Abstract
Heterotrimeric G proteins, composed of Gα and Gβγ subunits, transduce extracellular signals via G-protein-coupled receptors to modulate many important intracellular responses. The Gβγ subunits hold a central position in this signaling system and have been implicated in multiple aspects of physiology and the pathophysiology of disease. The Gβ subunit belongs to a large family of WD40 repeat proteins with a circular β-bladed propeller structure. This structure allows Gβγ to interact with a broad range of proteins to play diverse roles. How Gβγ interacts with and regulates such a wide variety of partners yet maintains specificity is an interesting problem in protein-protein molecular recognition in signal transduction, where signal transfer by proteins is often driven by modular conserved recognition motifs. Evidence has accumulated that one mechanism for Gβγ multitarget recognition is through an intrinsically flexible protein surface or “hot spot” that accommodates multiple modes of binding. Because each target has a unique recognition mode for Gβγ subunits, it suggests that these interactions could be selectively manipulated with small molecules, which could have significant therapeutic potential.
Introduction
G protein-coupled receptors (GPCRs) mediate multiple physiological processes and represent the largest single family of cell surface receptors (Lagerström and Schiöth, 2008). GPCRs respond to a wide array of ligands, including hormones, peptides, proteins, lipids, neurotransmitters, nucleotides, ions, and photons (Lagerström and Schiöth, 2008). Because of their central role in biology and physiology, they are major targets of current pharmaceuticals and continue to be very important drug targets (Flower, 1999; Ma and Zemmel, 2002). There has been an explosion of structural information about the nature of these receptors that promises to move drug discovery targeted at these receptors at an increasingly rapid pace (Rosenbaum et al., 2009).
GPCRs transduce extracellular information through a number of mechanisms, but classic GPCR signaling is through direct coupling to heterotrimeric G proteins, consisting of an α subunit that binds GDP and GTP and a constitutive dimer of β and γ subunits (Gilman, 1987; Hamm, 1998). In the classic model for GPCR-dependent G protein activation, GPCRs undergo ligand binding-dependent conformational changes to catalyze GDP release and subsequent binding of GTP to the G protein α subunit, leading to dissociation of Gα-GTP from Gβγ (Gilman, 1987). This dissociation event releases two signaling proteins, Gα and Gβγ, that drive downstream signaling through direct protein-protein interactions (Milligan and Kostenis, 2006; Oldham and Hamm, 2008). Gα subunit signaling is terminated by hydrolysis of GTP, and Gβγ signaling is terminated by reassociation with Gα subunits in a way that sequesters the protein recognition surface on both subunits.
The Gβγ subunits are involved in multiple aspects of GPCR-mediated signaling and regulation (Smrcka, 2008; Dupré et al., 2009). In addition to their role in downstream signaling, Gβγ subunits interact with GPCRs and Gα subunits and are critical for GPCR-dependent G protein activation. The diverse and expanding roles for Gβγ in cell signaling are numerous and have been reviewed (Smrcka, 2008; Dupré et al., 2009). Rather, we will focus on new concepts relating to the nature of molecular recognition by Gβγ and how pharmacological targeting of Gβγ capitalizes on these ideas.
Gβγ Interaction with Effectors
As discussed above, Gβγ interacts directly with a wide range of effectors and regulators to modulate diverse downstream cellular responses. The first example was discovered in 1987 when purified Gβγ was shown to activate a cardiac potassium channel normally activated by a muscarinic cholinergic receptor after stimulation by acetylcholine (Logothetis et al., 1987). Additional evidence for Gβγ-dependent downstream pathway activation came from genetic analysis of the pheromone signaling pathway in yeast, indicating that Gβγ is the key activator of the pheromone response downstream from the G protein coupled pheromone receptor (Whiteway et al., 1989). Since then, many Gβγ effectors have been identified, including adenylyl cyclase (AC) isoforms (Tang and Gilman, 1991; Sunahara and Taussig, 2002), G protein-coupled receptor kinase 2 (GRK2) (Pitcher et al., 1992), phospholipase C (PLC) β2 and β3 isoforms (Camps et al., 1992; Park et al., 1993; Smrcka and Sternweis, 1993), inwardly rectifying potassium channels (GIRK) (Logothetis et al., 1987; Nakajima et al., 1996), phosphoinositide 3-kinase γ (PI3Kγ) (Stephens et al., 1994, 1997), and N-type calcium channels (Ikeda, 1996). Proteomic methods and yeast two-hybrid screening have revealed multiple novel Gβγ binding proteins. These include PDZ domain containing proteins (Li et al., 2006); guanine exchange factors (GEFs) for small G proteins such as P-Rex1 (Mayeenuddin et al., 2006), FLJ00018, also known as pleckstrin homology domain containing family G member 2 (a Gβγ-activated Rac and Cdc42 guanine nucleotide exchange factor) (Ueda et al., 2008), and p114-RhoGEF (Niu et al., 2003); protein kinase D (PKD) (Jamora et al., 1999); receptor for activated C kinase 1 (RACK1) (Dell et al., 2002); soluble NSF attachment protein (SNAP) receptor (SNARE) complex (Yoon et al., 2007); and a Radil-Rap1A complex (Ahmed et al., 2010). A striking observation for all of these Gβγ binders is that no readily apparent consensus sequence or structure mediates binding of these proteins to Gβγ. In the next sections, we will discuss ideas for how these binding partners can be accommodated by Gβγ.
