Abstract
Dravet syndrome is an epilepsy syndrome of infantile onset, frequently caused by SCN1A mutations or deletions. Its prevalence, long-term evolution in adults and neuropathology are not well known. We identified a series of 22 adult patients, including three adult post-mortem cases with Dravet syndrome. For all patients, we reviewed the clinical history, seizure types and frequency, antiepileptic drugs, cognitive, social and functional outcome and results of investigations. A systematic neuropathology study was performed, with post-mortem material from three adult cases with Dravet syndrome, in comparison with controls and a range of relevant paediatric tissue. Twenty-two adults with Dravet syndrome, 10 female, were included, median age 39 years (range 20–66). SCN1A structural variation was found in 60% of the adult Dravet patients tested, including one post-mortem case with DNA extracted from brain tissue. Novel mutations were described for 11 adult patients; one patient had three SCN1A mutations. Features of Dravet syndrome in adulthood include multiple seizure types despite polytherapy, and age-dependent evolution in seizure semiology and electroencephalographic pattern. Fever sensitivity persisted through adulthood in 11 cases. Neurological decline occurred in adulthood with cognitive and motor deterioration. Dysphagia may develop in or after the fourth decade of life, leading to significant morbidity, or death. The correct diagnosis at an older age made an impact at several levels. Treatment changes improved seizure control even after years of drug resistance in all three cases with sufficient follow-up after drug changes were instituted; better control led to significant improvement in cognitive performance and quality of life in adulthood in two cases. There was no histopathological hallmark feature of Dravet syndrome in this series. Strikingly, there was remarkable preservation of neurons and interneurons in the neocortex and hippocampi of Dravet adult post-mortem cases. Our study provides evidence that Dravet syndrome is at least in part an epileptic encephalopathy.
Keywords: SCN1A, Na+ channel, epilepsy, neuropathology, encephalopathy
Introduction
Dravet syndrome (severe myoclonic epilepsy of infancy; MIM 607208), first described ∼30 years ago, is a severe epilepsy with onset in infancy (Dravet, 1978; Dravet et al., 2005). Dravet syndrome includes severe myoclonic epilepsy of infancy and severe myoclonic epilepsy of infancy-borderland, where one or two cardinal features of severe myoclonic epilepsy of infancy may be missing. Dravet syndrome is characterized by onset of recurrent febrile and/or afebrile hemiclonic or generalized seizures, or status epilepticus, in a previously healthy infant, followed by appearance of multiple seizure types generally resistant to anti-epileptic drugs with developmental arrest or regression (Dravet et al., 2005; Jansen et al., 2006; Wolff et al., 2006). Onset up to 15 months of age may occur (Depienne et al., 2009b). Mortality may be up to 15% by 20 years (Dravet et al., 2005).
Of the cases with Dravet syndrome, 70–80% are caused by SCN1A mutations, 90% of which occur de novo (Depienne et al., 2009b; Marini et al., 2009; Mullen and Scheffer, 2009). Haploinsufficiency is thought to be the mechanism underlying most cases (McArdle et al., 2008; Depienne et al., 2009b; Mullen and Scheffer, 2009). Genetic modifiers (Meisler et al., 2010) and environmental factors probably contribute to the variable phenotype of patients with SCN1A mutations. Other genes involved in Dravet syndrome include SCN1B (Patino et al., 2009) and GABRG2 (Harkin et al., 2002). PCDH19 (Dibbens et al., 2008; Depienne et al., 2009a) and SCN2A (Kamiya et al., 2004; Shi, 2009) mutations, and deletions involving the chromosome 2q SCN cluster (Pereira et al., 2004; Davidsson et al., 2008; Lossin, 2009; Meisler et al., 2010) have been reported in Dravet syndrome-like syndromes.
SCN1A knockout or -in animal models of Dravet syndrome manifest spontaneous seizures, motor deficits, ataxia and premature death (Yu et al., 2006; Kalume et al., 2007; Ogiwara et al., 2007; Tang et al., 2009; Martin et al., 2010). Sodium currents are significantly reduced in inhibitory interneurons in both hippocampus and cortex, but less so in hippocampal pyramidal cells (Yu et al., 2006; Ogiwara et al., 2007). In a knock-in mouse model of Dravet syndrome, excitatory cortical pyramidal neurons were shown to be mostly unaffected, while inhibitory cortical interneurons had impaired sodium channel activity (Martin et al., 2010). Reduced sodium currents in hippocampal and cortical GABAergic interneurons led to altered firing patterns and overall hyperexcitability (Yu et al., 2006; Catterall et al., 2008; Tang et al., 2009; Martin et al., 2010). The reduced expression of voltage-gated sodium channel type 1.1 (Nav1.1) in Purkinje cells, leading to abnormal sodium flux, may contribute to ataxia observed in animal models (Yu et al., 2006). Further parallels between animal models and human Dravet syndrome include sensitivity to body temperature elevation, causing seizures and interictal epileptiform discharges, and age dependence of seizure frequency and severity (Oakley et al., 2009).
Immune-inflammatory mediators have received attention in epileptogenesis, febrile seizures and some chronic epilepsies (Vezzani and Granata, 2005; Ravizza et al., 2008; Vezzani et al., 2008). Dravet syndrome may provide a model to advance understanding of inflammation in epileptogenesis and fever as a seizure-provoking factor (Baulac et al., 2004; Oakley et al., 2009). The influence in Dravet syndrome of additional environmental factors such as vaccination may provide another window into investigation of immune factors in epileptogenesis (Berkovic et al., 2006; McIntosh et al., 2010).
In childhood, Dravet syndrome has been well studied. Dravet syndrome is comparatively uncommon, with an estimated incidence of <1:40 000 children (Hurst, 1990; Dravet et al., 2005), but important to diagnose because it is considered at least in part an epileptic encephalopathy, though other factors may contribute to outcomes (Ragona et al., 2011). Thus, seizures and frequent epileptiform activity on EEG are held in part responsible for cognitive, behavioural and other impairments (Dravet et al., 2005); both seizures and interictal discharges are potentially treatable and their control might improve outcomes in Dravet syndrome (Scheffer et al., 2009). In contrast to this knowledge in children with Dravet syndrome, the place of Dravet syndrome in adults with epilepsy is less well understood. Dravet syndrome is under-diagnosed and under-reported in adulthood (Scheffer et al., 2009). For patients with chronic epilepsy who are long-standing attendees at clinic, details of the early history may become obscured, and the diagnosis of Dravet syndrome may not be considered. The long-term course of Dravet syndrome has therefore not been fully characterized, particularly in patients in their forties and over.
We aimed to gather more information on Dravet syndrome in adults in order to inform management. We undertook an observational study that was not intended to be a systematic study of prevalence in adults with severe epilepsy. Using both post-mortem and surgical brain tissue resources, we also aimed to undertake a detailed systematic neuropathological investigation of Dravet syndrome. We hypothesized that in the long term, Dravet syndrome would cause further broad neurological decline, and that years of encephalopathy would eventually lead to associated brain tissue damage and loss identifiable on neuropathological examination. By analysing clinical and neuropathological data, we sought to determine if Dravet syndrome could still be considered an epileptic encephalopathy later in life.
Materials and methods
This project was approved by the relevant local (Human) Research Ethics Committees with appropriate consent, or assent from relatives or legal guardians in the case of minors, adults with intellectual impairment and study of post-mortem tissue.
Patient ascertainment and phenotyping
We included adult patients from National Hospital for Neurology and Neurosurgery clinics. All available clinical and investigational information was reviewed.
Genetic testing
Details of DNA extraction and molecular analysis of the SCN1A gene with DNA sequencing and gene dosage analysis are given in the supplementary material. Parents of patients with a mutation were tested where possible with direct sequencing of the mutated SCN1A region or multiplex ligation-dependent probe amplification.
Genotype–phenotype analysis
The design of our study limits such analysis. We divided our cohort into: (i) paediatric cases with Dravet syndrome, with death before 12 years; (ii) adult cases with Dravet syndrome with death after 45 years; (iii) living adult Dravet patients; and (iv) living children with generalized epilepsy with febrile seizure plus. We looked at each of these groups for type of SCN1A mutations, and distribution of SCN1A missense mutations.
Neuropathology
The whole brain of three adult post-mortem cases with Dravet syndrome [who all met established criteria for Dravet syndrome (Commission, 1989)], two adult post-mortem disease controls with hippocampal sclerosis and three adult post-mortem controls with no known neurological disease were studied. Adult disease cases were former residents at the National Society for Epilepsy, Chalfont (Sander et al., 1993). As comparators for older post-mortem cases, we studied four paediatric post-mortem cases with Dravet syndrome, one anterior temporal lobectomy specimen from a child with intractable childhood epilepsy with generalized tonic–clonic seizures, left hippocampal sclerosis, operated at 12 years and a SCN1A mutation (referred to as SCN1A+ surgical case; Livingston et al., 2009), and one post-mortem brain from a child with severe febrile seizures in the genetic epilepsy with febrile seizures plus spectrum. We also had access to a brain biopsy obtained in childhood from an individual ascertained as an adult (Case 4).
Studies were undertaken to look for subtle malformations, hippocampal sclerosis (using standard qualitative, quantitative and immunohistochemical examination), cortical neuronal loss (qualitative examination), loss of specific cell populations (qualitative and semi-quantitative immunohistochemistry for interneurons), abnormalities of brainstem nuclei or tracts, distribution and quantitation of cells labelled with antibodies to Nav1.1 and for evidence of inflammatory and other disease processes [examination with antibodies to human leucocyte antigen (HLA)-DR and connexin-43 (Cx-43)].
Formalin-fixed post-mortem whole brains were sliced coronally along the anteroposterior axis and each slice was carefully re-examined for macroscopic abnormalities. Systematic histological sampling using blocks of 5 mm thickness were taken from several regions where possible: frontal (F1/F2), parietal, temporal and occipital cortex, insula, cingulate gyrus, cerebellum, hippocampus, amygdala, thalamus, basal ganglia, midbrain, pons, medulla and spinal cord at the cervical level. For two adult post-mortem cases with Dravet syndrome (Cases PM1/EP039 and PM3/EP099), additional blocks were taken from medial and orbital frontal cortex (Brodmann areas 6, 8 and 11), and insula. For the paediatric post-mortem cases, available sample blocks are shown in the supplementary material. Surgically resected temporal neocortex and hippocampal tissues were available for the SCN1A+ surgical case.
All blocks were processed in alcohol then xylene and embedded in paraffin within 1 week of sampling. Haematoxylin and eosin and Luxol fast blue stains were performed on sections from all regions.
Immunohistochemistry was performed on the post-mortem hippocampal, frontal cortical (F1/F2), cerebellar, pontine, medullary and spinal cord sections, and the surgically resected hippocampal and temporal neocortical sections. Details of the techniques and primary antibodies, including the panel used as markers of neurodegenerative processes, are given in the supplementary material.
Quantitative analysis
Pyramidal cell density was stereologically evaluated in the hippocampal cornu ammonis-1 and cornu ammonis-4 subfields of the adult post-mortem cases with Dravet syndrome and post-mortem hippocampal sclerosis controls. Areal Nav1.1-immunopositive counts were also undertaken in the hippocampal formation (dentate gyrus, cornu ammonis and subiculum) and one gyrus of the frontal cortex in the same cases. To obtain more information on patterns of cell loss, given that Dravet syndrome is considered an interneuronopathy (Mullen and Scheffer, 2009), we undertook interneuron counts. Details of methods are in the supplementary material.
Results
Demographic and clinical data are summarized in Table 1. Median age at last follow-up for the 22 adult cases with Dravet syndrome was 39 years (range 20–66 years). Detailed case histories of the adult post-mortem cases are given in the supplementary material.
Table 1.
