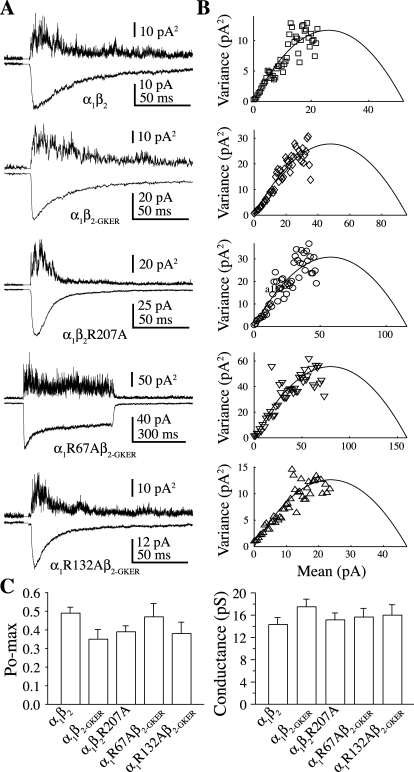
Fig. 6.
None of the mutants altered the peak open probability (Po-max) of the channel. A, mean (below) and variance (above) of consecutive responses to 2 or 500 ms pulses of 10 mM GABA. B, plots of normalized mean current versus variance for the traces shown in A fit with a parabola (black line) describing the single-channel conductance, Po-max, and the number of channels present in each patch (Sigworth, 1980). C, summary of Po-max and single-channel conductance from the fits in B. No differences were found by one-way ANOVA with post hoc Tukey's test, p < 0.05.
Results
Arginines α1Arg67, α1Arg132, and β2Arg207 Are Critical for Prolonged Receptor Activation.
Responses to rapid ligand application were recorded in outside-out patches from HEK-293 cells transfected with either α1β2, α1β2R207A, α1β2-GKER, α1R67Aβ2-GKER, or α1R132Aβ2-GKER subunit combinations. Receptor kinetics were characterized by macroscopic deactivation after brief pulses (2–4 ms), somewhat similar to that occurring during synaptic transmission, and desensitization during long pulses (500 ms) of saturating GABA (10–30 mM). The β2 subunit GKER mutation was employed to rescue receptor assembly in the presence of α1R67A or α1R132A (see Materials and Methods). Compared with α1β2 receptors, α1β2-GKER did not alter macroscopic deactivation or desensitization kinetics, or GABA EC50 (Fig. 1, B and C; Table 1). Thus, we treated α1β2-GKER as a “wild-type” control for the α1R67A and α1R132A mutants, whereas the β2R207A mutant was compared with α1β2.
TABLE 1.
Summary of biexponential fits to deactivation and desensitization
Arginine-to-alanine mutations α1R67A, α1R132A, and β2R207A sped deactivation after brief (2–4 ms) pulses of 10 to 30 mM GABA, with little effect on desensitization during longer (500-ms) pulses. Data are mean ± S.E.M. “Remaining” indicates fraction of peak current remaining at the end of a 500-ms pulse.
| τfast | τfast | τslow | τslow | τweighted | Remaining | n | |
|---|---|---|---|---|---|---|---|
| ms | % | ms | % | ms | % | ||
| Deactivation after brief GABA pulses (2–4 ms, 10 mM) | |||||||
| α1β2 | 26 ± 2 | 72 ± 5 | 298 ± 18 | 29 ± 5 | 103 ± 14 | N.A. | 9 |
| α1β2-GKER | 22 ± 3 | 71 ± 3 | 281 ± 31 | 29 ± 3 | 96 ± 12 | N.A. | 9 |
| α1β2R207A | 6 ± 1* | 90 ± 5* | 31 ± 5* | 10 ± 5* | 8 ± 1* | N.A. | 4 |
| α1R67Aβ2-GKER | 4 ± 1* | 98 ± 2* | 15 ± 3* | 2 ± 2* | 4 ± 1* | N.A. | 7 |
| α1R132Aβ2-GKER | 13 ± 2* | 81 ± 1 | 161 ± 31* | 19 ± 1 | 42 ± 8* | N.A. | 4 |
| Desensitization during long GABA pulses (500 ms, 10 mM) | |||||||
| α1β2 | 13 ± 1 | 50 ± 3 | 159 ± 14 | 20 ± 2 | 57 ± 10 | 27 ± 2 | 32 |
| α1β2-GKER | 18 ± 1 | 49 ± 1 | 239 ± 30 | 23 ± 2 | 88 ± 11 | 28 ± 2 | 25 |
| α1β2R207A | 11 ± 1 | 62 ± 3 | 184 ± 18 | 15 ± 2 | 42 ± 5 | 23 ± 2 | 39 |
| α1R67Aβ2-GKERa | 27 ± 3* | 37 ± 4 | 217 ± 26 | 27 ± 3 | 107 ± 13 | 36 ± 3 | 15 |
| α1R132Aβ2-GKER | 11 ± 1 | 62 ± 3 | 267 ± 61 | 17 ± 2 | 70 ± 15 | 21 ± 2 | 16 |
N.A., not available.