Molecular Recognition by Gβγ
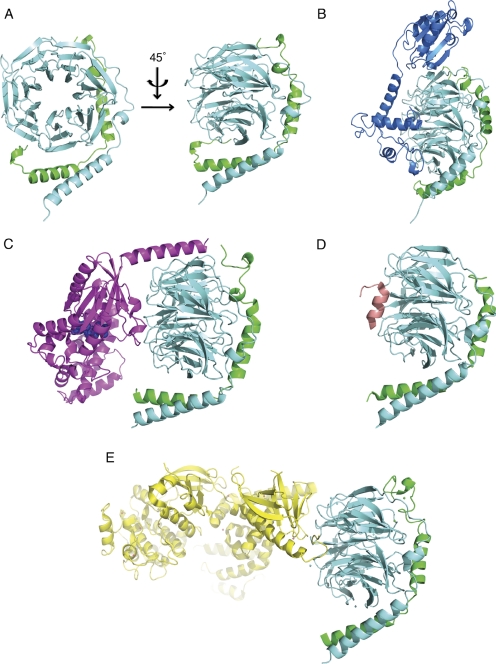
Two major approaches have been used to understand the nature of molecular recognition by Gβγ: X-ray crystal structure determination of free and complexed Gβγ, and mutagenic analysis of effector binding sites on Gβγ. The first and only Gβγ structure free of any binding partner was the crystal structure of the Gβ1γ1 dimer of transducin published by Sondek et al. (1996) (Fig. 1A). As in all the X-ray structures of Gβγ, the Gβ subunit folds with an N-terminal α-helix that makes extensive contacts to Gγ2 and a seven-bladed β propeller domain in which each blade comprises a four-stranded β sheet. In addition to the free Gβ1γ1 structure, crystal structures of Gβγ-associated with Gα subunits (Fig. 1C) (Wall et al., 1995; Lambright et al., 1996), GRK2 (Fig. 1E) (Lodowski et al., 2003), phosducin (Fig. 1B) (Gaudet et al., 1996), and the peptide SIGKAFKILGYPDYD (SIGK) (Fig. 1D) (Davis et al., 2005) have been solved. The associated Protein Data Bank (PDB) codes are compiled in Table 1. The overall structure of Gβγ is unperturbed in all of the crystal structures (Fig. 1). An exception is the structural change observed in Gβ1γ1-phosducin (Gaudet et al., 1996; Loew et al., 1998), where a cavity is introduced between blades 6 and 7 of the Gβ propeller by a movement of these two blades (Loew et al., 1998). It is noteworthy that this structural change has not been observed in other complexes thus far, but the number of Gβγ-cocomplexes remains limited. On the basis of the apparently unchanging nature of Gβγ in the various solved cocrystal structures and thermal denaturation studies (Thomas et al., 1993), Gβγ has been thought of as a relatively rigid scaffold for protein binding that can general undergo only limited conformational changes.
Fig. 1.
Gβγ crystal structures. A, Gβ1γ1 (PDB code: 1TBG) (Sondek et al., 1996). B, Gβ1γ1-phosducin (PDB code: 2TRC) (Gaudet et al., 1996). C, Gαt/iβ1γ1 heterotrimer (PDB code: 1GOT) (Lambright et al., 1996). D, Gβ1γ2-SIGK peptide (PDB code: 1XHM) (Davis et al., 2005). E, Gβ1γ2-GRK2 (PDB code: 1OMW) (Lodowski et al., 2003). Gβ subunits are all colored in aquamarine; Gγ subunits are all colored in green; Gαt/i is colored in magenta with bound GDP shown as space fill in blue; phosducin is colored in marine; GRK2 is colored in yellow; and SIGK peptide is colored in salmon.
TABLE 1.
Gβγ structures
| PDB Code | Complex | Resolution | Reference |
|---|---|---|---|
| Å | |||
| 1TBG | Gβ1γ1 | 2.1 | Sondek et al., 1996 |
| 1GOT | Gαt/iβ1γ1 heterotrimer | 2.0 | Lambright et al., 1996 |
| 1GP2 | Gαi1β1γ2 heterotrimer | 2.4 | Wall et al., 1995 |
| 1GG2 | G203A-Gαi1β1γ2 heterotrimer | 2.7 | Wall et al., 1995 |
| 2BCJ | Gαi/q(GDP-AlF4−)-GRK2-Gβ1γ2 | 3.1 | Tesmer et al., 2005 |
| 2TRC | Gβ1γ1-phosducin | 2.4 | Gaudet et al., 1996 |
| 1A0R | Gβ1γ1-phosducin | 2.8 | Loew et al., 1998 |
| 1B9X | Gβ1γ1S73E-phosducin | 3.0 | Gaudet et al., 1999 |
| 1B9Y | Gβ1γ1-phosducin | 3.0 | Gaudet et al., 1999 |
| 1OMW | Gβ1γ2-GRK2 | 2.5 | Lodowski et al., 2003 |
| 3CIK | Gβ1γ2-GRK2a | 2.75 | Tesmer et al., 2010 |
| 3KRW | Gβ1γ2-GRK2-balanol | 2.9 | Tesmer et al., 2010 |
| 1XHM | Gβ1γ2-SIGK peptide | 2.7 | Davis et al., 2005 |
GRK2 is from human to distinguish it from 1OMW, where GRK2 is from bovine.