Demographic and clinical features of the 22 adult (PM1-3, 4-22) and four paediatric cases with Dravet syndrome (PM23-26), and two other SCN1A mutation-carrying paediatric cases with other epilepsy syndromes (PM27, and 28/SCN1A+ surgical case)
| Case ID | Gender/age at follow-up or ‡age at death (yrs) | Age at onset (months), seizure type at onset | Identifiable trigger at seizure onset | Seizure types in childhood | Seizure types in adulthood | Development/autistic features/behavioural problems | Psychometry data | Intellectual outcome at last follow-upd | Other neurological signs | Functional outcome at last follow-up | SCN1A mutation/deletion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM1/EP039 | F/46‡ | 3, GTC | Vaccination (no further details) | GTC, CP | GTC, My, Fo, ‘drops’, SE, NCSE/fever sensitivity | Development delayed after seizure onset/no autistic features/behavioural problems | No formal neuropsychometry data | Severe | Pyramidal signs | Deceased | Missense |
| PM2/EP213 | M/66‡ | 11, GTC | None | My, GTC, NCSE | GTC, My, ‘drops’, NCSE | Development delayed after seizure onset/no autistic features/behavioural problems | Progressive cognitive decline, dementia from 55 yrs | Severe | Progressive ataxia, parkinsonism, dementia, cerebellar signs | Deceased | Not possible |
| PM3/EP099 | M/46‡ | 18, GTC | None | My, GTC, Fo | GTC, My, Fo | Development delayed after seizure onset/autistic features/ behavioural problems | 10 yrs, FSIQ 77, 17 yrs, FSIQ 57 | Severe | Cognitive slowing, dysarthria, ataxia | Deceased | Not possible |
| 4 | M/39 | 6, ND | Vaccination (whooping cough, 24 h) | GTC, My, hemiclonic, dyscognitive | GTC, My, CP, ‘drops’, dyscognitive, NCSE | Development regression after seizure onset/no autistic features/behavioural problems | No formal neuropsychometry data | Severe | Extra-pyramidal signs (choreoathetosis, dystonia), fixed contractures | No speech, institutionalized, full care, PEG, incontinent, wheelchair-bound | None detected |
| 5 | M/25 | 10, FS, hemiclonic | Fever | CP, My, GTC, SE, Dyscognitive, ‘drops’ | GTC, CP, ‘drops’, My, dyscognitive, SE | Development regression after seizure onset/autistic features/no behavioural problems | No formal neuropsychometry data; progressive, slow cognitive decline | Severe | Pyramidal signs (spasticity) | Lives at home with parents, behavioural problems, minimal speech (only repeats words) | Missense |
| 6 | M/60 | 12, GTC | Vaccination (whooping cough, 8 h) | GTC, clonic, dyscognitive, ‘drops’, SE | GTC, CP, My, T, SE, NCSE | Development regression from 6 yrs/ autistic features/behavioural problems | At 6 yrs went to mainstream school; At 27 yrs, VIQ 51, PIQ 58 | Severe | Not documented | Recognizes basic words, able to tell the time, PEG, recurrent respiratory infections, wheelchair-bound, incontinent, institutionalized | Truncating, del |
| 7 | M/41 | 9, GTC | Slight increased temperature | GTC, MJ, dyscognitive, SE | GTC, dyscognitive | Development regression from 15 mo/no autistic features/ behavioural problems | No formal neuropsychometry data | Severe | Marked scoliosis, gait abnormality | Walks unaided, with stooped posture and legs in semi-flexion; performs one-stage command | Splice donor, del |
| 8 | F/43 | 12, ND | No trigger documented | Dyscognitive, My | CP, dyscognitive, My | Development delayed after seizure onset/autistic features/ behavioural problems | No formal neuropsychometry data | Severe | Lives with parents, walks unaided but uses wheelchair for longer distances, speaks in short phrases, but mainly sign language, eats unaided, with spoon, recurrent respiratory infections | Missense | |
| 9 | F/27 | 8, FS | Fever | GTC, CP, dyscognitive, My, F, NCSE | GTC, dyscognitive, My, T | Development delayed after seizure onset/autistic features/ behavioural problems not documented | No formal neuropsychometry data, Cognitive decline in adulthood | Severe | Truncal ataxia, pyramidal signs, hand tremor, wide-based gait | Missense | |
| 10 | M/20 | 7.5, FS | Fever, vaccination (whooping cough, hours) | CP, GTC | CP, GTC, dyscognitive, SE/fever sensitivity | Development delayed after seizure onset/autistic features/ behavioural problems |
|
Moderate | Cerebellar signs, truncal and gait ataxia, action and postural tremor | Lives with parents, needs constant one-to-one care | Splice site |
| 11 | F/29 | 7, Feb SE | Fever, whooping cough infection | GTC, CP, SE, ‘drops’ | GTC, dyscognitive, CP, T, SE, NCSE | Development delayed after seizure onset/autistic features/no behavioural problems | No formal neuropsychometry data | Severe | Abnormal gait, pyramidal signs (hyper-reflexia) | Lives with parents, requires help for activities of daily living; able to walk unaided, occasional single words | Missense |
| 12 | M/43 | 7, GTC | Vaccination (whooping cough, timeline not documented) | GTC, CP | GTC, CP | Development delayed after seizure onset/no autistic features/no behavioural problems | At 42 yrs, MMSE = 20/30 | Mild | Extra-pyramidal signs (dystonic tremor, hypomimia, bradykinesia) | Lives with parents, self-caring with some help | None detected |
| 13 | M/21 | 12, ND | Vaccination (third dose of triple vaccination, 12 h) | My, GTC | GTC, dyscognitive, CP | Development regression after seizure onset/autistic features/behavioural problems | At 19 yrs, MMSE = 13/30 | Moderate | Not documented | Residential care, still behavioural problems | None detected |
| 14 | F/40 | 15, GTC | Vaccination (measles vaccination, several days) | GTC, My, dyscognitive | GTC, My, ‘drops’, dyscognitive | Development regression after seizure onset/autistic features/behavioural problems | No formal neuropsychometry data | Severe | Kyphosis, pyramidal signs | Residential care, speaks one or two words, performs simple orders, walks unaided | None detected |
| 15 | M/31 | 6, GTC | Vaccination (triple vaccine, 9 days) | GTC, dyscognitive, NCSE, My | GTC, dyscognitive, My | Development regression after seizure onset/autistic features/behavioural problems | No formal neuropsychometry data, But gradual decline | Severe | Gait ataxia | Nursing home, minimal communication, walks with help | None detected |
| 16 | F/48a | 2.5, hemiclonic | Vaccination (triple vaccine, 2 days) | Hemiclonic, CP, My, GTC | GTC, My, hemiclonic, ‘drops’, T, NCSE | Development delayed after seizure onset/no autistic features/behavioural problems | No formal neuropsychometry data, But gradual decline | Severe | Pyramidal signs | Deceased | None detected |
| 17 | M/21 | 3, FS | Fever | GTC, dyscognitive, ‘drops’, My | GTC, My, dyscognitive, SE, NCSE | Development delayed after seizure onset/no autistic features/no behavioural problems | No formal neuropsychometry data | Moderate | Action tremor, extra-pyramidal signs | Residential care, does basic domestic chores with prompting | None detected |
| 18 | F/26 | 3, FS | Fever, vaccination (no details) | My, CP, ‘drops’, T | GTC, T, CP | Development delayed after seizure onset/autistic features/behavioural problems | No formal neuropsychometry data | Severe | Intention tremor | Institutionalized | None detected |
| 19 | F/44 | 6, FS | Fever vaccination (pertussis, 2 days) | GTC, dyscognitive | GTC, CP | Development regression after seizure onset/autistic features/behavioural problems | No formal neuropsychometry data | Severe | Gait ataxia | Lives with parents, has carers, entirely dependent, doubly incontinent | Missense |
| 20 | F/39 | 10, FS | Fever | FS, GTC, ‘drops’, My | GTC, My, ‘drops’, T, SE | Development delayed after seizure onset/autistic features/behavioural problems | At 40 yrs, MMSE = 14/30 | Moderate | None documented | Institutionalized, feeds herself, requires help with domestic chores | Missense |
| 21 | F/23 | 4.5, Feb SE | Fever | CP, GTC, dyscognitive, ‘drops’ | GTC, T, CP | Development delayed from 9 mo/autistic features/behavioural problems | No formal neuropsychometry data | Severe | Kyphosis | Institutionalized, speech limited to one or two phrases, able to walk independently | One splice site del + two missense |
| 22 | M/33 | 4, GTC | No trigger documented | GTC, CP, My | T, GTC, My | Development delayed from 3 yrs/autistic features/behavioural problems | No formal neuropsychometry data; at 23 yrs, no speech, carries out some one-step commands | Severe | Wide-based gait | Institutionalized; no speech, walks with help, requires help with all activities of daily living | Delins |
| PM23 | M/2 | 5, a febrile GTC | No trigger documented | GTC, My. No FS | Not applicable | Development delayed from 18 mo | No formal neuropsychometry data | Mild global cognitive delay. Limited expressive language | None documented | Deceased | Whole gene deletion |
| PM24a | F/10 | 2, Feb SE | Fever | FS, My, CP, Abs, GTC, SE, At, Hemiclonic | Not applicable | Development never normal, regression at 5 yrs | No formal neuropsychometry | Severe (nonverbal) | Crouch gait | Deceased | Truncation |
| PM25a | M/11 | 8, SE | No trigger documented | GTC, recurrent SE, My, At, T (nocturnal), My Status, Fo | Not applicable | Developmental regression with seizure onset/autistic features/behavioural problems | No formal neuropsychometry | Severe | Ataxia and spasticity | Deceased | Splice site |
| PM26 | F/11 | 10, FS | Fever | FS, Abs, My, GTC, SE, CP, Hemiclonic | Not applicable | Developmental slowing from 10 months 4 yrs/behavioural problems | No formal neuropsychometry | Severe | Ataxia and tremulous | Deceased | No mutation detected; MLPA not done |
| PM27b | M/5 | 18, Feb SE | Fever | FS, Fo, GTC, SE | Not applicable | Normal development | No formal neuropsychometry | Normal | None | Deceased | Missense |
| 28/SCN1A+ surgicalc | M/12 | 10, FS | Fever | GC, CPS, F, GTC/fever sensitivity | Not applicable | Development delayed clear from 3 yrs/autistic features/behavioural problems | No formal neuropsychometry data | Moderate | None documented | In a special school, moderate global intellectual disability | Missense |
Abs = absence; At = ; CP = complex partial; delins = deletion/insertion; ‘drops’ = ‘drop attacks’; F = female; Feb SE = febrile status epilepticus; Fo = focal; FS = febrile seizure; FSIQ = full-scale IQ; GC = generalized clonic; GTC = generalized tonic–clonic; HS = hippocampal sclerosis; IED = interictal epileptiform discharges; M = male; MJ = myoclonic jerks; MLPA = Multiplex Ligation-dependent Probe Amplification; mo = months; My = myoclonic; NCSE = non-convulsive status epilepticus; PEG = percutaneous endoscopic gastrostomy; PIQ = performance IQ; PM = post-mortem; SE = convulsive status epilepticus; T = tonic; VIQ = verbal IQ; WM = white matter; yrs = years; ND = undetermined seizure type.
a Described in Wallace et al., 2003.
b Described in Harkin et al., 2007; and Deng et al., 2007.
c Described in Livingston et al., 2009.
d Classification of intellectual outcome at last follow-up as described in McIntosh et al., 2010.
‡age at death.
For 11 patients with Dravet syndrome, a close temporal relation of seizure onset with vaccination (Table 1) was documented, as previously described (Berkovic et al., 2006; McIntosh et al., 2010).
Family history
There was a family history of epilepsy and/or febrile seizures in nine adult patients (Supplementary Table 3), and another adult patient had a sibling who had had one isolated seizure. Case 20 comes from a family with genetic epilepsy with febrile seizures plus. Case 6 has a 15-year-old sister with microcephaly, quadriparesis, profound cognitive impairment and spasms, who is on anti-epileptic drugs, but does not carry the SCN1A mutation found in her brother (Case 6), nor SCN1A deletion or duplication.
Clinical condition and evolution in adulthood
From onset in infancy, there was no period of seizure freedom recorded. In two patients, recognition of a false ‘seizure-free period’ in childhood led to anti-epileptic drug cessation, but increased seizure severity and frequency led to recommencement of anti-epileptic drugs, and in retrospect the parents could recognize subtle seizures had never ceased to occur. All patients had multiple anti-epileptic drugs with differential control of different seizure types (Table 2), but not complete seizure freedom.
Table 2.
Anti-epileptic drug history
| Case ID | Anti-epileptic drug changes after diagnosis of Dravet syndrome | Improvement with anti-epileptic drug changes after diagnosis (seizure control/cognition) | All known previous anti-epileptic drug history | Other treatment | Improvementa (seizure types) | Documented worseninga (seizure types) |
|---|---|---|---|---|---|---|
| PM1/EP039 | N/A | N/A | CBZ, CLB, GBP, LTG, PB, PHT, VPA | PHT (GTC), VPA | PHT (My) | |
| PM2/EP213 | N/A | N/A | CBZ, CLB, PB, PHT, PRM, VPA | CBZ, PHT | ||
| PM3/EP099 | N/A | N/A | ACZ, CBZ, CLB, PB, PHT, PRM, VGB, VPA | |||
| 4 | No new anti-epileptic drug started | N/A | CBZ,CLB, CNZ, LTG, PHT, PRM, SLT, VGB, VPA | PB, VPA | ||
| 5 | Stopped CBZ; reintroduced VPA; started STP + VPA; decreased LTG | Seizure control improved cognition | CBZ, GBP, LEV, LTG, OXC, PHT, STP, TGB, TPM, VGB, VPA | PHT (GTC), STP + VPA | CBZ, OXC (‘drop attacks’) | |
| 6 | Started LEV; reduced CBZ | Seizure control improved cognition improved | CBZ, GBP, PB, PHT, PRM, SLT, VGB, VPA | Stereotactic anterior thalamotomy, mephenytoin, phenacemide, benuride | LEV (GTC), PRM,VPA | |
| 7 | Stopped CBZ | Seizure control unchanged cognition N/A Short follow-up | CBZ, CNZ, DZP, LEV, PB, PHT, PRM, VPA | VNS | CNZ | |
| 8 | No changes made | N/A | PRM, TPM, VPA | PRM, VPA | ||
| 9 | No new anti-epileptic drug started | N/A | CBZ, CLB, LEV, LTG, NTZ, OXC, PB, TPM, VGB, VPA | KD | CLB, KD, VPA | |
| 10 | Increased ZNS: suggested STP, not yet started | N/A | CBZ, CLB, ESX, LEV, LTG, PB, PGB, PHT, TPM, VGB, VPA, ZNS | VPA, ZNS | LTG (‘drop attacks’), PGB | |
| 11 | No new anti-epileptic drug started | N/A | ACZ, CBZ, ESX, GBP, LEV, LTG, NTZ, PB, PHT, PRM, VGB, VPA | ACTH, corticosteroids, VNS, KD, GOS exclusion diet | TPM | LTG |
| 12 | No new anti-epileptic drug started | N/A | ACZ, CBZ, CNZ, LEV, LTG, PB, PHT, PRM, SLT, VGB, VPA | SLT, VPA | ||
| 13 | No new anti-epileptic drug started | N/A | CLB, LEV, LTG, OXC, VGB, VPA | Prednisolone | VPA, LEV (stopped GTC) | |
| 14 | N/A | N/A | CBZ, CNZ, DZP, ESX, LTG, NTZ, PHT, VGB | KD, ethotoin | ||
| 15 | No new anti-epileptic drug started | N/A | CBZ, CLB, CNZ, ESX, LTG, NTZ, PB, VPA | CLB, ESX (dyscognitive), LEV, VPA | ||
| 16 | N/A | N/A | CBZ, CLB, DZP, LEV, LTG, NTZ, OXC, PB, PGB, PHT, VPA | CBZ, VPA | OXC (My) | |
| 17 | No new anti-epileptic drug started | N/A | CLB, CNZ, DZP, ESX, LEV, LTG, TPM, VPA, PIR | pyridoxine, biotin | VPA (GTCS) | |
| 18 | Started VPA |
|
CBZ, CLB, GBP, LEV, LTG, TPM, VPA | VPA | ||
| 19 | No new anti-epileptic drug started. Stopped LCM; suggested STP, not yet started | N/A | CBZ, CLB, LCM, LEV, LTG, VPA, TPM, ZNS | LCMa | ||
| 20 | No new anti-epileptic drug started | N/A | CBZ, CLB, CNZ, ESX, LEV, LTG, NTZ, PB, PHT, PIR, TPM, VGB, VPA | KD | ||
| 21 | Started STP (+CLB), later stopped, tapered RUF; restarted VPA. |
|
CBZ, CLB, CNZ, GBP, LEV, LTG, PB, PHT, RUF, STP, TGB, TPM, VGB, VPA | pyridoxine | TPM, VPA, STP | RUFa, VGBa, CBZa, LTGa |
| 22 | Stopped PGB; started ZNS | Seizure control improved cognition improved | ACZ, CBZ, CLB, CNZ, DZP, GBP, LEV, LTG, NTZ, PGB, PIR, VGB, VPA, ZNS | CBZ, (GTC), CLB, LEV, PIR (My), VPA, ZNS | CBZ (My), GBP (My), LTGa, PGBa | |
| PM23 | N/A | N/A | VPA | VPA (My) | ||
| PM24 | N/A | N/A | CLB, CNZ, LEV, LTG, STP, TPM, VPA | pyridoxine | STP | LTG |
| PM25 | N/A | N/A | CBZ, CNZ, DZP, LTG, PB, PHT, STP, TPM, VGB, VPA | Steroids, VNS, KD | STP, VNS | – |
| PM26 | N/A | N/A | CBZ, CNZ, GBP, LTG, TPM, VPA | None | LTG | GBP |
| PM27 | N/A | N/A | LTG, VPA | None | ||
| 28/SCN1A+ surgical | N/A | N/A | No data available | Ant TLx |
a Data on which specific seizure types improved or worsened are not always available for every antiepileptic drug.