Currents were elicited with 30 mM GABA.
Differences between mutants and their appropriate control (see Results) were calculated using one-way ANOVA with post-hoc Tukey test at p < 0.05 (Prism 4).
All three mutations accelerated deactivation (speeding of τw: α1R67A, 24-fold; α1R132A, 2-fold; β2R207A, 13-fold), with little or no effect on desensitization (Fig. 1B, Table 1). Although α1R67A exhibited a slower initial component of desensitization, it sped deactivation to a much larger degree than it slowed desensitization. Both macroscopic deactivation and desensitization emerge from the interactions of numerous microscopic transitions. However, only deactivation depends on ligand unbinding, because in the presence of saturating ligand, any unbound receptor will immediately rebind (it is “as if” the ligand never unbinds). Speeding deactivation without appreciably altering desensitization therefore suggests that all three mutations increase the unbinding rate of GABA, with little or no effect on gating (further evidence for this conclusion for α1R67A and β2R207A is presented below). Faster unbinding should reduce the time spent in ligand-bound states, which predicts a lower apparent affinity. Consistent with this interpretation, all three mutations shifted the GABA dose-response curve to the right (shift in GABA EC50: α1R67A, 183-fold; α1R132A, 5-fold; β2R207A, 23-fold) (Fig. 1C, Table 2).
Arginines Are Differentially Involved in Competitive Antagonist Binding.
Competitive antagonists are likely to bind at the same location as GABA, thus preventing GABA from binding, but there must be a difference in the way they interact with the binding site because they do not induce channel opening. We therefore asked whether α1Arg67, α1Arg132, or β2Arg207 were involved in binding the competitive antagonist SR-95531 by examining the effect of their individual alanine mutations on SR-95531 microscopic binding and unbinding rates.
The unbinding rate of a competitive antagonist can be obtained from macroscopic currents using a deconvolution based method described in detail by Jones et al. (2001). In brief, receptors are pre-equilibrated in antagonist and then rapidly switched to a solution with saturating GABA alone. The resulting current is due to antagonist unbinding from receptors that are then free to bind GABA and open (Fig. 2A). This current is the convolution of the antagonist unbinding time course with the response to GABA alone. Therefore, the antagonist unbinding time course can be obtained by deconvolving the currents after antagonist equilibration with a control response to saturating GABA (Fig. 2B). In our hands, the unbinding time course of SR-95531 from the GABAA receptor obtained by this and other methods (e.g., Jones et al., 1998) has always been monoexponential, suggesting unbinding from a single site. Thus, we take the inverse of this time constant to be the microscopic antagonist unbinding rate (Table 2).
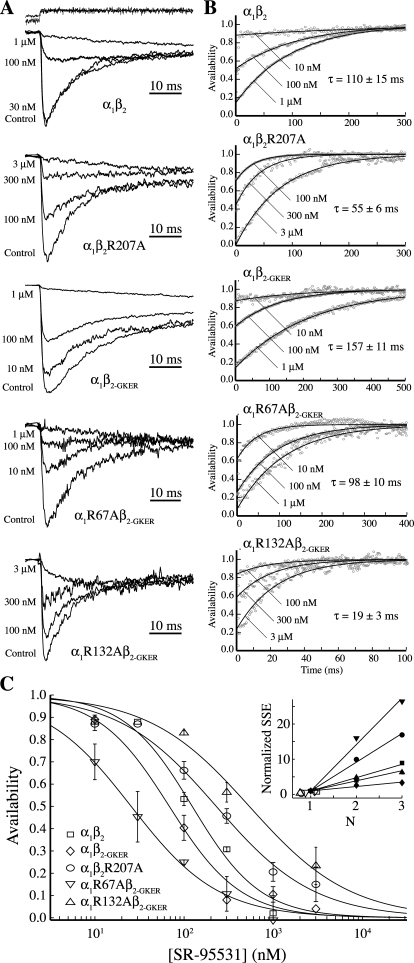
Fig. 2.