The cocrystal structures reveal that effector/binding proteins share a critical interaction interface on the top of the torus of Gβ created by the β propeller fold that binds to switch II helix of the Gα subunits (Fig. 1). Alanine-scanning mutagenesis of Gβ confirms the notion that effectors such as PLCβ2, ACII, GRK2, and GIRK channels share a common binding surface on Gβγ but also reveals that Gβγ-interacting proteins use unique combinations of residues within this common binding surface to mediate binding (Ford et al., 1998; Li et al., 1998; Panchenko et al., 1998). Overall, despite a common surface being used for α subunits and effectors, the unique nature of binding for each partner suggested that approaches could be developed that would allow for selective manipulation of Gβγ protein-protein interactions.
As an alternative approach to understanding the nature of molecular recognition by Gβγ, we conducted a random peptide phage display screen with Gβγ as the target (Scott et al., 2001). It is noteworthy that the peptides appeared to “select” the common effector interaction surface on Gβγ suggesting that the binding site had intrinsic physicochemical properties as a preferred protein-protein interaction surface or “hot spot.” One peptide, SIGK, was cocrystallized with Gβγ, identifying the peptide binding site and the hot spot as the Gα subunit switch II binding site and the major effector-binding surface. (Davis et al., 2005). Protein interaction hot spots tend to be targeted in random peptide phage display screens (Fairbrother et al., 1998) and are generally thought to contain various types of amino acids that can participate in multiple types of binding interactions (Ma et al., 2001). Hot spots are also thought to be structurally flexible to be able to accommodate different structures (DeLano, 2002). Both of these characteristics would make sense in terms of molecular recognition by Gβγ, where multiple proteins with diverse sequence and structure are accommodated in a single binding site.
As discussed above, Gβγ is thought to be relatively rigid based on comparison of X-ray structures between different Gβγ-target complexes. However, X-ray crystallography is not an ideal approach for studies involving molecular flexibility because X-ray structures represent space and time averaged structure and are subject to lattice constraints. It is possible to compare the temperature factors for different regions but this gives limited information. An additional drawback of comparing different structures is that because of different conditions for crystallization, it is difficult to determine to what extent subtle differences in structure represent relevant differences in solution.
NMR spectroscopy is more ideally suited to measurement of protein flexibility and dynamics in solution than is X-ray crystallography. An NMR method was developed for monitoring Gβγ conformational alterations and dynamics (Smrcka et al., 2010). In part because of protein size limitations in NMR, a specific labeling protocol was adopted in which all of the Trp positions in Gβγ were labeled with 15N at both indole and amide positions. The labeled protein was then analyzed by two-dimensional transverse relaxation optimized spectroscopy-heteronuclear single quantum correlation NMR. Peaks in the spectra were assigned by site-directed mutagenesis and mapped to specific positions in the three-dimensional structure of Gβ. Thus, changes in dynamics and chemical shift position upon protein/ligand binding could be interpreted in the context of specific regions of the Gβγ dimer. Supporting the concept of a Gβγ hot spot, Trp residues in the Gβγ hot spot were unusually dynamic. Two tryptophan residues in the hot spot seemed to be in motion in different time scales. Trp99 moves in a very rapid time scale and Trp332 moves in an intermediate time scale with backbone and indole amides moving in different time regimens. It should be cautioned that this interpretation was made based on peak intensities and awaits rigorous confirmation by NMR relaxation methods to accurately determine dynamics. Nevertheless, these data together create a picture in which amino acids in the hot spot are in motion in the absence of binding partners. Only Trp residues are monitored by this method, but it is likely that all of the amino acids in the hot spot are unusually dynamic. A hypothesis that arises from these measurements is that the Gβγ subunit hot spot surface explores a range of conformations in the uncomplexed state. This range of conformations could then present a range of structures that can accommodate different partners and provides evidence that one of the properties that allow the hot spot to be a preferred protein-protein interaction surface is an inherent flexibility.
Further NMR analyses of Gβγ dynamics in the presence of three different binding partners revealed different alterations at the Gβγ hot spot surface and supports the idea that the Gβγ hot spot and perhaps all of Gβγ is more conformationally flexible than is generally presumed. These molecules, Gαi1-GDP, a phage display derived Gβγ-binding peptide SIGK, and phosducin, have all been cocrystallized with Gβγ. They share a binding surface at the hot spot but have significantly different effects on Gβγ structure and dynamics, as assessed by NMR, that are not reflected in the cocrystal structures with these molecules. Gαi1-GDP subunit binding to Gβγ did not significantly alter Gβγ surface dynamics at the hot spot. This was unexpected because Gα binding to Gβγ buries much of the hot spot surface, including Trp99 and Trp332 residues (Wall et al., 1995; Park et al., 2011). In contrast to binding of Gαi1-GDP, binding of SIGK to much of the same surface as Gα largely suppressed Trp99 and Trp332 dynamics. SIGK seems to select, and lock in, a particular conformation of the Gβγ hot spot, supporting the idea that the hot spot can flexibly adapt to accommodate different binding partners. In addition, there were chemical shift changes of Trp residue signals at some distance from the Gβγ-SIGK interface. These changes were subtle and their biological significance was unclear, but they indicate that conformational information can be transmitted allosterically throughout the Gβγ molecule. Finally, binding of phosducin both altered the dynamics and induced large chemical shift changes throughout Gβ. It is noteworthy that much, but not all, of this alteration can be accounted for by binding of the N-terminal domain of phosducin at the hot spot. These data highlight the fact that many of the assumptions about Gβγ structural flexibility and conformational alteration is based on cocrystal structures with relatively few binding partners and interpretations can be hampered by the inherent limitations of X-ray crystallography in defining physiologically relevant yet subtle alterations in structure and dynamics. It also highlights the idea that three different binding partners that interact with the same surface on Gβ have very different effects on the overall dynamics of Gβγ.