Abs = absences; ACTH = adrenocorticotrophic hormone; ACZ = acetazolamide; Ant TLx = anterior temporal lobectomy with amygdalo-hippocampectomy; CBZ = carbamazepine; CLB = clobazam; CNZ = clonazepam; DZP = diazepam; ESX = ethosuximide; GBP = gabapentin; GOS = Great Ormond Street; GTC = generalized tonic–clonic; KD = ketogenic diet; LCM = lacosamide; LEV = levetiracetam; LTG = lamotrigine; My = myoclonic; N/A = not available; NTZ = nitrazepam; OXC = oxcarbazepine; PB = phenobarbital; PGB = pregabalin; PHT = phenytoin; PIR = piracetam; PRM = primidone; RUF = rufinamide; SLT = sulthiame; STP = stiripentol; TGB = tiagabine; TPM = topiramate; VGB = vigabatrin; VNS = vagal nerve stimulator; VPA = sodium valproate; ZNS = zonisamide.
There was an evolution of seizure semiology and predominance of certain seizure types with time (Supplementary Table 3). There was no single pattern for seizure evolution for all patients.
All patients had multiple seizure types in adulthood (Table 1). For 10 patients, seizures were mostly nocturnal and comprised brief tonic or tonic–clonic seizures. Seizures were recorded in video-EEG telemetry for 10 adults; seizures observed were complex motor, dyscognitive, tonic or secondarily generalized with focal EEG onset pattern or no recognizable EEG change. Myoclonus was not prominent in adulthood, though its frequency may have been under-reported. No adult patient in our series had documented absences; all ‘absence-like’ (dyscognitive) seizures recorded in adulthood had focal EEG onset or no EEG change documented. Fever sensitivity persisted into adulthood, with even slight variations of temperature sufficient to trigger seizures in nine patients. No patient had any meaningful seizure-free period. Non-convulsive status epilepticus was documented with EEG on at least one occasion in seven patients. Triggers included inter-current infections and slight increases in body or ambient temperature.
Behavioural problems or ‘autistic-like’ features were observed at some time of the evolution in most patients in our series (Table 1).
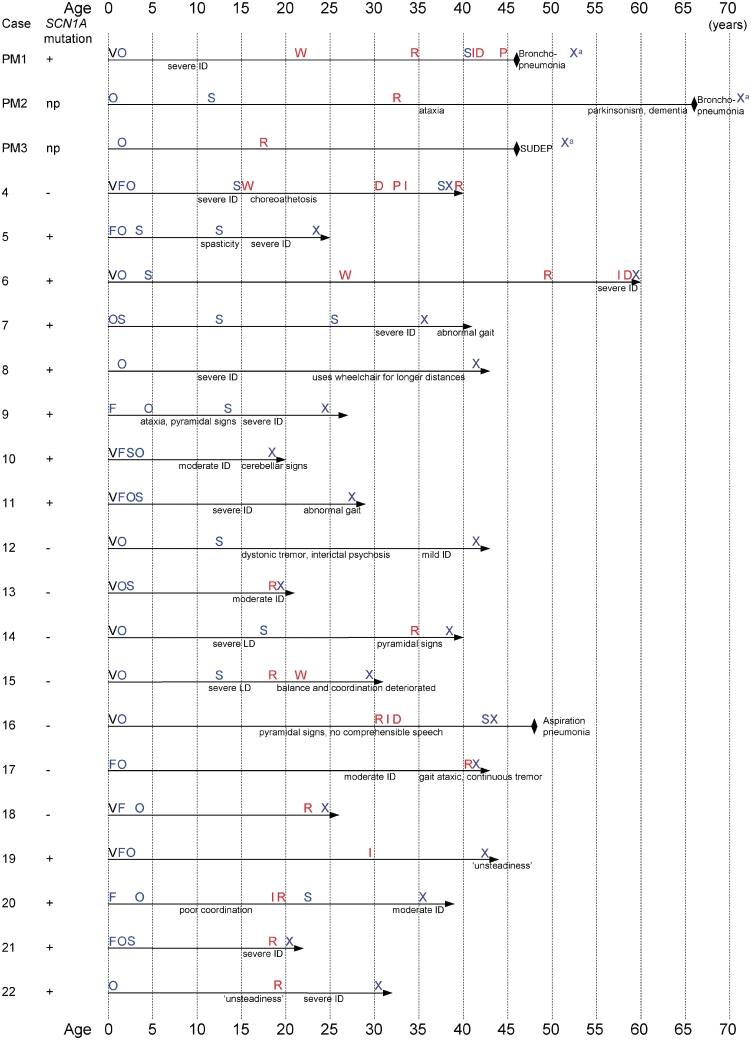
At last follow-up, the oldest living patient was 60 years of age. Sixteen patients were in residential care; the remainder lived at home with support. Neurological deterioration continued throughout life in all patients, with further impairment of speech, mobility and ability for daily activities (Table 1 and Fig. 1). Kyphoscoliosis was documented in six patients. Cerebellar signs were found in five patients, pyramidal signs in seven and extra-pyramidal in four patients. Non-ictal urinary incontinence occurred late in the evolution. The majority (18/22) of adult patients had severe intellectual disability (as classified in McIntosh et al., 2010) at last follow-up (Table 1).
Figure 1.
Timelines in Dravet syndrome—milestones in disease evolution. D = dysphagia; F = febrile seizure; I = incontinence; ID = intellectual disability; np = not possible; O = onset of afebrile seizures; P = percutaneous endoscopic gastrostomy (PEG); R = residential care; S = status epilepticus; SUDEP = sudden unexplained death in epilepsy; V = vaccination; X = diagnosis; W = wheelchair-dependent; a = diagnosis made after death; black diamond = death; horizontal arrow = living patient; + = SCN1A change found; − = no SCN1A change found.
Recurrent respiratory infections were documented in six patients. Dysphagia emerged as a late feature in five patients, documented in or after the fourth decade of life, leading eventually to percutaneous endoscopic gastrostomy. One adult patient died during the follow-up period from repeat aspiration pneumonia. No post-mortem brain tissue was available for review from this case.
Anti-epileptic drugs and non-pharmacological treatments are listed for each case in Table 2, as well as changes to anti-epileptic drugs after the diagnosis of Dravet syndrome was made, and their impact on seizure control, cognitive function and quality of life. Seven patients had already had drug changes instituted following diagnosis, but in only three had sufficient follow-up elapsed to evaluate the effect of the changes. There was improvement in seizure control even after years of drug resistance in three cases, with significant additional improvement in cognition and quality of life in adulthood in two. In the four of the seven patients who had had drug changes with a shorter period of follow-up, some early indication of benefit for some seizure types at least was apparent in three (the one patient with no or minor change in seizure frequency had stopped carbamazepine, but not yet started any new anti-epileptic drug).
At last follow-up, most patients were on anti-epileptic drug polytherapy. No patient was seizure-free, but in several cases secondarily generalized seizures were controlled with medication.
Causes of death in our adult series (Table 3) included three cases of bronchopneumonia, and one case of sudden unexplained death in epilepsy. In the paediatric Dravet syndrome group, three died from sudden unexplained death in epilepsy and one had global ischaemic brain injury; it is unclear for the latter case whether there was a seizure followed by cardiorespiratory arrest. No adult case in our series died of convulsive status epilepticus.
Table 3.
Summary of neuropathological findings: macroscopic findings, and results of histological staining with haematoxylin and eosin, Luxol fast blue and cresyl violet
| Case ID | Macroscopic findings (brain weight post-fixation) | Cortex: frontal (F1/F2, medial, orbital), parietal, temporal and occipital | Medial and subcortical structures: hippocampus, amygdala, thalamus, basal ganglia | Cerebellum: vermis and cerebellar hemispheres | Brainstem: midbrain, pons, medulla, and cranial nerve nuclei; cervical spinal cord | Cause of death (age at death, in years) |
|---|---|---|---|---|---|---|
| PM1/EP039 | Cerebellar atrophy, with preferential involvement of the anterior lobe and vermis (1331 g) | Normal | Normal | Loss of Purkinje cells | Myelin loss in dorsal columns of spinal cord | Bronchopneumonia and recurrent NCSE (46 yrs) |
| PM2/EP213 | Mild cerebellar atrophy; discolouration and loss of periventricular white matter; old frontobasal contusion (1100 g) | Focal periventricular white matter and myelin loss | Normal | Mild Purkinje cells loss | Myelin loss in dorsal columns of spinal cord | Bronchopneumonia (66 yrs) |
| PM3/EP099 | Cerebellar atrophy (1380 g) | Frontopolar, dorsal frontal and occipital cortex, with ‘micro-columnar’ architecture | Normal | Loss of Purkinje cells | Normal | Sudden unexplained death in epilepsy (46 yrs) |
| PM23 | Normal. Some leptomeningeal congestion (1273 g) | Normal | Mild bilateral endfolium hippocampal gliosis. No mossy fibre sprouting. | Mild patchy gliosis but no discernable Purkinje cell loss. | Normal brainstem. Cord not available | Sudden unexplained death in epilepsy (2 yrs) |
| PM24 | Normal (1062 g) | Frontal and occipital cortex: normal | Hippocampus (one side): no sclerosis, cornu ammonis-1 hyperconvoluted. | Purkinje cells preserved. Mild vacuolation of white matter noted. | Normal | Sudden unexplained death in epilepsy during a 46°C day in Australia (10 yrs) |
| PM25 | Swollen brain with herniation (1300 ga) | Frontal and temporal: widespread ischaemic neurons. No MCD or evidence of chronic atrophy | Not all subfields available for histology. Cornu ammonis-1 shows acute neuronal changes but no evidence of chronic sclerosis | Acute injury of Purkinje cells superimposed on mild chronic loss | No malformation. Ischaemic neurons noted in medulla | Sudden unexplained death in epilepsy (11 yrs) |
| PM26 | Swollen brain (1245 ga) | Frontal and temporal. No MCD and no atrophy | No sclerosis (mild endfolium gliosis) | Autolytic changes but no evidence of chronic atrophy | No histology | Global ischaemic brain injury (11 yrs) |
| PM27 | Leptomeningeal congestion and uncal grooving but no tonsillar herniation (1266 g) | Frontal cortex: normal architecture but pan cortical necrosis and reactive changes consistent with cerebral infarction of 10 days | Hippocampus (one side): no evidence of chronic hippocampal sclerosis but acute anoxic changes to end-folium neurons | Autolytic changes but no evidence of atrophy/Purkinje cell loss | Normal | Convulsive status epilepticus (5 yrs) |
| 28/SCN1A+ surgicalb | Not applicable | Normal temporal neocortex | Pyramidal cell loss in left hippocampus | Not applicable | Not applicable | Not applicable |
| Control 1/EP296 | Modest dilatation of lateral ventricles, left hippocampal formation significantly smaller than right (1156 g) | Normal | Pyramidal cell loss in the left hippocampus | Loss of Purkinje cells | Normal | Sudden unexplained death in epilepsy (49 yrs) |
| Control 2/EP038 | Not available | Cell loss in upper cortical layers of parietal and temporal cortices | Pyramidal cell loss in both hippocampi | Loss of Purkinje cells | Normal | Pulmonary oedema (74 yrs) |
| Control 3 | Normal (1185 g) | Normal | Normal | Normal | Normal | Cardiac arrest (36 yrs) |
| Control 4 | – | Normal | Normal | Normal | Normal | Not available (58 yrs) |
| Control 5 | Normal (1540 g) | Normal | Normal | Loss of some Purkinje cells | Normal | Not available (57 yrs) |
a For these cases, pre-fixation brain weight is presented, no post-fixation brain weight available.
b For case 28/SCN1A+ surgical, only the resected hippocampus and temporal neocortex were available for study.