Unbinding kinetics and affinity of the competitive antagonist SR-95531. A, currents evoked with 10 to 30 mM GABA both without (control) and with pre-equilibration in the competitive antagonist SR-95531 (concentrations given to the left of each trace). B, the antagonist unbinding time course was obtained by deconvolving the control (no antagonist pre-equilibration) currents and the currents after equilibration in antagonist. Deconvolutions (gray circles) were fit to the equation A(t) = [P∞ − (P∞ − P0) exp(−t/τ)]N, where A(t) is the fraction of available receptors (antagonist not bound at any site), P0 and P∞ are the probabilities that a single binding site is available initially at t = 0 and at steady state as t → ∞, τ is the time constant of antagonist unbinding from each site (koff-SR = 1/τ), and N is the number of antagonist binding sites (Jones et al., 2001). Best fits were always obtained with N = 1. Note the different time scales for each construct. C, dose-response curves for the equilibrium antagonist occupancy in the absence of GABA, A(t = 0), were fitted to the normalized Hill equation I/Imax = 1 − 1/[(KD-SR/[SR − 95531])N + 1]. Unconstrained fits (shown) had Hill coefficients (N) near unity for all five constructs (α1β2, 1.1; R207A, 0.8; α1β2-GKER, 1.1; R67A, 0.9; R132A, 0.8). The goodness of fit as judged by the sum-of-squared errors (SSE) decreased for increasing integer N (inset, SSE normalized to value at N = 1; solid symbols, N constrained to integers 1, 2, or 3; open symbols, N unconstrained).
In addition to the unbinding rate, the amount of current elicited immediately upon agonist application reflects the equilibrium fraction of receptors having bound antagonist during the pre-equilibration, which depends on the antagonist concentration. All three mutants altered the affinity of the receptor for SR-95531, as evidenced by their shifted inhibition dose-response curves, but not in the same direction (Fig. 2C, Table 2). Consistent with the monoexponential nature of SR-95531's unbinding time course (Fig. 2B), the inhibition dose-response curves for each construct were best fitted with a Hill slope near unity (Fig. 2C, inset), suggesting that antagonism occurs upon binding of a single molecule of SR-95531. For a single binding site, as seems to be the case for SR-95531 (see Discussion), the antagonist binding rate can be computed as kon-ant = koff-ant/KD, where the microscopic dissociation constant KD is the antagonist concentration required to block half of the channels.
The lower antagonist affinities conferred by β2R207A and α1R132A were entirely due to a 2- and 9-fold increase in the SR-95531 unbinding rate, respectively (Table 2). On the other hand, the higher affinity conferred by α1R67A was due to a 5-fold increase in the SR-95531 binding rate. This indicates that α1Arg67 acts as a barrier to binding SR-95531, whereas α1Arg132 and to a lesser extent β2Arg207 stabilize the SR-95531-bound complex.
Arginines α1Arg67 and β2Arg207 Are Required for Fast Agonist Binding.
To determine the roles of α1Arg67, α1Arg132, and β2Arg207 in GABA binding, we examined the effect of their individual alanine mutations on the microscopic GABA binding rate using a macroscopic measure that involves “racing” GABA against the competitive-antagonist SR-95531. In brief, control responses to saturating GABA were interleaved with responses to the simultaneous application of GABA and SR-95531. Coapplication of agonist and antagonist leads to a reduction in the peak agonist-evoked response as a result of some of the receptors binding antagonist and contributing no current. The ratio Irace of the peak current in the presence of antagonist to that in control depends on the relative concentrations and binding rates of the agonist and antagonist as they “race” against each other for the binding site. If the antagonist binding rate (kon-ant) is known, the agonist binding rate can be computed as kon-ag = [ant] kon-ant/([ag](1/Irace − 1)), where [ant] and [ag] are the antagonist and agonist concentrations, respectively (Jones et al., 1998).
In wild-type receptors, simultaneous application of 10 mM GABA and 300 μM SR-95531 blocked ∼15% of the peak current obtained by application of GABA alone (Fig. 3). This suggests that SR-95531 “out-raced” GABA for its binding site at 15% of the receptors. In contrast, peak current for the β2R207A mutant was blocked by ∼45% under the same conditions. Because this mutation does not affect the binding rate of SR-95531, it must be that β2R207A slows GABA binding. Peak current for the α1R67Aβ2-GKER mutant was 10-fold more sensitive to SR-95531 than that for β2R207A. However, part of this enhanced sensitivity is due to the faster binding rate of SR-95531, which when taken into account yields a 14- and 6-fold slowing of the GABA binding rate compared with wild type for the α1R67A and β2R207A mutants, respectively. In contrast, α1R132A did not confer a detectable difference in the GABA binding rate. Therefore, α1Arg67 and β2Arg207, but not α1Arg132, are required for rapid formation of the GABA-bound complex.
Fig. 3.
The mutations β2R207A and α1R67A slow the GABA binding rate. Responses to simultaneous application of GABA and the competitive antagonist SR-95531 reflect their relative binding rates. The larger amplitude traces are responses to GABA alone, whereas the smaller traces are responses to coapplication of GABA and antagonist. The ratio of the peak currents was used to compute kon-GABA (Jones et al., 1998). Each trace is the average of between 5 and 20 sweeps. The top traces are recordings from open pipette tips made at the end of each experiment.