Small Molecule Targeting of the Gβγ “Hot Spot”
The random peptide phage display screen with Gβγ as the target led to identification of Gβγ-binding peptides that were selective blockers of effector regulation (Scott et al., 2001). One peptide, SIRKALNILGYPDYD, blocked Gβγ-dependent activation of PLCβ and PI3Kγ in vitro but not Gβγ-mediated inhibition of voltage-gated calcium channels. This selectivity of SIRKALNILGYPDYD suggests that small molecules might be found that bind to the hot spot and display effector selectivity.
On the basis of these data demonstrating selective modulation of signaling downstream of Gβγ by peptides and studies on the nature of the molecular recognition surface of Gβγ, we initiated a screen to identify small molecules that would bind to the hot spot on Gβγ and block downstream signaling (Bonacci et al., 2006). Compounds that bind Gβγ were identified through a combination of computational virtual screening and testing of the National Cancer Institute (NCI) chemical diversity set in a competition enzyme-linked immunosorbent assay for the SIGK peptide. This NCI diversity set is a collection of compounds that represent the chemical diversity present in the larger NCI chemical library. Nine candidate compounds that inhibited SIGK binding with IC50 values ranging from 100 nM to 60 μM were identified. These compounds blocked effector interactions in vitro and in intact cells. The Gβγ inhibitory compounds could be divided into two general classes on the basis of binding mechanism. One class, which included M119 (NSC119910; 2-(3,4,5-trihydroxy-6-oxoxanthen-9-yl)cyclohexane-1-carboxylic acid) and the highly related molecule gallein (3′,4′,5′,6′-tetrahydroxyspiro[2-benzofuran-3,9′-xanthene]-1-one) (Lehmann et al., 2008), referred to together as M119/gallein, bound via a reversible noncovalent mechanism (Seneviratne et al., 2011), whereas another class, represented by selenocystamine, formed redox-reversible covalent adducts with Gβγ (Dessal et al., 2011). Many of these redox-dependent compounds targeted a cysteine residue (Cys204) in the Gβ hot spot to form reversible mixed disulfides. The M119/gallein class of compound has been analyzed extensively for selective blocking of Gβγ-target interactions, the results of which are compiled in Table 2.
TABLE 2.
Selectivity of M119/gallein in blocking downstream effector interactions
| Blocked by M119/gallein | |
| PLC β2, β3 | Bonacci et al., 2006; Mathews et al., 2008 |
| pREX guanine nucleotide exchange factor | Zhao et al., 2007; Lehmann et al., 2008; Qin et al., 2009 |
| PI3K γ | Bonacci et al., 2006; Lehmann et al., 2008 |
| GRK2 | Bonacci et al., 2006; Casey et al., 2010 |
| Not blocked by M119/gallein | |
| N-type Ca2+ channel | P. Kammermeier, unpublished observations |
| Inwardly rectifying K+-channel | P. Kammermeier, unpublished observations |
| ERK1/2 | Bonacci et al., 2006 |
| ACII, IV, VI | C. Dessauer and V. Watts, unpublished observations |
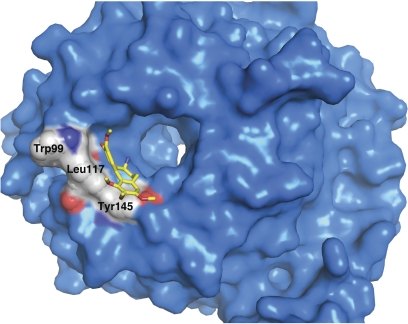
To understand the detailed nature of compound binding, and how specificity is generated, we applied a combination of structure activity relationship (SAR) analysis, site-directed mutagenesis, and X-ray structure determination to identify specific binding modes for compound interactions with Gβγ. The structure of a complex between Gβγ and a reversibly binding compound, M201 (N-deacetyl colchicine; 7-amino-1,2,3,10-tetramethoxy-6,7-dihydro-5H-benzo[a]heptalen-9-one) has recently been determined (P. Seneviratne, J. Krucinska, Y. Lin, J. Wedekind, and A. V. Smrcka, in preparation) (Fig. 2). This structure and associated mutagenic analysis show that M201 binds to the hot spot with contacts primarily along the sides of the central pore in the Gβγ propeller; a portion of the compound extends beyond the protein surface. It is noteworthy that an important part of the binding mechanism may involve hydrogen bonding of the compound to tightly bound water in the core of the molecule. Because the binding contacts for M201 are within the core of the propeller, many of the contacts for binding are below the direct protein interaction surface of the hot spot. This has the potential to allow for high-affinity protein binding while occluding only a small subset of the surface amino acids in the hot spot. For example, only two amino acids within the hot spot, Tyr145 and Leu117 are occluded by M201. Site directed mutagenesis studies indicate that these amino acids are not required for PLC activation. Thus, as might be expected, M201 binding to Tyr145 and Leu117 does not inhibit PLC activation. On the other hand, these amino acids are required for GRK2 binding activation, and M201 inhibits Gβγ interaction with GRK2. These data support the idea that individual compounds selectively interfere with effectors because they interact with amino acids critical for activation of specific effectors. Although a structure of bound M119/gallein was not determined, mutating amino acids at the binding site observed in the structure eliminates binding and functional effects of M201 but does not alter gallein binding, indicating that these compounds have different binding sites on the Gβγ surface and may form the basis for the differences in effector selectivity for these two compounds.