MCD = malformation of cortical development; NCSE = non-convulsive status epilepticus; yrs = years.
Neuroimaging findings
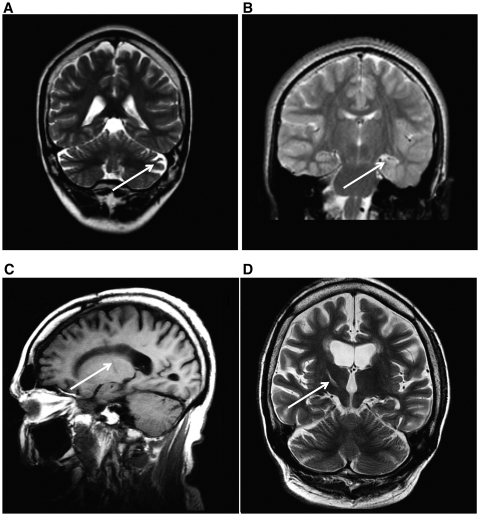
MRI with or without light sedation was successful in all but four of the adult cases with Dravet syndrome. Most frequently, brain imaging was normal, or showed non-specific findings, including cerebral and cerebellar atrophy, or cerebellar atrophy alone (Fig. 2A). One adult case with SCN1A mutation had unilateral hippocampal sclerosis on MRI performed at 22 years of age (Fig. 2B). Evidence of the anterior thalamotomy performed at the age of 16 years was seen for Case 6 (Fig. 2C and D).
Figure 2.
Brain MRI findings in adults with Dravet syndrome and SCN1A mutation. Cerebellar atrophy (A, sagittal T1, Case 6) was a feature in some cases. Case 21 was the only adult case with Dravet syndrome in our series with hippocampal sclerosis (left in this case) evident on MRI (B, coronal T2). Case 6 had a stereotactic thalamotomy at the age of 16 years (C, sagittal T1 and D, coronal T2). Arrows show the location of the main abnormalities in each image.
Electroencephalography findings
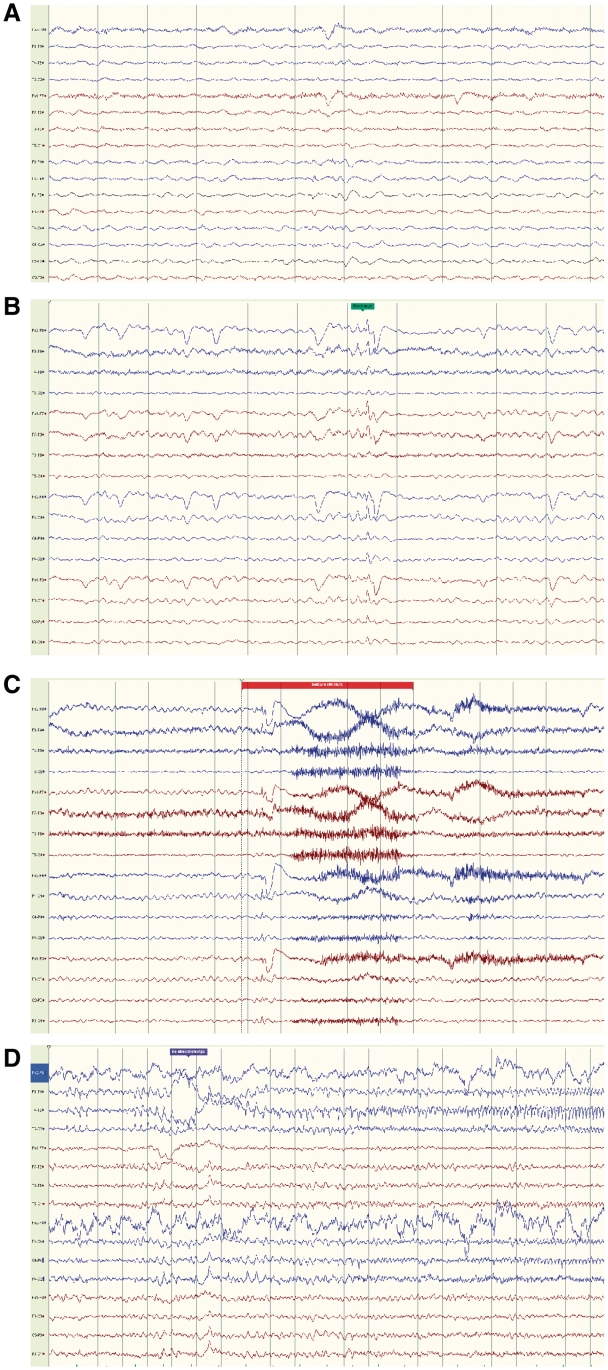
Serial EEG data were available for 21 adult patients. At least 1 seizure was recorded with video-EEG for 10 patients; seizure types recorded included tonic, focal motor, dyscognitive and secondarily generalized. Focal EEG features (Fig. 3A–D) were recorded in 17 of our adult cases. Ictal EEG onset was maximal in the frontocentral regions in four cases (Fig. 3C).
Figure 3.
EEG findings. For Case 6, routine EEG showing background of bilateral diffuse slow activity at 3–5 Hz, and very rare low amplitude sharp waves/spikes, more apparent in frontal regions, right > left (A, bipolar montage). For Case 5, video–EEG telemetry at the age of 26 years, showed bihemispheric cortical dysfunction and bifrontal interictal epileptiform discharges (B, bipolar longitudinal montage). Several complex motor seizures were recorded, some with non-lateralized frontocentral EEG onset (C, combined longitudinal and transverse bipolar montage). Electrographic seizures were also recorded with right posterior temporal pattern (D, bipolar longitudinal montage).
Interictal EEG in all adult cases showed slow background activity. For 10 adults, childhood EEG data were available: four had one previous EEG in early childhood with generalized epileptiform discharges. No generalized epileptiform discharges were seen in the EEGs in adulthood; focal features were seen (focal or multifocal interictal epileptiform discharges; focal ictal discharges; Supplementary Table 3).
Non-convulsive status epilepticus was documented on video-EEG in two patients, for whom subtle seizures with predominant impairment of consciousness had previously been confused with behavioural problems.
Genetic findings
Twenty adult patients had genetic analysis: SCN1A mutations were found in 12 adult cases (Table 4; Figure 11). The mutations were all different, and all but one patient had novel mutations. One patient (Case 21) was found to have three SCN1A mutations, which to the best of our knowledge has not been previously described in the literature. We have not screened other genes for mutations in our patients. For the four adults where both parents have been tested, the mutations were de novo. We were unable to extract DNA of adequate quality from formalin-fixed paraffin-embedded brain tissue for two adult cases (PM2/EP213 and PM3/EP099). Of the four paediatric post-mortem cases with Dravet syndrome, two had SCN1A mutation, one had a whole gene deletion and one was not found to have a mutation but has not yet been checked for deletions. The two other paediatric cases, one surgical case with intractable childhood epilepsy with generalized tonic–clonic seizures, and one post-mortem case in the genetic epilepsy with febrile seizures plus spectrum, both had SCN1A mutations previously documented (Table 4).
Table 4.
SCN1A structural variation identified in this study
| Case ID | Nucleotide changes | Exon/intron | Mutation type | Inheritance | Amino acid change | Protein domain | Variation in the same position on the SCN1A variant database (http://www.molgen.ua.ac.be/SCN1AMutations) |
|---|---|---|---|---|---|---|---|
| PM1/EP039 | c.677C > A | Exon 5 | Missense | Not determined (parents unavailable) | p.Thr226Lys | DI-S4 | c.677C > T, p.Thr226Met, de novo (Harkin et al., 2007) |
| 5 | c.4913T > C | Exon 26 | Missense | De novo (parents and one sister analysed) | p.Ile1638Thr | DIV-S4 | None in that position; one c.4911_4914delGATC,p.I1638VfsX11 (Depienne et al., 2009b) |
| 6 | c.992delT | Exon 7 | Truncating | Not determined (no parent analysed) | p.Leu331X | DI-S5-S6 | Two: c.992dupT,p.Leu331fs, de novo; 992[T]993ins,L331fsX339 (Mancardi et al., 2006) |
| 7 | c.264 + 3delAGTG | Intron 1 | Splice donor, deletion | Not determined (no parent analysed) | p.? | – | One c.264 + 5G > A, de novo (Mancardi et al., 2006) |
| 8 | c.5639G > A | Exon 26 | Missense | Not determined (one parent analysed, mother negative) | p.Gly1880Glu | COOH terminal | None found in this position |
| 9 | c.3797A > C | Exon 19 | Missense | De novo | p.Glu1266Ala | DIII-S2 | None found in this position |
| 10 | c.603-2A > G | Intron 4 | Splice site | De novo | p.? | – | None found in this position |
| 11 | c.4384T > C | Exon 23 | Missense | De novo | p.Tyr1462His | DIII-S6 | one c.4385A > G,p.Tyr1462Cys (Zucca et al., 2008) |
| 19 | c.2792G > Aa | Exon 15 | Missense | Not determined | p.Arg931His | DII-S5-S6 | Löfgren and DeJonghe, personal communication, 2010 |
| 20 | c.4568T > C | Exon 24 | Missense | Not determined (no parent analysed) | p.Ile1523Thr | DIII-DIV | None found in this position |
| 21 | c.80G > C; c.3749C > T; c.3706-2A > Gb | Intron 18 | Missense; missense; one splice acceptor mutation | Not determined (no parent analysed) | p.Arg27Thr; p.Thr1250Met; aberrant splicing (p.?) | N-terminal; DIII- S2; - | None found in this position; none found in this position; c.3706-2A > G, inheritance not determined (Singh et al., 2009; Löfgren and DeJonghe, personal communication, 2010) |
| 22 | c.2717_2727delinsAC | Exon 15 | In-frame deletion mutation | Not determined (no parent analysed) | p.Val906_Met909delinsAsp | DII-S5 | None found in this position |
| PM23 | N/A | Whole SCN1A gene | Whole SCN1A gene deletion | De novo | N/A | N/A | (Marini et al. 2009; Depienne et al., 2009b) |
| PM24 | c.5536_5539delAAAC | Exon 26 | Truncation | De novo | p.Lys1846fsX1856 | COOH terminal | (Case previously reported in Wallace et al., 2003). Claes et al., 2001; Kearney et al., 2006; Mancardi et al., 2006; Harkin et al., 2007; Zucca et al., 2008; Depienne et al., 2009b; Löfgren and DeJonghe, personal communication, 2010) |
| PM25 | IVS22-14T > G | Intron 22 | Splice site | De novo | p.? | DIIIS5-S6 | (Case previously reported in Wallace et al., 2003) |
| PM27 | c.4970G > A | Exon 26 | Missense | De novo | p.Arg1657His | DIV-S4 | (Case previously reported in Harkin et al., 2007; Deng et al., 2007) |
| 28/SCN1A+ surgical | c.652T > C | Exon 5 | Missense | Inherited (mother and sister have the same mutation) | p.Phe218Leu | DI-S4 | (Case previously reported in Livingston et al., 2009) |
SCN1A variant database (http://www.molgen.ua.ac.be/SCN1AMutations) (Claes et al., 2009).
Intronic changes nomenclature: ex. c.xx + 1G > C refers to the +1 intron position following coding base xx, with + or − sign denoting the intronic 5′-beginning or 3′-ending, respectively. p.? denotes an unknown effect on the protein, an effect is expected but difficult to predict.
All mutations found are novel, except: a c.2792G > A, previously reported by Löfgren A, DeJonghe P, personal communication, 2010.
b c.3706-2A > G (Singh et al., 2009).
del = deletion; dup = duplication; ins = insertion; N/A = not applicable or not available.
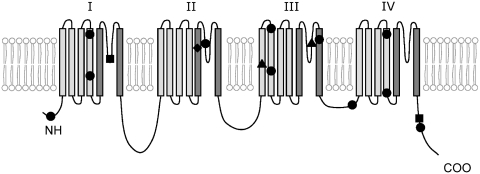
Figure 11.
Schematic representation of the SCN1A mutations found in our study (Table 4). SCN1A protein scheme adapted from Harkin et al. (2007). The protein has four domains, I–IV, each consisting of six transmembrane segments, S1–S6. Circle = missense; square = truncating; triangle = splice-site mutation; diamond = in-frame deletion. Positioning of the mutations within segments is approximate.
Genotype–phenotype associations are summarized in Table 5. In the paediatric Dravet post-mortem subgroup, we did not observe missense mutations (Table 5); in the adult Dravet deceased subgroup for whom genetic analysis was possible, 50% had an SCN1A missense mutation. Both children with genetic epilepsy with febrile seizures plus phenotype had missense mutations. For the 17 adult patients living with Dravet syndrome, eight had missense mutations. Additional information is provided in the supplementary material.