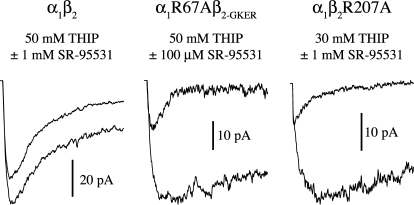
To test whether α1Arg67 and β2Arg207 are specifically involved only in binding GABA, or if they are more generally involved in agonist binding, we examined the effect of their mutation to alanine on the microscopic binding rate of the lower affinity agonist THIP. Similar to their effect on GABA binding, both α1R67A and β2R207A slowed THIP binding, suggesting that these residues may play a generic role in agonist binding at the GABAA receptor (Fig. 4, Table 2).
Fig. 4.
The mutations β2R207A and α1R67A slow the binding rate of THIP. Responses to simultaneous application of THIP and the competitive antagonist SR-95531 reflect their relative binding rates. Pulses were 500 ms and the first 100 ms are shown. The larger amplitude traces are responses to THIP alone, whereas the smaller traces are responses to coapplication of THIP and antagonist. The ratio of the peak currents was used to compute kon-THIP (Jones et al., 1998).
Mutations α1R67A and β2R207A Do Not Alter Peak Open Probability, Open Time, or Conductance.
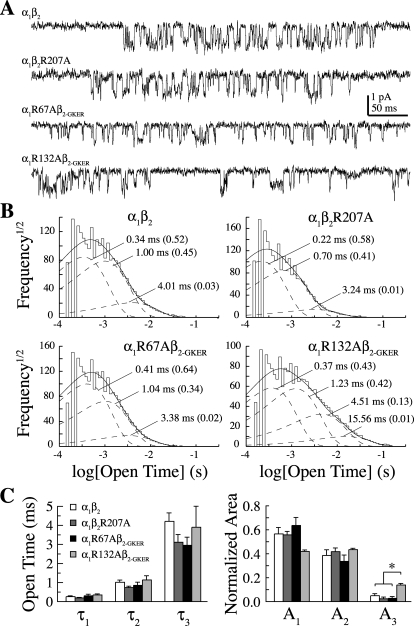
Because none of the mutations had much of an effect on macroscopic desensitization, we hypothesized that they did not greatly affect gating. To test this, we examined the effect of each mutation on multiple aspects of channel gating including single-channel conductance, open dwell time distributions, and maximal open probability (Po-max). Open dwell time distributions from single-channel recordings in the presence of 30 mM GABA at a holding potential of −80 mV for α1β2, α1R67Aβ2-GKER, and α1β2R207A were all fitted with three exponential components whose time constants and relative areas were not different (Fig. 5). Although the first three time constants of the open dwell time distribution for α1R132Aβ2-GKER were also similar to wild type, we observed a small fraction of longer openings not seen in the other constructs. Mean open times (and relative areas) were the following: for α1β2 (eight patches, mean ± S.E.M.), 0.3 ± 0.03 ms (0.57 ± 0.05), 1.0 ± 0.1 ms (0.39 ± 0.05), and 4.2 ± 0.4 ms (0.05 ± 0.01); α1R67Aβ2-GKER (four patches), 0.3 ± 0.1 ms (0.64 ± 0.06), 0.9 ± 0.2 ms (0.34 ± 0.05), and 3.0 ± 0.4 ms (0.03 ± 0.01); α1R132Aβ2-GKER (three patches), 0.4 ± 0.04 ms (0.42 ± 0.01), 1.1 ± 0.2 ms (0.43 ± 0.01), 3.9 ± 1.1 ms (0.14 ± 0.01), and 16.1 ± 3.2 ms (0.01 ± 0.003); and α1β2R207A (four patches), 0.2 ± 0.02 ms (0.56 ± 0.03), 0.7 ± 0.1 ms (0.42 ± 0.03), and 3.1 ± 0.4 ms (0.02 ± 0.01). In addition, none of the mutations altered the channel's main conductance level, which was the following: for α1β2 (mean ± S.E.M.), 10.7 ± 0.6 pS; R67Aβ2-GKER, 9.3 ± 0.3 pS; R132Aβ2-GKER, 8.6 ± 0.4 pS; and α1β2R207A, 9.9 ± 0.8 pS. Differences were assessed by one-way ANOVA with post hoc Tukey's test at p < 0.05.
Fig. 5.
Single-channel properties of arginine mutants. A, representative single channel events. Recordings were filtered at 2 kHz for analysis and 1 kHz for display. B, open dwell time distributions across patches were fit with the sum of three to four exponentials (time constants and relative areas are labeled). The first three time constants were not different in the mutants, but α1R132A receptors exhibited a small number of additional long-lived openings not seen for the other three constructs. C, summary of the first three open dwell time constants and relative areas. *, different by one-way ANOVA with post hoc Tukey's test at p < 0.05.