Fig. 2.
Binding of M201 to the Gβγ hot spot. M201 (NSC201400) is depicted in yellow. Gβ is in blue with some of the key amino acids in the hot spot shown in Corey-Pauling-Koltun form and labeled.
Therapeutic Potential of Selective Targeting Gβγ-Effector Interface
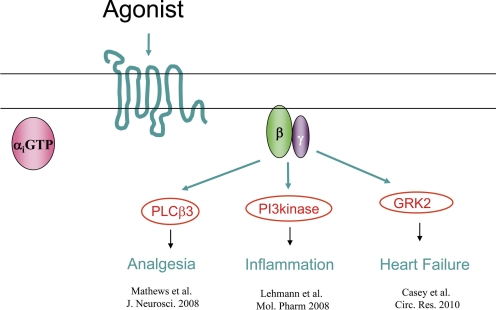
Studies on the nature of molecular recognition of targets by Gβγ subunits are physiologically important because many of the Gβγ-target couplings are involved in diseases and disruption of these interactions has been shown to be of potential therapeutic benefit. For example, various studies have shown that blocking Gβγ protein-protein interactions is an effective approach to preventing heart failure (Rockman et al., 1998), arterial restenosis (Iaccarino et al., 1999), hypertension (Koch et al., 1995), drug addiction (Yao et al., 2003), cancer metastasis (Müller et al., 2001), and prostate cancer (Bookout et al., 2003) in animal models. Most of these studies used GRK2ct (C terminus of GRK2) and the Gβγ binding peptide QEHA (sequence derived from ACII) for pharmacological targeting of Gβγ. The details of these studies have been the subject of previous reviews (Smrcka, 2008; Smrcka et al., 2008). Small molecules that bind to Gβγ (M119/gallein) are effective in animal models of inflammation, analgesia and heart failure (Fig. 3). In addition to the direct potential benefits in the specific indications discussed above, there are other theoretical advantages to targeting Gβγ as discussed below.
Fig. 3.
Therapeutic targets for Gβγ inhibitors.
Multitarget Inhibition May Be More Therapeutically Efficacious
A major advantage of targeting GPCRs directly is pharmacological specificity. There are many GPCRs and subtypes involved in a variety of physiologies that have the potential to be selectively targeted, thus limiting side effects of a more broadly based pharmacological strategy. A downside to this approach is that high specificity can limit therapeutic efficacy in complex diseases. If multiple GPCRs are involved in the development of disease, targeting a single GPCR may not be effective; rather, inhibiting the therapeutically relevant signaling pathway(s) downstream of a group of receptors could achieve this goal. An example is chemokine receptors in rheumatoid arthritis, where common Gβγ signaling systems are downstream of multiple chemokine receptor subtypes (Johnson et al., 2004). Thus inhibiting Gβγ signaling may be more efficacious than targeting a single GPCR. Although Gβγ binding compounds are somewhat selective for downstream signaling pathways, it is unlikely that compounds will be found that bind to Gβγ and only inhibit single effector because of the overlapping nature of the binding surface. As things currently stand, compounds tend to inhibit groups of Gβγ targets. This is likely to limit to some extent the specificity of this approach therapeutically; on the other hand, it could provide some benefits in terms of efficacy.
Biased Agonist Signaling
Biased agonist signaling by GPCRs is a property of GPCRs that is currently an important research direction that has possible therapeutic applications (Kenakin, 2011). The overall idea is that GPCRs sample multiple conformations that signal downstream to different signaling pathways. Agonists that select particular conformations of the receptor direct the receptor to favor activation of select pathways downstream. This has important therapeutic implications because GPCRs are major drug targets, and selectively modulating pathways that are therapeutically relevant could improve pharmacological specificity and efficacy. An alternate approach to biasing GPCR signaling down a particular pathway is to identify compounds that selectively interfere with pathways downstream from the receptors. Compounds identified in this way could be combined with existing GPCR agonists to alter signaling specificity and would obviate the need for identifying biased agonists for individual receptors. In this regard, small-molecule Gβγ inhibitors that selectively modify signaling downstream could act to bias GPCR signaling. Such molecules inhibit only a portion of the Gβγ-dependent component (see Table 2 for example) of the GPCR signal, leaving the remainder of the GPCR signaling pathway intact.