Table 5.
Genotype-phenotype analysis: SCN1A mutation type, and distribution of SCN1A missense mutations
| Case ID | Type of SCN1A mutation | Distribution of SCN1A missense mutations |
|---|---|---|
| Children with Dravet syndrome, death between 2 and 11 years (n = 4, PM23–PM26) | Truncating—1 | No missense mutation found |
| Whole-gene deletion—1 | ||
| Splice site—1 | ||
| No mutation, no result yet for deletion—1a | ||
| Children with genetic epilepsy with febrile seizures plus, one alive, 12 years, one death at 5 years (n = 2, 28 and PM27) | Missense—2 | S4—2 |
| Adults with Dravet syndrome, death between 46 and 66 years (n = 4, PM1–PM3 and 16) | Missense—1 | S4—1 |
| No mutation, no deletion—1 | ||
| No genetic analysis possible—2b | ||
| Adults with Dravet syndrome, alive, 20–60 years (n = 18, Patients 4–15 and 16–22) | Missense—8c | S4—2 |
| Truncating deletion—1 | S5–S6—1 | |
| Splice site —3c | S6—1 | |
| Insertion/deletion—1 | Others—4c | |
| No mutation or deletion found—7 | – S2—2c | |
| – DIII–DIV—1 | ||
| – C-terminal—1 |
a For one child with Dravet, who died, the result was not available regarding the presence of deletion, after a negative mutation analysis.
b For two adults with Dravet, who died, it was not possible to perform genetic analysis on the post-mortem material.
c Patient 21 had three SCN1A mutations found, two missense and one splice acceptor.
D = (SCN1A protein) domain; genetic epilepsy with febrile seizures plus = genetic epilepsy with febrile seizures plus; S = (SCN1A protein) segment.
Neuropathology
The macroscopic findings and results from histological and immunohistochemical studies are summarized in Tables 3 and 6, respectively.
Table 6.
Summary of neuropathological findings: immunohistochemistry
| Case ID | Brain region | Neuronal nuclei | Nav1.1 | Calretinin | Calbindin | Parvalbumin | Neuropeptide Y | GFAP | HLA-DR | Cx43 | von Willebrand factor | Dynorphin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM1/EP039 | Brainstem: midbrain, pons, medulla, cervical spinal cord | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND |
| PM2/EP213 | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND | |
| PM3/EP099 | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND | |
| 28/SCN1A+ surgicala | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Control 1/EP296 | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND | |
| Control 2/EP038 | ND | ND | + | + | + | ND | ND | ND | ND | ND | ND | |
| PM1/EP039 | Cerebellum: one of the cerebellar hemispheres | *loss | + | + | *loss | + | + | ++ | ++ | + | + | ND |
| PM2/EP213 | + | + | + | + | + | + | + | + | + | + | ND | |
| PM3/EP099 | *loss | + | + | *loss | + | + | ++ | ++ | + | + | ND | |
| 28/SCN1A+ surgicala | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ND | |
| Control 1/EP296 | *loss | + | + | *loss | + | ++ | + | + | + | + | ND | |
| Control 2/EP038 | *loss | + | + | *loss | + | + | ++ | ++ | + | + | ND | |
| PM1/EP039 | Hippocampus | + | + | + | + | + | + | + | + | ++ | + | + |
| PM2/EP213 | + | + | + | + | + | + | ++ | + | ++ | + | + | |
| PM3/EP099 | + | + | + | + | + | + | + | + | ++ | + | + | |
| 28/SCN1A+ surgicala | *loss | + | + | + | *loss | ++ | ++ | ++ | ++ | + | ++ | |
| Control 1/EP296 | *loss | + | + | + | + | ++ | ++ | ++ | ++ | + | + | |
| Control 2/EP038 | *loss | + | + | + | + | ++ | ++ | ++ | ++ | + | ++ | |
| PM1/EP039 | Frontal cortex: F1,F2 | + | + | + | + | + | + | + | + | + | + | ND |
| PM2/EP213 | + | + | + | + | + | + | + | + | + | + | ND | |
| PM3/EP099 | + | + | + | + | + | + | + | + | + | + | ND | |
| 28/SCN1A+ surgicala | + | + | + | + | + | ++ | + | + | + | + | ND | |
| Control 1/EP296 | + | + | + | + | + | ++ | + | + | + | + | ND | |
| Control 2/EP038 | *loss | + | + | + | + | ++ | ++ | + | + | + | ND |
Immunolabelling appeared increased (++), similar (+) or decreased (−) compared with controls.
*loss = cell loss; N/A = tissue unavailable for examination; ND = immunohistochemistry not performed.
a For SCN1A+ surgical case, only the resected hippocampus and temporal neocortex were available for study.
Routine histological stains
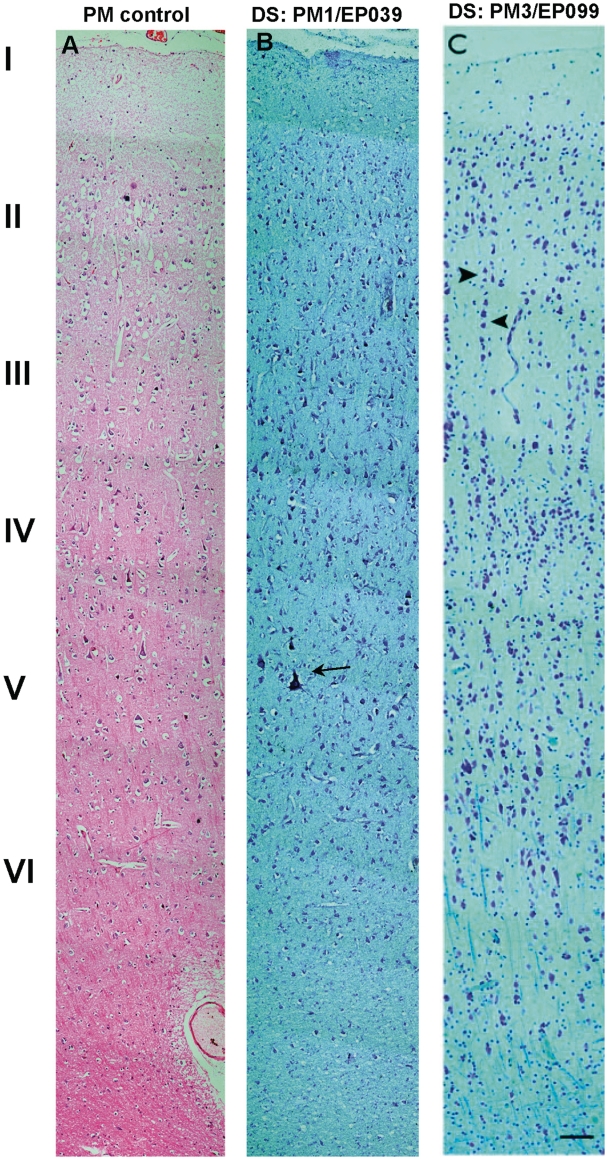
The frontal cortex of two adult post-mortem cases with Dravet syndrome (PM1/EP039 and PM2/EP213) showed an ordered and preserved hexalaminar architecture with no neuronal cell loss, similar to the frontal cortex of post-mortem controls with no known neurological disease (Fig. 4A and B). The cortex in the frontopolar, dorsal frontal and occipital regions of one adult post-mortem case with Dravet syndrome (PM3/EP099) showed a ‘micro-columnar’ architecture, with exaggeration of the vertical alignment of cortical neurons (Fig. 4C), but these changes did not amount to focal cortical dysplasia type I (Blümcke et al., 2011). The cytoarchitecture of the parietal, temporal and occipital cortices of all adult post-mortem cases with Dravet syndrome and controls appeared normal, apart from cell loss observed in the upper cortical layers of the parietal and temporal cortex of the hippocampal sclerosis post-mortem control case (Control 2/EP296). The temporal cortex of the SCN1A+ surgical case was well preserved, retaining hexalaminar architecture with no neuronal cell loss noted.
Figure 4.
Frontal cortex—histological staining. (A) Haematoxylin and eosin shows the normal frontal cortex from a post-mortem control with no known neurological disease. (B) Cresyl violet shows the motor cortex of the adult Dravet syndrome (DS) case, PM1/EP039, with good preservation of the cortical laminae and Betz cells (arrow). (C) Cresyl violet and Luxol fast blue show the frontal cortex from the adult post-mortem Dravet syndrome case, PM3/EP099, with a focal ‘micro-columnar’ appearance (arrowheads to columnar alignment). Haematoxylin and eosin-stained section is 7 µm thick while Luxol fast blue and cresyl violet-stained sections are 14 µm. Scale bar = 100 µm.
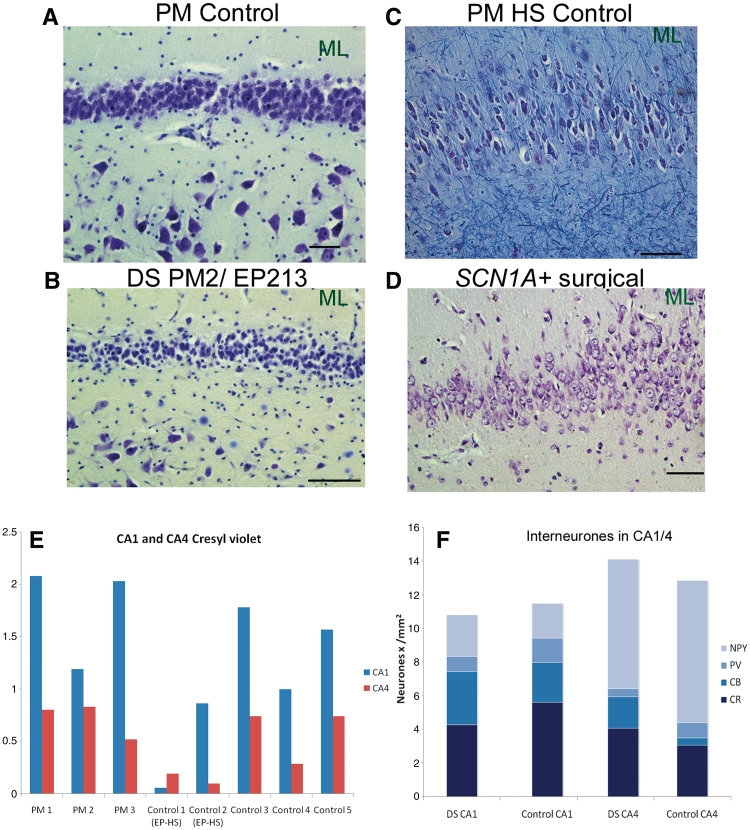
The hippocampi of all adult post-mortem cases with Dravet syndrome showed preservation of neurons in all cornu ammonis subfields, similar to post-mortem controls with no known neurological disease (Fig. 5A and B), and distinct from hippocampal sclerosis post-mortem controls (Fig. 5C) and the SCN1A+ surgical case (Fig. 5D). Neuronal preservation in Dravet syndrome hippocampi was confirmed by stereological quantification of cresyl violet-stained pyramidal cells in cornu ammonis-1 and cornu ammonis-4 (Fig. 5E). The dentate gyrus of all adult post-mortem cases with Dravet syndrome also appeared normal, with a distinct, densely packed granule cell layer, as in post-mortem controls with no known neurological disease (Fig. 5A and B). In contrast, the granule cell layer of the hippocampal sclerosis post-mortem controls and the SCN1A+ surgical case showed dispersion of granule cells into cornu ammonis-4 and dentate molecular layer (Fig. 5C and D).
Figure 5.