Closed dwell time distributions were fit with four exponential components (data not shown). Mean closed times (and relative areas) for α1β2 were 0.9 ± 0.1 ms (0.53 ± 0.04), 7.4 ± 0.8 ms (0.28 ± 0.04), 183 ± 52 ms (0.14 ± 0.05), and 1856 ± 686 ms (0.04 ± 0.01). None of the mutants differed in any of these components. However, because patches probably contained multiple channels, the observed closed times include apparent closures that may be due to the closing of one channel and the opening of another. Because these apparent closures are less likely to occur during sojourns to short-lived closed states, we examined closed time distributions from within bursts of openings separated by closures longer than 10 ms, excluding those bursts containing stacked openings. Within-burst closed-time distributions were fit with three exponential components. Mean within burst closed times (and relative areas) for α1β2 were 0.3 ± 0.04 (0.26 ± 0.05), 1.5 ± 0.2 (0.45 ± 0.03), and 8.7 ± 0.6 ms (0.30 ± 0.04). None of the mutants differed in any of these components.
We used nonstationary variance analysis (Sigworth, 1980) to estimate both the single-channel conductance and Po-max of the receptor (Fig. 6). Because Po-max is a measure that depends on the interplay between numerous microscopic transitions, it is useful not only as a general measure of microscopic kinetic changes but also as a constraint on any kinetic model of the receptor (see below). None of the mutations altered either conductance (γ) at −60 mV or Po-max (α1β2: γ = 14.3 ± 1.2 pS, Po-max = 0.49 ± 0.03, n = 10; α1β2-GKER: γ = 17.5 ± 1.3 pS, Po-max = 0.35 ± 0.05, n = 5; α1R67Aβ2-GKER: γ = 15.7 ± 1.2 pS, Po-max = 0.47 ± 0.07, n = 19; α1R132Aβ2-GKER: γ = 16 ± 1.8 pS, Po-max = 0.38 ± 0.06, n = 3; α1β2R207A: γ = 15.2 ± 1.2 pS, Po-max = 0.39 ± 0.03; n = 17). The larger single-channel conductances obtained from nonstationary variance analysis than those measured directly in the single-channel recordings above is probably due to the fact that the former reflects a weighted average of all conductance levels, whereas the latter only measures openings to the most frequent level. We were not able to quantitatively confirm this, however, because subconductances did not appear as distinct peaks in all-points amplitude histograms. These data suggest that α1Arg67 and β2Arg207 are not involved in channel gating, whereas α1Arg132 has an effect on the stability of the open channel.
The Kinetic Effects of α1R67A and β2R207A Are Due to Slower GABA Binding and Faster Unbinding.
Given that those mutations having the largest effects on the microscopic GABA binding rate also had the largest effects on macroscopic deactivation, and also that binding and unbinding rates are often inversely correlated (Jones et al., 1998, 2001; Mozrzymas et al., 1999; Barberis et al., 2000), we hypothesized that the macroscopic effects of the mutations on deactivation were entirely due to faster GABA unbinding. Unlike the binding rate, we could not examine this directly, but instead we asked whether such a hypothesis could explain our observations using a kinetic model shown previously to account for multiple aspects of GABAA receptor behavior (Fig. 7A) (Jones et al., 1998; Wagner et al., 2004; Goldschen-Ohm et al., 2010).
The model shown in Fig. 7A was optimized for individual patches by fitting current responses to 2 to 4 ms and 500-ms pulses of 10 to 30 mM GABA for α1β2 (7 patches), α1β2R207A (7 patches), and α1R67Aβ2-GKER (10 patches) receptors (Fig. 7C, see Materials and Methods). We were able to quantitatively replicate all observed macroscopic effects of β2R207A and α1R67A on responses to brief and long GABA pulses by 1) decreasing the GABA binding rate as measured above (see Table 2), and 2) increasing the GABA unbinding rate. The final rate constants (mean ± S.E.M.) are listed in Fig. 7B. Thus, the model illustrated in Fig. 7 is consistent with the idea that α1Arg67 and β2Arg207 are involved in GABA binding and unbinding and have little or no effect on channel gating.