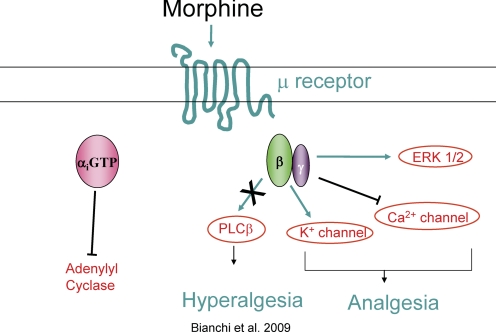
An example of such a pathway where it has been proposed that M119/gallein biases GPCR signaling is in μ-opioid receptor-dependent analgesia (Fig. 4). Administration of M119/gallein by either intracerebroventricular or intraperitoneal injections into mice potentiates the action of μ-opioid receptor agonists (Bonacci et al., 2006; Mathews et al., 2008). Because opioid receptor efficacy is largely dependent on Gβγ signaling, we propose that M119/gallein selectively blocks a Gβγ-dependent inhibitory pathway downstream of the μ-opioid receptor (PLCβ3) (Bianchi et al., 2009), while leaving other signaling pathways required for analgesia intact (N-type Ca2+ and GIRK channels). Thus M119/gallein apparently biases signaling downstream of the μ-opioid receptor. Further confirmation of this hypothesis requires more detailed testing of these signaling pathways, but these data support the idea that Gβγ inhibitors could be used to bias signaling pathways downstream of GPCRs.
Fig. 4.
Gβγ inhibitors bias the action of μ opioid receptor agonists. M119/gallein potentiates the analgesic potency of morphine in vivo. It blocks PLC activation but not calcium channel regulation in vitro. We propose that Gβγ inhibitors bias the action of morphine by blocking a hyperalgesic pathway, dependent on PLC activation, downstream of the μ-opioid receptor without blocking Gα subunit signaling or Gβγ-dependent Ca2+ channel or K+ channel regulation, thus potentiating opioid analgesia.
Critical Role of Gβγ in the G Protein Cycle and Target Specificity
To consider Gβγ as a feasible therapeutic target, several issues associated with its central role in the GPCR signaling cascade must be considered. Gβγ is required for interaction of the G protein heterotrimer with GPCRs. Therefore, a potential therapeutic strategy must target Gβγ without disruption of this G protein cycling. Another major problem is that Gβγ expression is nearly ubiquitous, so blocking all Gβγ functions might have unwanted side effects. Although Gβγ subunits are universally expressed, individual effectors, and Gβγ-effector couples, have tissue-specific expression and/or restricted subcellular location. With small-molecule inhibitors that selectively target specific Gβγ-effector coupling, without ablating general Gβγ function, selectivity issues associated with ubiquitous Gβγ expression may be overcome.
As discussed, small-molecule Gβγ inhibitors (M119/gallein) have been used extensively to investigate Gβγ functions in cell biological and animal models of diseases. In the course of these studies, many questions concerning off-target effects have been addressed. For example, in the presence of M119/gallein, the following were observed:
Unimpaired isoproterenol- and Gαs-dependent cAMP production (Casey et al., 2010).
Unimpaired [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (a μ-opioid receptor-specific agonist), Gαi-dependent decrease in cAMP and unimpaired μ-opioid receptor, Gβγ-dependent analgesia, as discussed under Biased Agonist Signaling (Mathews et al., 2008).
No effect of compounds on δ and κ opioid receptor signaling (Mathews et al., 2008).
Unimpaired fMLP- and Gβγ-dependent extracellular signal-regulated kinase activation in HL60 neutrophil-like cells (Bonacci et al., 2006).
Unimpaired M3 muscarinic acetylcholine receptor, Gαq- dependent Ca2+ regulation (Bonacci et al., 2006).
Unimpaired stromal cell-derived factor-1- and Gαi-dependent inhibition of cAMP levels (Kirui et al., 2010).
All these data indicate that GPCRs function normally in the presence of Gβγ inhibitors and that there is selectivity for different Gβγ targets. These experiments do not address unanticipated off-target effects unrelated to the G protein signaling machinery that could complicate interpretation of cellular and in vivo experiments. Thus far, results from published in vivo experiments are consistent with a Gβγ-dependent mechanism of action, and other experiments have not revealed significant off-target effects of gallein. For example, daily intraperitoneal injections of gallein in mice for three months were without significant observable physiological effects, other than those expected for inhibition of Gβγ in cardiac function. Thus, if there are off-target effects, they are not major. Nevertheless, a more thorough investigation is warranted.
Summary
Studies of the protein recognition properties of Gβγ have led to insights into the mechanisms by which Gβγ recognizes multiple different protein targets through a flexible binding surface that presents multiple types of potential bonding interactions. These insights have led to the development of a novel strategy for targeting Gβγ signaling that may have therapeutic potential for treatment of specific diseases but may also open up a new pharmacological approach to manipulating GPCR signaling in a more general sense.
This work was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM081772] (to A.V.S.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073072.
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinase
- PLC
- phospholipase C
- GIRK
- G protein-coupled inwardly rectifying potassium channels
- PI3K
- phosphoinositide 3 kinase
- PDB
- Protein Data Bank
- AC
- adenylyl cyclase
- SIGK
- SIGKAFKILGYPDYD
- NCI
- National Cancer Institute
- M119
- 2-(3,4,5-trihydroxy-6-oxoxanthen-9-yl)cyclohexane-1-carboxylic acid
- M201
- N-deacetyl colchicine; 7-amino-1,2,3,10-tetramethoxy-6,7-dihydro-5H-benzo[a]heptalen-9-one.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Lin and Smrcka.