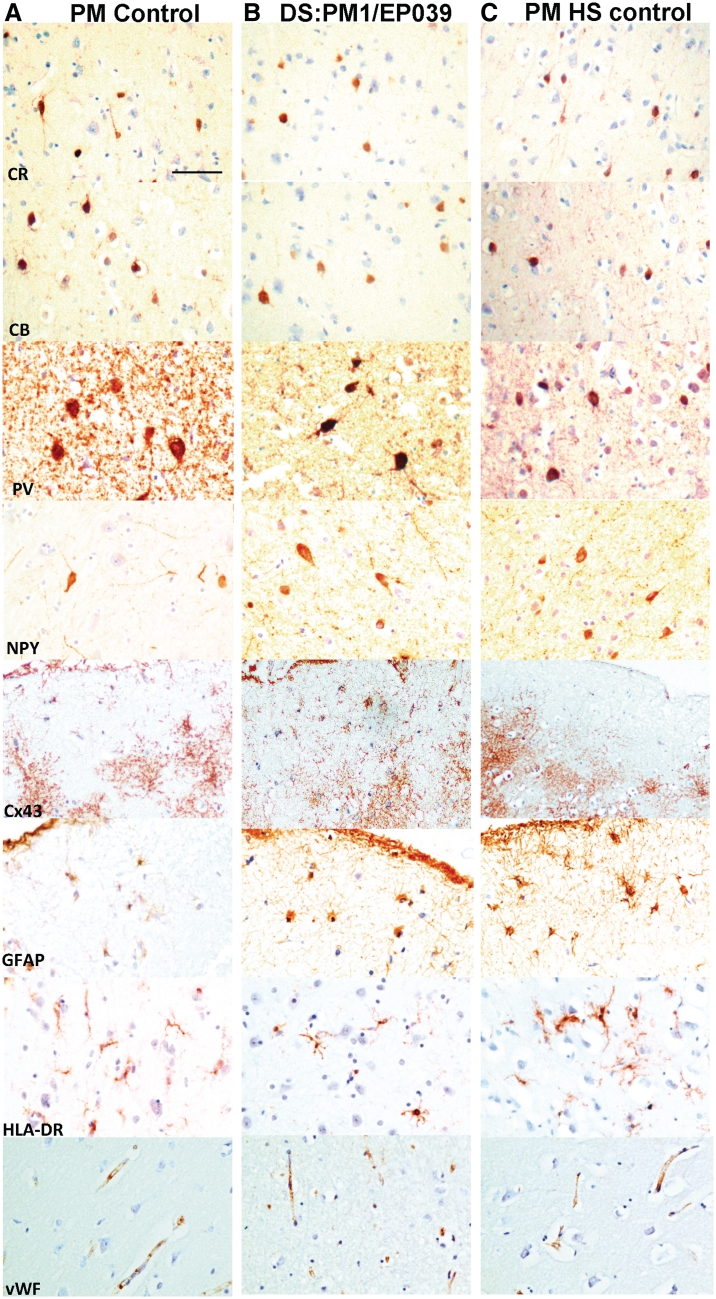
Hippocampus, histological staining and interneuronal cell counts. Cresyl violet shows the normal hippocampus from a post-mortem (PM) control with no known neurological disease (A), and the adult post-mortem case with Dravet syndrome (DS), PM2/EP213 (B). In contrast, pyramidal cell loss in the left cornu ammonis-4 and granule cell dispersion are seen in the hippocampal sclerosis post-mortem (PM HS) control (C), and the SCN1A+ surgical case (D). (E) Stereological quantification of cresyl violet-stained neurons shows lower numbers of pyramidal cells in cornu ammonis-1 and -4 for hippocampal sclerosis post-mortem controls (Control 1 and 2 EP-HS) compared with adult post-mortem cases with Dravet syndrome (PM1–3) and post-mortem controls with no known neurological disease (Controls 3–5). (F) Areal 2D counts of calbindin (CB), calretinin (CR), parvalbumin (PV) and neuropeptide Y (NPY)-immunopositive cells in the cornu ammonis-1 and -4 show that the average number of hippocampal interneurons in the adult post-mortem Dravet syndrome (n = 3) and controls with no known neurological disease (n = 2) is not markedly different. Refer to Fig. 10 (hippocampus immunolabelling) for images of calbindin, calretinin, parvalbumin and neuropeptide Y immunoreactivities in the hippocampus of cases with Dravet syndrome and controls. Scale bar = 50 µm. CA = cornu ammonis; ML = molecular layer.
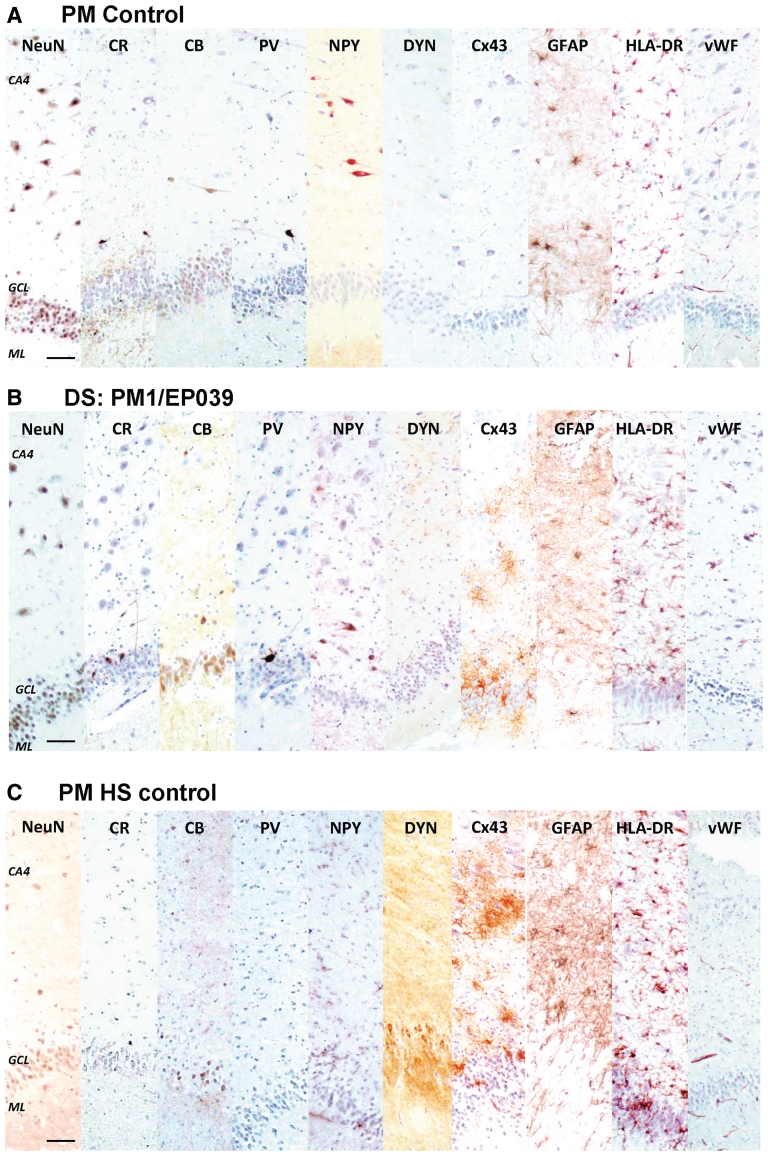
We investigated the interneuronal population within the hippocampi of all adult post-mortem cases with Dravet syndrome using immunohistochemistry for calbindin, calretinin, parvalbumin and neuropeptide Y. The appearance and localization of the calbindin-, calretinin-, parvalbumin- and neuropeptide Y-immunopositive interneurons in adult post-mortem cases with Dravet syndrome were similar to that observed in the post-mortem controls with no known neurological disease (Fig. 10). While case numbers are obviously small, 2D counts of calbindin-, calretinin-, parvalbumin- and neuropeptide Y-immunopositive cells in cornu ammonis-1 and cornu ammonis-4 showed no clear difference between adult post-mortem cases with Dravet syndrome and post-mortem controls (Fig. 5F), in keeping with evidence of neuronal preservation in the Dravet syndrome hippocampi on the basis of total cell counts. Other subcortical structures (amygdala, thalamus, basal ganglia), of all adult post-mortem cases with Dravet syndrome were intact.
Figure 10.
Hippocampus—immunolabelling. The hippocampi of a control with no known neurological disease (A), the adult post-mortem Dravet syndrome case, PM1/EP039 (B), and a hippocampal sclerosis post-mortem control (C), are immunolabelled with a panel of interneuronal, inflammatory and vascular markers. The distribution and morphology of neuronal nuclei, calretinin, calbindin, parvalbumin, and neuropeptide Y-immunopositive cells in the hippocampus are similar between the case with Dravet syndrome and post-mortem control with no known neurological disease, while expected loss of these cells is detected in the hippocampal sclerosis post-mortem control. The immunoreactivity of dynorphin (DYN), a marker that demonstrates mossy fibre sprouting, which is often associated with hippocampal sclerosis, is intense in the inner to outer molecular layer of the hippocampal sclerosis post-mortem case but not in the case with Dravet syndrome or the post-mortem control with no neurological disease. The immunoreactivity of Cx43, a gap junction marker that has been reported to be upregulated in astrocytes from resected epileptic human brain tissue, is higher in the hippocampus of the case with Dravet syndrome and the hippocampal sclerosis post-mortem control compared with the post-mortem control with no neurological disease. The immunoreactivity of GFAP, HLA-DR and von Willebrand factor is not greatly different between cases with Dravet syndrome and post-mortem controls, whilst GFAP and HLA-DR differ from the hippocampal sclerosis post-mortem control. Scale bars = 50 µm. CA = cornu ammonis; CB = calbindin; CR = calretinin; GCL = granule cell layer; ML = molecular layer; NeuN = neuronal nuclei; NPY = neuropeptide Y; PV = parvalbumin.
Routine histological stains and calbindin- and parvalbumin immunohistochemistry confirmed cerebellar atrophy with Purkinje cell loss and gliosis in all adult post-mortem cases with Dravet syndrome (Fig. 6 and Table 3). Cerebellar atrophy (without reported ataxia in life) was also noted in both hippocampal sclerosis post-mortem controls (Control 1/EP296, Control 2/EP038), and in one post-mortem control with no known neurological disease (Control 5).
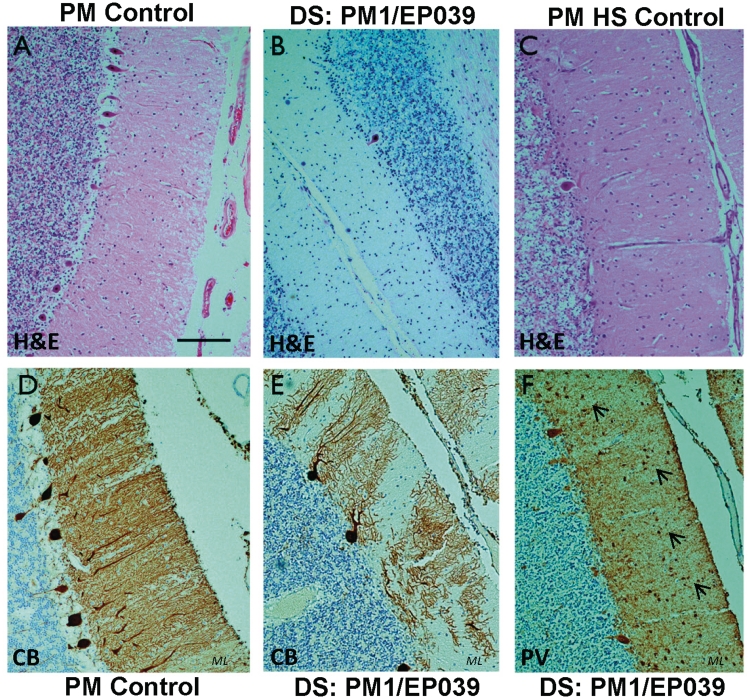
Figure 6.
Cerebellum, histological staining and immunolabelling. (A) Haematoxylin and eosin (H&E) shows a normal cerebellum from a post-mortem control with no known neurological disease. The same stain shows Purkinje cell loss in the cerebellum of the adult post-mortem Dravet syndrome case, PM1/EP039 (B), and a hippocampal sclerosis post-mortem control (C). The loss of Purkinje cells and their processes, which normally extend into the molecular layer as observed in D, is evident in calbindin- and parvalbumin-immunolabelled cerebellar sections from the case with Dravet syndrome, PM1/EP039 (E and F). Small, parvalbumin-immunopositive cells are still observed in the molecular layer of the Dravet syndrome cerebellum (F, arrows). Scale bar = 100 µm. ML = molecular layer.
The paediatric post-mortem case, PM23, showed mild bilateral end folium gliosis only. For the other paediatric post-mortem cases, only preterminal event-associated changes were found at neuropathological examination of the brain with no other significant abnormality (Table 3).
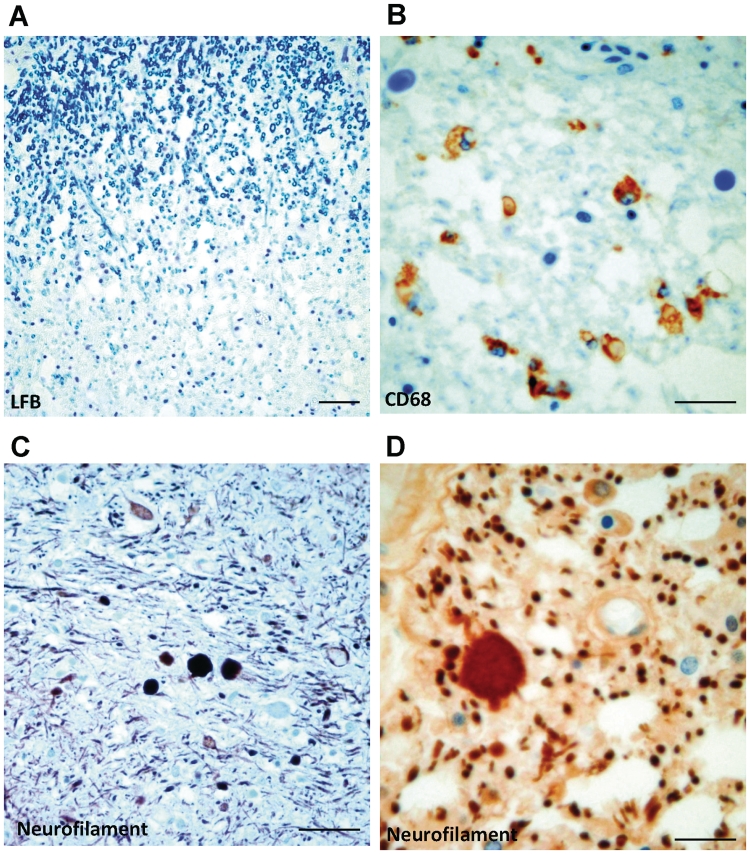
Serial sections through the brainstem, including midbrain, pons and medulla, of all adult post-mortem cases with Dravet syndrome, showed no significant pathology. In two adult post-mortem cases with Dravet syndrome, PM1/EP039 and PM2/EP213 (Table 3), loss of myelin in the dorsal columns of the medulla and cervical spinal cord was apparent (Fig. 7A). Both cases had dysphagia and ataxia. Further immunohistochemical investigation using CD68 and neurofilament antibodies showed focal macrophage infiltration (Fig. 7B) and axonal swelling (Fig. 7C and D) in the pathological areas of both cases with Dravet syndrome.