Discussion
Despite the wealth of information gained from mutagenesis, functional assays, and crystallography of homologous proteins (Sigel et al., 1992; Amin and Weiss, 1993; Smith and Olsen, 1994; Boileau et al., 1999, 2002; Westh-Hansen et al., 1999; Brejc et al., 2001; Wagner and Czajkowski, 2001; Cromer et al., 2002; Holden and Czajkowski, 2002; Newell and Czajkowski, 2003; Celie et al., 2004; Wagner et al., 2004; Hansen et al., 2005; O'Mara et al., 2005), the exact structure of the GABA binding site and the roles of individual residues in ligand binding and the accompanying conformational changes remain unclear. Most previous studies of the binding site have relied on changes in dose-response curve macroscopic measures (e.g., EC50) that are influenced by both binding and gating and thus cannot separate them (Colquhoun, 1998). We therefore used kinetic methods to separate the roles in binding versus gating of three arginines lining the GABA-binding intersubunit interface of the human GABAA receptor (α1Arg67, α1Arg132, and β2Arg207). Individual alanine mutations lowered affinity for GABA and accelerated deactivation with little or no effect on desensitization. A combination of macroscopic and single-channel measurements with kinetic modeling demonstrated that for the mutations α1R67A and β2R207A, these effects can be entirely explained by a 10- to 14-fold slower microscopic GABA binding rate and 8- to 20-fold faster unbinding rate. Therefore, these two residues contribute to both the rapid formation and stability of the agonist-bound complex, with little or no involvement in channel gating. A parsimonious interpretation is that one or both of these arginines interacts directly with the carboxylate group of the GABA molecule, although it is also possible that they contribute structurally to the integrity of the binding site.
Unlike the other two mutations, α1R132A did not affect the GABA binding rate. Given that α1R132Aβ2-GKER deactivates more quickly than wild type, this seems to suggest that α1Arg132 influences only GABA unbinding but not binding. However, because agonist binding and unbinding rates are typically inversely correlated (Jones et al., 1998, 2001; Mozrzymas et al., 1999; Barberis et al., 2000), the modest speeding of deactivation by α1R132A suggests that a slowing of GABA binding may be present but too small to detect with our methods. Interestingly, single-channel recordings from α1R132Aβ2-GKER receptors exhibited a small number of openings to a new long-lived open state. Although these long openings comprised only ∼1% of all openings, they account for ∼11% of the observed charge. These long openings could prolong deactivation, potentially masking some of the effects of faster ligand unbinding.
Two mutations (β2R207A and α1R132A) reduced SR-95531 affinity by speeding its unbinding rate 2- to 9-fold without altering its binding rate, indicating that these arginines maintain the stability of the antagonist-receptor complex but do not influence its formation. In contrast, α1R67A increased antagonist affinity by speeding binding 5-fold without changing unbinding, indicating that this arginine hinders formation of the antagonist-receptor complex but does not influence its stability once formed. Therefore, α1Arg67 is part of the energy barrier to formation of the antagonist-receptor complex, whereas β2Arg207 and α1Arg132 are part of the energy well that stabilizes this complex. The SR-95531 molecule may thus encounter steric or electrostatic resistance to entering the pocket from α1Arg67 but once in the pocket may interact directly with β2Arg207 and α1Arg132 to form the antagonist-bound state. Alternatively, β2Arg207 and α1Arg132 could contribute structurally to the stability of the antagonist binding site.
Antagonism upon Binding of a Single Molecule of SR-95531.
Despite the presence of two agonist sites, the monoexponential unbinding time courses of several antagonists suggest that antagonism is relieved upon unbinding from a single site (Jones et al., 1998, 2001; Wagner et al., 2004). In addition, antagonist Hill slopes near unity suggest that antagonism occurs upon binding to only a single site. One explanation for these results is that SR-95531 binds to only one of the two GABA binding sites. However, a study of receptors formed from concatenated subunits containing mutations in none, both, or one or the other GABA binding site suggest that both sites have a similar affinity for the competitive antagonists SR-95531 and bicuculline (Baumann et al., 2003). This suggests that SR-95531 can bind at either site, but allosterically inhibits its binding to the other site. Indeed, competitive antagonists can exert allosteric effects on channel activation (Ueno et al., 1997), and can alter the accessibility of residues at both of the β/α GABA binding interfaces (Boileau et al., 2002) and the α/γ benzodiazepine binding interface (Sharkey and Czajkowski, 2008). Interestingly, competitive antagonists for the homologous nACh receptor known to bind preferentially to one or the other nonidentical binding site illustrate that binding of a single antagonist molecule is sufficient to prevent channel opening in a cysteine-loop receptor (Wenningmann and Dilger, 2001; Dilger et al., 2007). Furthermore, pairs of nACh receptor antagonists often exhibit cooperative effects, possibly because antagonist binding at one site allosterically influenced antagonist binding at the other site (Liu and Dilger, 2008). We conclude that SR-95531 either preferentially binds to only one of the two GABA binding sites or allosterically inhibits itself from binding to both sites simultaneously.
Arginines Are Similarly Involved in GABA and THIP Binding.
Interestingly, the two mutations having the largest effect on the GABA binding rate (α1R67A and β2R207A) had similar effects on the binding rate of the lower affinity agonist THIP, suggesting that those arginines may be part of a generic mechanism underlying agonist binding. Consistent with the idea that α1Arg67 is generally involved in agonist binding, molecular dynamics simulations with GABA and glycine docked to homology models of the GABAC and glycine receptors, respectively, show the carboxylate group of both ligands in direct contact with the amide head group of an arginine in a homologous position to α1Arg67 in the GABAA receptor (Grudzinska et al., 2005; Melis et al., 2008). A glutamate-gated chloride channel having 34% sequence identity to the human α1 glycine receptor has been crystallized with glutamate bound in the agonist binding site, in which its carboxylate groups are coordinated by two arginines, one of which is located similarly to α1Arg67 in the GABAA receptor (Hibbs and Gouaux, 2011).