References
- Ahmed SM, Daulat AM, Meunier A, Angers S. (2010) G protein βγ subunits regulate cell adhesion through Rap1a and its effector radil. J Biol Chem 285:6538–6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Norcini M, Smrcka A, Ghelardini C. (2009) Supraspinal Gβγ-dependent stimulation of PLCβ originating from G inhibitory protein-μ opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J Neurochem 111:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. (2006) Differential targeting of Gβγ-subunit signaling with small molecules. Science 312:443–446 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Finney AE, Guo R, Peppel K, Koch WJ, Daaka Y. (2003) Targeting Gβγ signaling to inhibit prostate tumor formation and growth. J Biol Chem 278:37569–37573 [DOI] [PubMed] [Google Scholar]
- Camps M, Hou C, Sidiropoulos D, Stock JB, Jakobs KH, Gierschik P. (1992) Stimulation of phospholipase C by G-protein βγ subunits. Eur J Biochem 206:821–831 [DOI] [PubMed] [Google Scholar]
- Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, et al. (2010) Small molecule disruption of G βγ signaling inhibits the progression of heart failure. Circ Res 107:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Bonacci TM, Sprang SR, Smrcka AV.(2005) Structural and molecular characterization of a preferred protein interaction surface on G protein βγ subunits. Biochemistry 44:10593–10604 [DOI] [PubMed] [Google Scholar]
- DeLano WL. (2002) Unraveling hot spots in binding interfaces: progress and challenges. Curr Opin Struct Biol 12:14–20 [DOI] [PubMed] [Google Scholar]
- Dell EJ, Connor J, Chen S, Stebbins EG, Skiba NP, Mochly-Rosen D, Hamm HE. (2002) The βγ subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem 277:49888–49895 [DOI] [PubMed] [Google Scholar]
- Dessal AL, Prades R, Giralt E, Smrcka AV. (2011) Rational design of a selective covalent modifier of G protein βγ subunits. Mol Pharmacol 79:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré DJ, Robitaille M, Rebois RV, Hébert TE. (2009) The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol 49:31–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother WJ, Christinger HW, Cochran AG, Fuh G, Keenan CJ, Quan C, Shriver SK, Tom JY, Wells JA, Cunningham BC. (1998) Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry 37:17754–17764 [DOI] [PubMed] [Google Scholar]
- Flower DR. (1999) Modelling G-protein-coupled receptors for drug design. Biochim Biophys Acta 1422:207–234 [DOI] [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, et al. (1998) Molecular basis for interactions of G protein βγ subunits with effectors. Science 280:1271–1274 [DOI] [PubMed] [Google Scholar]
- Gaudet R, Bohm A, Sigler PB. (1996) Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell 87:577–588 [DOI] [PubMed] [Google Scholar]
- Gaudet R, Savage JR, McLaughlin JN, Willardson BM, Sigler PB. (1999) A molecular mechanism for the phosphorylation-dependent regulation of heterotrimeric G proteins by phosducin. Mol Cell 3:649–660 [DOI] [PubMed] [Google Scholar]
- Gilman AG. (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 [DOI] [PubMed] [Google Scholar]
- Hamm HE. (1998) The many faces of G protein signaling. J Biol Chem 273:669–672 [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Smithwick LA, Lefkowitz RJ, Koch WJ. (1999) Targeting Gβγ signaling in arterial vascular smooth muscle proliferation: a novel strategy to limit restenosis. Proc Natl Acad Sci USA 96:3945–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. (1996) Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature 380:255–258 [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. (1999) Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98:59–68 [DOI] [PubMed] [Google Scholar]
- Johnson Z, Power CA, Weiss C, Rintelen F, Ji H, Ruckle T, Camps M, Wells TN, Schwarz MK, Proudfoot AE, Rommel C., Wells TNC, Schwarz MK, Proudfoot AEI, Rommel C. (2004) Chemokine inhibition – why, when, where, which and how? Biochemical Society Transactions 32:366–377 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302 [DOI] [PubMed] [Google Scholar]
- Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. (2010) Gβγ signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther 333:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. (1995) Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science 268:1350–1353 [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Schiöth HB. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7:339–357 [DOI] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. (1996) The 2.0 Å crystal structure of a heterotrimeric G protein. Nature 379:311–319 [DOI] [PubMed] [Google Scholar]
- Lehmann DM, Seneviratne AM, Smrcka AV.M.P.B., Smrcka AV. (2008) Small molecule disruption of G protein βγ subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol 73:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. (1998) Sites for Gα binding on the G protein β subunit overlap with sites for regulation of phospholipase Cβ and adenylyl cyclase. J Biol Chem 273:16265–16272 [DOI] [PubMed] [Google Scholar]
- Li Z, Benard O, Margolskee RF. (2006) Gγ13 interacts with PDZ domain-containing proteins. J Biol Chem 281:11066–11073 [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300:1256–1262 [DOI] [PubMed] [Google Scholar]
- Loew A, Ho YK, Blundell T, Bax B. (1998) Phosducin induces a structural change in transducin. Structure 6:1007–1019 [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. (1987) The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325:321–326 [DOI] [PubMed] [Google Scholar]
- Ma B, Wolfson HJ, Nussinov R. (2001) Protein functional epitopes: hot spots, dynamics and combinatorial libraries. Curr Opin Struct Biol 11:364–369 [DOI] [PubMed] [Google Scholar]
- Ma P, Zemmel R. (2002) Value of novelty? Nat Rev Drug Discov 1:571–572 [DOI] [PubMed] [Google Scholar]