Figure 7.
Brainstem and spinal cord—histological staining and immunolabelling. (A) Luxol fast blue (LFB) section shows a cord area with myelin pallor in the dorsal column of the adult post-mortem Dravet syndrome case, PM1/EP039, where no myelin debris is observed. (B) The same area immunolabelled with the CD68 antibody shows infiltration of CD68-immunopositive macrophages into the myelin pallor. Neurofilament immunohistochemistry shows axonal swelling in the spinal cord of the Dravet syndrome case, PM1/EP039, which is presented here, in low (C) and high (D) magnification. The other Dravet case, PM2/EP213, shows similar findings as PM1, while the spinal cord was normal for Dravet syndrome case PM3/EP099 (data not shown). Scale bar = 50 µm (A and C); 25 µm (B and D).
Immunohistochemistry
The frontal cortex (F1/F2), hippocampus and cerebellum of all adult post-mortem cases with Dravet syndrome and controls, and the temporal neocortex and hippocampus of the SCN1A+ surgical case, were examined using immunohistochemistry for a range of neuronal, interneuronal, inflammatory, vascular and neurodegenerative markers. In particular, the frontal cortex was examined as ictal electroclinical patterns suggested frontal onset in several adult cases with Dravet syndrome (Fig. 3C and Table 1).
Neocortex—neuronal and interneuronal markers
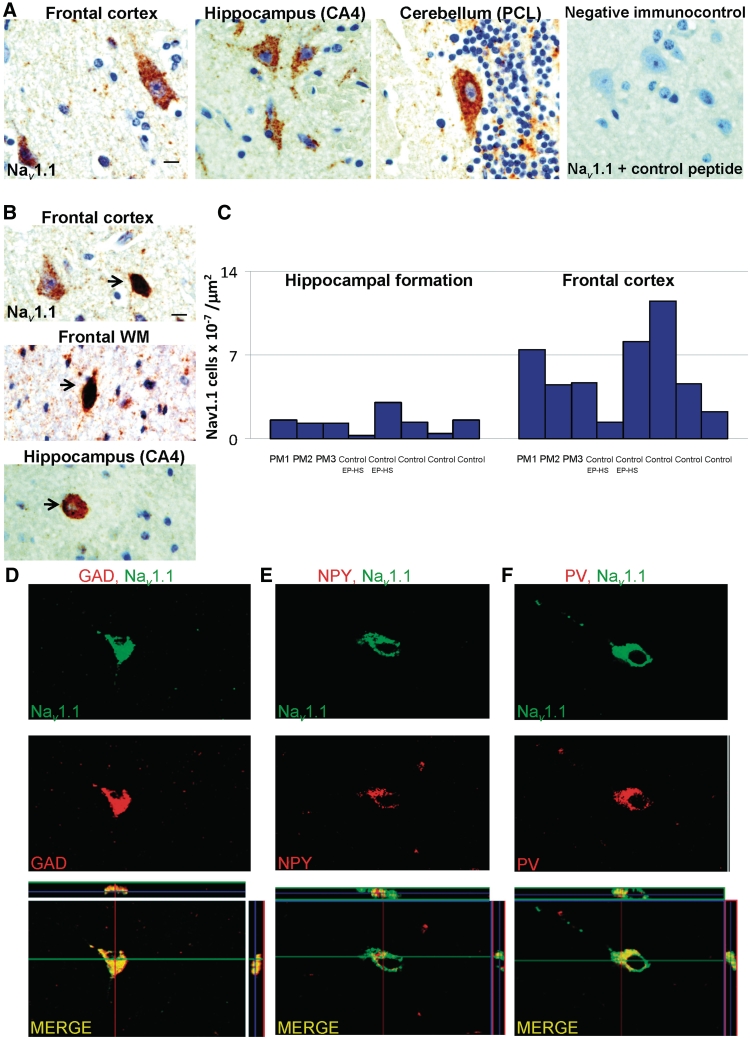
Neuronal nuclei-immunopositive neurons were organized in a well-defined, hexalaminar structure in the frontal cortex of all adult post-mortem cases with Dravet syndrome or post-mortem controls. No focal neuronal loss in the frontal cortex was evident in any cases with Dravet syndrome and post-mortem controls with no known neurological disease. Round, small to medium-sized calretinin-, calbindin- and parvalbumin-immunopositive cells were predominantly found in cortical layers II–IV of all adult post-mortem cases with Dravet syndrome with similar distribution and morphology to post-mortem controls (Fig. 8A–C; CR, CB and PV). Neuropeptide Y-immunopositive cells and fibres were observed throughout the frontal cortex of all adult post-mortem cases with Dravet syndrome and post-mortem controls (Fig. 8A–C, NPY). In particular, one adult post-mortem Dravet syndrome case, PM2/EP213, and both hippocampal sclerosis post-mortem controls showed higher numbers of neuropeptide Y-immunopositive cells and fibres in the frontal cortex compared with post-mortem controls with no known neurological disease. Nav1.1-immunopositive pyramidal cells were detectable throughout the frontal cortex of all adult post-mortem cases with Dravet syndrome (Fig. 9A), hippocampal sclerosis post-mortem controls and post-mortem controls with no neurological disease. A population of small, intensely labelled Nav1.1 cells was noted in the lower cortical layers and in the white matter of all adult post-mortem cases with Dravet syndrome and post-mortem controls (Fig. 9B). The number of these intensely labelled Nav1.1-immunopositive cells in the grey and white matter of the frontal cortex was not obviously different between cases with Dravet syndrome, hippocampal sclerosis post-mortem controls and post-mortem controls with no known neurological disease (Fig. 9C). To confirm the nature of intensely labelled Nav1.1-immunopositive cells in the frontal cortex specifically, double-labelling immunofluorescent studies were undertaken with three different markers of interneurons (glutamic acid decarboxylase, neuropeptide Y and parvalbumin), confirming that these cells are likely to be inhibitory cells (Fig. 9D–F).
Figure 8.
Frontal cortex—immunolabelling. The frontal cortex of a post-mortem control with no known neurological disease (A), the adult post-mortem Dravet syndrome case, PM1/EP039 (B) and a hippocampal sclerosis post-mortem control (C) is immunolabelled with a panel of interneuronal, inflammatory and vascular markers. The distribution and morphology of immunolabelled cells in the frontal cortex are not markedly different between post-mortem cases with Dravet syndrome and controls. Apart from images of Cx43 and GFAP immunolabelling, which are taken from subpial or layer I, images for all other markers are taken in frontal cortical layers II and III of the post-mortem cases with Dravet syndrome and control. Scale bar = 50 µm. CB = calbindin; CR = calretinin; NPY = neuropeptide Y; PV = parvalbumin; vWF = von Willebrand factor.
Figure 9.
Nav1.1-immunoreactivity in frontal cortex, hippocampus and cerebellum. (A) Nav1.1-immunolabelling is observed in the cytoplasm of pyramidal cells in frontal cortex, hippocampal pyramidal cells, and cerebellar Purkinje cells, in all adult post-mortem cases with Dravet syndrome. No Nav1.1-immunopositive cells are observed in sections that are incubated with primary Nav1.1 antibody solution pre-mixed with control peptide. (B) A number of small, intensely labelled Nav1.1-immunopositive cells (arrows) are also found in the frontal lower cortical layers, frontal white matter, and hippocampal cornu ammonis-4, but not in the cerebellum. (C) The number of small, intensely labelled Nav1.1-immunopositive cells in frontal cortex and hippocampus is not markedly different between cases with Dravet syndrome, hippocampal sclerosis post-mortem controls and post-mortem controls with no known neurological disease. (D–F) Double-labelled immunofluorescent studies show small, intensely labelled Nav1.1 cells in the frontal cortex and hippocampi of cases with Dravet syndrome co-express glutamic acid decarboxylase (D), neuropeptide Y (E) and parvalbumin (F). Scale bars = 10 µm (A–C). CA = cornu ammonis; GAD = glutamic acid decarboxylase; PCL = Purkinje cell layer; WM = white matter.
Neocortex—connexin and inflammatory markers
Multipolar, connexin 43 (Cx43-) and glial fibrillary acidic protein (GFAP-) immunopositive cells were observed throughout the frontal cortex of all adult post-mortem cases with Dravet syndrome and controls, particularly in the subpial regions and cortical layer 1 (Fig. 8A–C). The distribution and morphology of HLA-DR-immunopositive microglial cells in the frontal cortex of all adult post-mortem cases with Dravet syndrome were similar to post-mortem controls with no known neurological disease (Fig. 8A and B, HLA-DR). In comparison, HLA-DR-immunopositive cells in the frontal cortex of both hippocampal sclerosis post-mortem controls appeared larger, more intensely labelled (Fig. 8C, HLA-DR) and formed clusters.
Neocortex—vascular cells and neurodegeneration processes
von Willebrand factor-immunopositive blood vessels were observed in all adult post-mortem cases with Dravet syndrome, hippocampal sclerosis post-mortem controls and controls with no known neurological disease, and the distribution and appearance of immunopositive vessels were not markedly different between cases (Fig. 8A–C, vWF). Immunohistochemistry using neurodegenerative process markers was not performed on frontal cortical tissue.
The same panel of markers as used to study adult post-mortem cases with Dravet syndrome showed that the temporal cortex of the SCN1A+ case retained a normal, hexalaminar cytoarchitecture with no focal neuronal cell loss. There were a higher number of neuropeptide Y-immunopositive cells and processes throughout the temporal cortex of the SCN1A+ case compared with post-mortem controls with no known neurological disease, and similar to immunolabelling evident in hippocampal sclerosis post-mortem controls. The immunoreactivity of Cx43, GFAP, HLA-DR and von Willebrand factor was not markedly different between temporal cortex of the SCN1A+ case and post-mortem controls with no known neurological disease.
Hippocampus—neuronal and interneuronal markers
The expected loss of large calretinin-, calbindin-, parvalbumin- and neuropeptide Y-immunopositive cells in the cornu ammonis-4 region, and loss of calbindin-immunopositive cells in the granule layer, was detected in the hippocampus of the SCN1A+ surgical case and the hippocampal sclerosis post-mortem controls, but not in any adult post-mortem cases with Dravet syndrome, or the controls with no known neurological disease (Fig. 10A–C), again demonstrating the preservation of neurons in this adult post-mortem Dravet syndrome series. Immunoreactivity for dynorphin (DYN), a marker of mossy fibre sprouting (Vezzani et al., 1999; Thom et al., 2009), was not observed in the molecular layer of the adult post-mortem cases with Dravet syndrome and controls with no known neurological disease, but was present in the SCN1A+ surgical case and both hippocampal sclerosis post-mortem cases (Fig. 10A–C; DYN). Nav1.1-immunopositive labelling was observed in the hippocampal pyramidal and granule cells of the cases with Dravet syndrome (Fig. 9B), and controls, and the SCN1A+ surgical case. A population of small, intensely labelled Nav1.1 cells, similar to those noted in the frontal cortex, was also found scattered throughout the hippocampal formation (dentate gyrus, cornu ammonis subfields and subiculum) of the SCN1A+ surgical case, adult post-mortem cases with Dravet syndrome and post-mortem controls. Quantification of these cells revealed that the number of small, intensely labelled Nav1.1 cells was lower in the hippocampus compared with the frontal cortex, within each case, but not markedly different between adult post-mortem cases with Dravet syndrome, hippocampal sclerosis post-mortem controls and controls with no known neurological disease (Fig. 9C).
Hippocampus—connexin and inflammatory markers
Cx43-immunoreactivity was not detected in the hippocampus of any post-mortem controls with no known neurological disease (Fig. 10A, Cx43). In contrast, Cx43-immunopositive cells were observed in the cornu ammonis regions, particularly cornu ammonis-4 and granule cell layer border, of adult post-mortem cases with Dravet syndrome, hippocampal sclerosis post-mortem controls (Fig. 10B and C, Cx43) and the SCN1A+ surgical case. The immunoreactivity of GFAP in the hippocampus of adult post-mortem cases with Dravet syndrome and controls with no neurological disease was not markedly different (Fig. 10A and B, GFAP); scattered GFAP-immunopositive cells were observed throughout the hippocampal formation. GFAP-immunopositive cells and a dense matrix of GFAP-immunopositive fibres were detected in the hippocampus of hippocampal sclerosis post-mortem controls (Fig. 10C, GFAP) and SCN1A+ surgical case. The distribution and morphology of HLA-DR immunopositive microglial cells in the hippocampus of Dravet syndrome and post-mortem controls with no known neurological disease were similar, while larger and more clustering of HLA-DR immunopositive cells were observed in hippocampal sclerosis post-mortem controls (Fig. 10A–C, HLA-DR) and the SCN1A+ surgical case.
Hippocampus—vascular cells and neurodegeneration processes
The immunoreactivity of von Willebrand factor was not markedly different between adult post-mortem cases with Dravet syndrome, controls (Fig. 10A–C, vWF), and the SCN1A+ surgical case. There were infrequent AT8-immunopositive neurons in the hippocampi of all adult post-mortem cases with Dravet syndrome (all Braak Stage 2 or less). There were no neuronal inclusions or plaques noted with any of the markers in any adult post-mortem cases with Dravet syndrome. Immunohistochemical labelling with markers for dementia and neurodegeneration (Supplementary Table 1) of post-mortem Dravet syndrome hippocampi was not markedly different from post-mortem controls.