Aromatic Residues in the Binding Site.
A number of highly conserved aromatic residues form the so-called “aromatic box” and have been widely implicated in GABA binding as a result of the large reduction in GABA affinity seen upon their mutation (Sigel et al., 1992; Amin and Weiss, 1993; Boileau et al., 1999, 2002; Wagner and Czajkowski, 2001). In particular, unnatural amino acid substitution showed that the apparent affinity for GABA in the GABAA and GABAC receptors correlates with the electronegativity of the π-electron orbitals of β2Tyr97 and ρ1Tyr198, respectively, suggesting that the GABA amine group may form cation-π bonds with these residues (Lummis, 2009). Given that these aromatics are on opposite sides of the interface, this requires that the orientation of GABA in the pocket is different for these two receptors. An alternative but so far unexplored possibility is that the correlation of aromatic electronegativity with apparent affinity reflects not the formation of cation-π bonds with the agonist but rather with the amide head group of a nearby arginine that stabilizes binding pocket structure. Interestingly, arginines involved in cation-π interactions with aromatics retain their hydrogen bonding capability. A survey of crystal structures found that whenever an arginine/aromatic pair interacted with a ligand, hydrogen bonding to the arginine was always involved, and direct contacts between the ligand and the aromatic were often seen as well (Flocco and Mowbray, 1994). Thus, aromatics may play an important role in the positioning of arginines for proper interaction with ligands.
Conclusions
We conclude that α1Arg67 and β2Arg207 participate primarily in agonist binding and unbinding, but not gating of the channel, and are thus good candidates to interact directly with the GABA and THIP molecules. Given that these two residues are on opposite sides of the β/α binding interface, it remains unclear whether they might simultaneously interact with the bound ligand or rather sequentially interact as part of a binding/unbinding pathway involving multiple steps or may simply contribute to the stability of the binding site. In addition, α1Arg67 poses a barrier to binding of the competitive antagonist SR-95531, whereas α1Arg132 and β2Arg207 stabilize the bound antagonist. In addition, α1Arg132 does influence the stability of the open channel and thus may participate in transducing binding to opening of the channel gate.
The microscopic binding and unbinding rates reported here are directly related to the energy landscape seen by these ligands during binding and unbinding and thus represent an important set of experimental constraints for validating atomic level models of the GABA binding site and future molecular dynamics simulations. Knowledge of the separate roles of residues in binding versus gating will be invaluable in improving our understanding of how ligand binding alters the receptor structure to cause channel activation.
This work was supported by the the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS046378]; and the American Epilepsy Society and the Lennox Trust Fund.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.072033.
- nACh
- nicotinic acetylcholine
- SR-95531
- 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)pyridazinium bromide (gabazine)
- THIP
- 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (gaboxadol)
- HEK
- human embryonic kidney
- ANOVA
- analysis of variance
- Po
- open probability.
Authorship Contributions
Participated in research design: Goldschen-Ohm, Wagner, and Jones.
Conducted experiments: Goldschen-Ohm and Wagner.
Performed data analysis: Goldschen-Ohm and Wagner.
Wrote or contributed to the writing of the manuscript: Goldschen-Ohm, Wagner, and Jones.