- Mathews JL, Smrcka AV, Bidlack JM. (2008) A novel Gβγ subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci 28:12183–12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeenuddin LH, McIntire WE, Garrison JC. (2006) Differential sensitivity of P-Rex1 to isoforms of G protein βγ dimers. J Biol Chem 281:1913–1920 [DOI] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147 (Suppl 1):S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56 [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Nakajima S, Kozasa T. (1996) Activation of G protein-coupled inward rectifier K+ channels in brain neurons requires association of G protein βγ subunits with cell membrane. FEBS Lett 390:217–220 [DOI] [PubMed] [Google Scholar]
- Niu J, Profirovic J, Pan H, Vaiskunaite R, Voyno-Yasenetskaya T. (2003) G protein βγ subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res 93:848–856 [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9:60–71 [DOI] [PubMed] [Google Scholar]
- Panchenko MP, Saxena K, Li Y, Charnecki S, Sternweis PM, Smith TF, Gilman AG, Kozasa T, Neer EJ. (1998) Sites important for PLCβ2 activation by the G protein βγ subunit map to the sides of the β propeller structure. J Biol Chem 273:28298–28304 [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG. (1993) Activation of phospholipase C isozymes by G protein βγ subunits. J Biol Chem 268:4573–4576 [PubMed] [Google Scholar]
- Park MS, Smrcka AV, Stern HA. (2011) Conformational flexibility and binding interactions of the G protein βγ heterodimer. Proteins 79:518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. (1992) Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science 257:1264–1267 [DOI] [PubMed] [Google Scholar]
- Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. (2009) Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene 28:1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. (1998) Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA 95:7000–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. (2009) The structure and function of G-protein-coupled receptors. Nature 459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, Taussig R, Smrcka AV. (2001) Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors. EMBO J 20:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne AM, Burroughs M, Giralt E, Smrcka AV. (2011) Direct-reversible binding of small molecules to G protein βγ subunits. Biochim Biophys Acta 1814:1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV. (2008) G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci 65:2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV, Kichik N, Tarragó T, Burroughs M, Park MS, Itoga NK, Stern HA, Willardson BM, Giralt E. (2010) NMR analysis of G-protein βγ subunit complexes reveals a dynamic Gα-Gβγ subunit interface and multiple protein recognition modes. Proc Natl Acad Sci USA 107:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV, Lehmann DM, Dessal AL. (2008) G protein βγ subunits as targets for small molecule therapeutic development. Comb Chem High Throughput Screen 11:382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV, Sternweis PC. (1993) Regulation of purified subtypes of phosphatidylinositol specific phospholipase Cβ by G protein α and βγ subunits. J Biol Chem 268:9667–9674 [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. (1996) Crystal structure of a G-protein βγ dimer at 2.1Å resolution. Nature 379:369–374 [DOI] [PubMed] [Google Scholar]
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. (1994) A novel, phosphoinositide 3-kinase activity in myeloid-derived cells is activated by G-protein βγ -subunits. Cell 77:83–93 [DOI] [PubMed] [Google Scholar]
- Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, et al. (1997) The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89:105–114 [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Taussig R. (2002) Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2:168–184 [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. (1991) Type-specific regulation of adenylyl cyclase by G protein βγ subunits. Science 254:1500–1503 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. (2010) Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem 53:1867–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. (2005) Snapshot of activated G proteins at the membrane: the Gαq-GRK2-Gβγ complex. Science 310:1686–1690 [DOI] [PubMed] [Google Scholar]
- Thomas TC, Sladek T, Yi F, Smith T, Neer EJ. (1993) G protein βγ subunit: physical and chemical characterization. Biochemistry 32:8628–8635 [DOI] [PubMed] [Google Scholar]
- Ueda H, Nagae R, Kozawa M, Morishita R, Kimura S, Nagase T, Ohara O, Yoshida S, Asano T. (2008) Heterotrimeric G protein betagamma subunits stimulate FLJ00018, a guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem 283:1946–1953 [DOI] [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iñiguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. (1995) The structure of the G protein heterotrimer Giα1β1γ2. Cell 83:1047–1058 [DOI] [PubMed] [Google Scholar]
- Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, Grant FJ, O'Hara P, MacKay VL. (1989) The STE4 and STE18 genes of yeast encode potential β and γ subunits of the mating factor receptor-coupled G protein. Cell 56:467–477 [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. (2003) Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-βγ dimers. Proc Natl Acad Sci USA 100:14379–14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. (2007) Gβγ interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol Pharmacol 72:1210–1219 [DOI] [PubMed] [Google Scholar]
- Zhao T, Nalbant P, Hoshino M, Dong X, Wu D, Bokoch GM. (2007) Signaling requirements for translocation of P-Rex1, a key Rac2 exchange factor involved in chemoattractant-stimulated human neutrophil function. J Leukoc Biol 81:1127–1136 [DOI] [PubMed] [Google Scholar]