References
- Amin J, Weiss DS. (1993) GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature 366:565–569 [DOI] [PubMed] [Google Scholar]
- Barberis A, Cherubini E, Mozrzymas JW. (2000) Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating. J Neurosci 20:8618–8627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. (2003) Individual properties of the two functional agonist sites in GABA receptors. J Neurosci 23:11158–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Evers AR, Davis AF, Czajkowski C. (1999) Mapping the agonist binding site of the GABAA receptor: evidence for a β-strand. J Neurosci 19:4847–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Newell JG, Czajkowski C. (2002) GABAA receptor β2 Tyr97 and Leu99 line the GABA-binding site. Insights into mechanisms of agonist and antagonist actions. J Biol Chem 277:2931–2937 [DOI] [PubMed] [Google Scholar]
- Bollan K, King D, Robertson LA, Brown K, Taylor PM, Moss SJ, Connolly CN. (2003) GABAA receptor composition is determined by distinct assembly signals within α and β subunits. J Biol Chem 278:4747–4755 [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276 [DOI] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41:907–914 [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. (1992) The time course of glutamate in the synaptic cleft. Science 258:1498–1501 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125:924–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. (1995) A Q-matrix cookbook. How to write only one program to calculate the single-channel and macroscopic predictions for any kinetic mechanism, in Single-Channel Recording, 2nd ed (Sakmann B, Neher E. eds) pp 589–633, New York, Plenum [Google Scholar]
- Colquhoun D, Sigworth FJ. (1995) Fitting and statistical analysis of single-channel records, in Single-Channel Recording, 2nd ed (Sakmann B, Neher E. eds) pp 483–587, New York, Plenum [Google Scholar]
- Cromer BA, Morton CJ, Parker MW. (2002) Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem Sci 27:280–287 [DOI] [PubMed] [Google Scholar]
- Dilger JP, Vidal AM, Liu M, Mettewie C, Suzuki T, Pham A, Demazumder D. (2007) Roles of amino acids and subunits in determining the inhibition of nicotinic acetylcholine receptors by competitive antagonists. Anesthesiology 106:1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flocco MM, Mowbray SL. (1994) Planar stacking interactions of arginine and aromatic side-chains in proteins. J Mol Biol 235:709–717 [DOI] [PubMed] [Google Scholar]
- Goldschen-Ohm MP, Wagner DA, Petrou S, Jones MV. (2010) An epilepsy-related region in the GABAA receptor mediates long-distance effects on GABA and benzodiazepine binding sites. Mol Pharmacol 77:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. (2005) The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45:727–739 [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24:3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JH, Czajkowski C. (2002) Different residues in the GABAA receptor α1T60-α1K70 region mediate GABA and SR-95531 actions. J Biol Chem 277:18785–18792 [DOI] [PubMed] [Google Scholar]
- Jones MV, Jonas P, Sahara Y, Westbrook GL. (2001) Microscopic kinetics and energetics distinguish GABAA receptor agonists from antagonists. Biophys J 81:2660–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Sahara Y, Dzubay JA, Westbrook GL. (1998) Defining affinity with the GABAA receptor. J Neurosci 18:8590–8604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm FM. (1996) Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 24:596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucken AM, Wagner DA, Ward PR, Teissére JA, Boileau AJ, Czajkowski C. (2000) Identification of benzodiazepine binding site residues in the γ2 subunit of the γ-aminobutyric acidA receptor. Mol Pharmacol 57:932–939 [PubMed] [Google Scholar]
- Liu M, Dilger JP. (2008) Synergy between pairs of competitive antagonists at adult human muscle acetylcholine receptors. Anesth Analg 107:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC. (2009) Locating GABA in GABA receptor binding sites. Biochem Soc Trans 37:1343–1346 [DOI] [PubMed] [Google Scholar]
- Melis C, Lummis SC, Molteni C. (2008) Molecular dynamics simulations of GABA binding to the GABAC receptor: the role of Arg104. Biophys J 95:4115–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Michalak K, Cherubini E. (1999) Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci 19:2474–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell JG, Czajkowski C. (2003) The GABAA receptor α1 subunit Pro174-Asp191 segment is involved in GABA binding and channel gating. J Biol Chem 278:13166–13172 [DOI] [PubMed] [Google Scholar]
- O'Mara M, Cromer B, Parker M, Chung SH. (2005) Homology model of the GABAA receptor examined using Brownian dynamics. Biophys J 88:3286–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey LM, Czajkowski C. (2008) Individually monitoring ligand-induced changes in the structure of the GABAA receptor at benzodiazepine binding site and non-binding site interfaces. Mol Pharmacol 74:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Baur R, Kellenberger S, Malherbe P. (1992) Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J 11:2017–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ. (1980) The variance of sodium current fluctuations at the node of Ranvier. J Physiol 307:97–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Olsen RW. (1994) Identification of a [3H]muscimol photoaffinity substrate in the bovine γ-aminobutyric acidA receptor a subunit. J Biol Chem 269:20380–20387 [PubMed] [Google Scholar]
- Taylor PM, Thomas P, Gorrie GH, Connolly CN, Smart TG, Moss SJ. (1999) Identification of amino acid residues within GABAA receptor β subunits that mediate both homomeric and heteromeric receptor expression. J Neurosci 19:6360–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. (1997) Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci 17:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C. (2001) Structure and dynamics of the GABA binding pocket: A narrowing cleft that constricts during activation. J Neurosci 21:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C, Jones MV. (2004) An arginine involved in GABA binding and unbinding but not gating of the GABAA receptor. J Neurosci 24:2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenningmann I, Dilger JP. (2001) The kinetics of inhibition of nicotinic acetylcholine receptors by (+)-tubocurarine and pancuronium. Mol Pharmacol 60:790–796 [PubMed] [Google Scholar]
- Westh-Hansen SE, Witt MR, Dekermendjian K, Liljefors T, Rasmussen PB, Nielsen M. (1999) Arginine residue 120 of the human GABAA receptor α1 subunit is essential for GABA binding and chloride ion current gating. Neuroreport 10:2417–2421 [DOI] [PubMed] [Google Scholar]