Cerebellum—neuronal and interneuronal markers
Focal reduction in calbindin- and parvalbumin-immunopositive Purkinje cells and dendrites was confirmed in the cerebellum of two adult cases with Dravet syndrome (PM1/EP039 and PM3/EP099; Fig. 6A and B), both hippocampal sclerosis post-mortem controls and one control with no known neurological disease (Control 5). Loss of calbindin- and parvalbumin-immunopositive Purkinje cells was only occasionally observed in the cerebellum of the Dravet syndrome case, PM2/EP213. Calretinin-immunopositive cells in the Purkinje and granule cell layers were preserved in adult post-mortem cases with Dravet syndrome as in controls. Similarly, small, parvalbumin-immunopositive cells were retained in the Purkinje and molecular layers of all adult post-mortem cases with Dravet syndrome, even in regions of cerebellar atrophy (Fig. 6F). Neuropeptide Y-immunoreactivity was not observed in the cerebellum of any adult post-mortem cases with Dravet syndrome or controls, except in one hippocampal sclerosis post-mortem control (Control 1/EP296), which showed a small number of neuropeptide Y-immunopositive cells and processes in the granule cell layer. While Nav1.1-immunopositive Purkinje cells were observed in the cerebellum of all cases (Fig. 9A), the small, intensely labelled Nav1.1-immunopositive neurons, which were observed in the frontal cortex and hippocampus, were not found in the cerebellum of any adult post-mortem cases with Dravet syndrome or post-mortem controls.
For the paediatric post-mortem cases with Dravet syndrome, the cerebellum was preserved; in some cases, there were ‘acute’ changes related to pre-terminal cerebral events, and in one (PM25), there was mild Purkinje cell loss (Table 3).
Cerebellum—connexin and inflammatory markers
A few Cx43-immunopositive cells were observed only in the cerebellar molecular layer of post-mortem cases with Dravet syndrome and controls. GFAP- and HLA-DR-immunopositive cells were mainly observed in the granule cell layer and white matter of the cerebellum of post-mortem cases with Dravet syndrome and controls. The distribution and appearance of these cells were not markedly different between post-mortem cases with Dravet syndrome and controls. In post-mortem cases with Dravet syndrome, PM1/EP039 and PM3/EP099, hippocampal sclerosis controls and one control with no known neurological disease (Control 5) that had marked Purkinje cell loss, GFAP and HLA-DR-immunopositive cells were also observed in the cerebellar molecular layer.
Cerebellum—vascular cells and neurodegeneration processes
The immunoreactivity of von Willebrand factor was similar between all cases. Immunohistochemistry using neurodegenerative markers was not performed in the cerebellum.
Brainstem
The immunoreactivities of the calcium-binding proteins, calretinin, calbindin and parvalbumin, were not markedly different between adult post-mortem cases with Dravet syndrome and controls. Immunohistochemistry for GFAP, ubiquitin, α-synuclein and non-phosphorylated neurofilaments did not reveal any pathological inclusions in the brainstem nuclei, neuronal loss or gliosis, in the adult post-mortem cases with Dravet syndrome.
Discussion
Dravet syndrome is an important and paradigmatic epilepsy syndrome, being among the first genetic epilepsy syndromes for which the molecular basis has been unravelled, enabling functional studies and animal models to reveal fundamental insights into the underlying pathophysiology (Catterall et al., 2008). Dravet syndrome is thought to be underestimated in prevalence and under-diagnosed in adults (Scheffer et al., 2009). There are many gaps in the understanding of the clinical evolution of Dravet syndrome in later ages, particularly after the fourth decade of life, as for many years Dravet syndrome has been considered to be of the remit of the child neurologist. As children with Dravet syndrome were prospectively followed, it became clear that some did reach adulthood (Dravet et al., 2005). More recently, adult patient series have been characterized (Jansen et al., 2006; Akiyama et al., 2010), but most adults were under 35 years of age at last follow-up. Surviving adults, over 35 years of age, with Dravet syndrome may have missed out on a diagnosis as the syndrome was only described 30 years ago (Dravet et al., 1978) and the diagnosis is often not considered in adult clinics.
We show that diagnosis even late(r) in life, in patients previously labelled as having drug-resistant epilepsy with intellectual disability of unknown cause, can carry important implications for affected patients; rational treatment changes can be instituted, with possible benefit as we and others have shown, even after years of drug resistance. In addition, recognition of the changes in language, cognition, swallowing and gait, and determining whether specific patterns exist, may help to improve diagnostic and prognostic information and may reinforce a mandate for treatment changes.
We identified 22 adult patients with Dravet syndrome who had not been diagnosed in childhood. Two-thirds were over 39 years of age at last follow-up, a greater proportion than for other studies to date (Table 7). Two adult cases with Dravet syndrome reached their sixties; survival to the seventh decade had not been previously reported. Ours is not a systematic evaluation of the prevalence of Dravet syndrome or SCN1A mutation in adults with severe epilepsy, but an observational study of a highly selected patient group from a tertiary centre. Together with the very detailed clinical records available and the neuropathology evaluation, this provided a unique opportunity for a study on the long-term follow-up and outcome of adult patients with Dravet syndrome.
Table 7.
Adults with Dravet syndrome in the literature
| Authors | Number of cases aged 18 yrs or older (total number in study) | Age range in study (yrs) | Dravet syndrome subtypes | SCN1A structural variation |
|---|---|---|---|---|
| Rossi et al., 1991 | Not specified (15) | 9-24 (mean 15) | SMEI | Not mentioned |
| Dravet et al., 2005 | Not specified (105) | 2.5-33.6 (median 11.5) | SMEI and SMEB | Not mentioned |
| Jansen et al., 2006 | 14 | 18-47 (median 26.5) | SMEI and SMEB | 10/14 mutations (+1 GABRG2 mutation) |
| Berkovic et al., 2006 | 2 | 17.5, 47 | 1 SMEI and 1 SMEB | 2/2 mutations |
| Depienne et al., 2006 | 4 | 23–40 | SMEI | 4/4 mutations |
| Fujiwara et al, 2006 | 2 | 19, 19 | SMEI | Not mentioned |
| Striano et al., 2007a | Not specified (58) | 0.3–25 | SMEI | Not mentioned |
| Striano et al., 2007b | Not specified (28) | 3–23 (mean 9.4) | SMEI | Not mentioned |
| Zucca et al., 2008 | 1 | 28 | SMEI | 1/1 deletions |
| Kassai et al., 2008 | Not specified (64) | 3–20 | SMEI | Not mentioned |
| Akiyama et al., 2010 | 31 | 18–43 (median 22) | 14 SMEI and 17 SMEB | 25/31 mutations |
| Marini et al., 2009 | 2 | 26 and 30 | SMEI | One duplication exon 26, one amplification exon 26 |
| Andrade et al., 2009 | 2 | 19, 34 | SMEI | Not mentioned |
| Ragona et al., 2010 | Not specified (37) | 0.5–28 (mean 16) | SMEI | 37/37 mutations |
SMEB = severe myoclonic epilepsy of infancy-borderland; SMEI = severe myoclonic epilepsy of infancy.
Genotype–phenotype analyses are often complex (Kanai et al., 2009; Scheffer, 2011; Zuberi et al., 2011), and more so in our selected series. Caution is required in interpretation. Considering the type of SCN1A mutations in the two extremes of age at death, a pattern may seem to emerge: in the four children with Dravet who died early, there were no missense mutations (Table 5); of the patients who died after the age of 45 years, out of the 2 in whom genetic analysis was possible, 1 had 1 SCN1A missense mutation, and the other was found not to have an SCN1A mutation or deletion. No truncating mutations were found in this group. Compared with published data, there seem to be more missense than truncating SCN1A mutations in the older Dravet group. We must emphasize limitations (ascertainment bias; selection bias; small numbers; predominance of paediatric cases in published data), but one could hypothesize that missense mutations are more frequent in patients with longer survival, testable with a prospective longitudinal study.
We acknowledge important limitations in our study. Though it includes some longitudinal data, it is a cross-sectional study. The numbers of post-mortem cases are small. We were unable to obtain DNA of sufficient quality from the two older post-mortem cases without a molecular genetic result (no frozen tissue was available). Neuropathological analyses at other levels, for example the electron microscopic, were not possible, as no appropriately fixed material was available. We cannot fully disentangle the natural history of Dravet syndrome and what may relate to other aspects, such as the chronic effects of anti-epileptic drugs. We note that for Unverricht-Lundborg disease, for example, previously reported progressive neurological deterioration was later attributed to the use of phenytoin (Eldridge et al., 1983); and avoidance has meant life expectancy may approach normal (Kalviainen et al., 2008). Despite these factors, the data available do generate new insights.
Features of Dravet syndrome in adults include drug-resistant seizures with a seizure repertoire that differs from that in childhood. Atypical absences and generalized interictal epileptiform discharges seen in childhood were not documented in our adult series. In many of our adult patients, the predominant seizures are nocturnal with focal semiological features and sometimes secondary generalization; focal onset was often documented on ictal EEG. This concurs with the findings of Akiyama et al. (2010), whose recent series of adult Dravet syndrome showed 35/40 apparently generalized seizures had frontal origin, with or without secondary generalization in the ictal EEG. No single clinical characteristic in our series allowed the distinction between SCN1A mutation-positive and -negative adult cases, but our numbers are small for subgroup comparisons.
Although long life is possible, long-term functional, seizure-related, cognitive and social outcomes appear unfavourable, with cognitive and physical decline, gait disturbance and later dysphagia, incontinence and increasing dependence for all activities of daily life. We cannot say how earlier recognition and treatment might influence these outcomes.
Dysphagia has emerged as a shared dysfunction in older cases with Dravet syndrome. This is a novel observation in Dravet syndrome, and not a feature of other chronic epilepsies, except some of the progressive myoclonic epilepsies, epilepsies associated with cerebrovascular disease and Lennox-Gastaut ‘syndrome’ (Ogawa et al., 2001). Dysphagia may manifest with unexplained cough, or recurrent respiratory infections, which may lead to neurological deterioration, and weight loss. Notably, for homozygous null Scn1a–/– knock-out mice, manual feeding extends survival (Yu et al., 2006). Awareness and early diagnosis of dysphagia may prevent complications, which include worsening of seizure control, poor nutrition and fluid intake, poor quality of life and life-threatening aspiration pneumonia. The neuropathological basis of the dysphagia is unclear, though visible changes in the brainstem were noted in two patients with Dravet syndrome and dysphagia.
The neuropathology of human Dravet syndrome has not been previously well characterized. To our knowledge, this is the first systematic neuropathological study in Dravet syndrome. We included three adult and four paediatric post-mortem cases with Dravet syndrome, and two other SCN1A mutation-carrying paediatric cases with other syndromes. Several findings are of interest.
Patients with Dravet syndrome often have autism-like behavioural features, and autism spectrum disorder has been associated with seizures in the first year of life (Saemundsen et al., 2008). In a recent report, the neuropathological examination of one Dravet paediatric case, who died of sudden unexplained death in epilepsy, showed multifocal micronodular dysplasia of the left temporal cortex and bilateral endfolium gliosis (Le Gal et al., 2010). We did not find other subtle malformation as reported in abstract form by Hayashi et al. (2004). In one of our adult cases, there was an exaggerated columnar architecture, or radial alignment of neurons involving frontal and occipital regions. This patient had a history of autistic spectrum disorder. Although studies in autism have also described abnormalities of cortical minicolumns (Casanova et al., 2010), neuropathological data in Dravet syndrome remain very limited; we simply make this observation, but cannot draw general conclusions from our single case.
One adult case with Dravet syndrome had unilateral hippocampal sclerosis on a MRI brain scan performed in his 20's; his previous MRIs were not available for review. The SCN1A+ surgical case had unilateral hippocampal sclerosis. Previous studies have shown that in a small proportion of patients with Dravet syndrome, hippocampal sclerosis is observed (Striano et al., 2007a), and this may not be present in the early childhood scans (Siegler et al., 2005). Prospective MRI studies in Dravet syndrome are required. It is of note that even on quantitative analysis, there was no neuropathological evidence of neuronal loss in the post-mortem adult cases with Dravet syndrome, showing that Dravet syndrome per se, and SCN1A mutation (one post-mortem adult case with Dravet syndrome), are not sufficient to cause hippocampal neuronal loss despite decades of drug-resistant seizures and recurrent episodes of status epilepticus. Rarely, significant clinical and imaging changes have been reported in Dravet syndrome following status epilepticus (Sakakibara et al., 2009; Chipaux et al., 2010; Tang et al., 2011). There may be age-dependent vulnerability of the brain to injury induced by seizures (Haut et al., 2004), but it is difficult to separate out effects of seizures on the brain from the effects of the disease process per se, and the effects of drugs and other factors. It has been suggested that SCN1A mutation may protect hippocampal neurons (Auvin et al., 2008), but more research is needed to determine whether (and which, if any) SCN1A mutations (or other causes of Dravet syndrome) are actually neuroprotective, and it should be noted that Dravet syndrome is not primarily a hippocampal epilepsy.
Cerebellar atrophy was a frequent finding in cases with Dravet syndrome but did not differ, either in pattern or distribution, to that previously described in patients with chronic epilepsy without Dravet syndrome (Crooks et al., 2000). The exact mechanism of selective Purkinje cell loss, as well as the potential relationship to observed ataxia, requires further study. In contrast to a previous post-mortem report in a paediatric Dravet syndrome case (Renier and Renkawek, 1990), no cerebellar dysplasia was seen in any of our cases.
Vacuolar demyelinating myelopathy of the dorsal columns of the cervical cord was noted in two patients with Dravet syndrome. This is not a typical finding in patients with epilepsy, and although a toxic or metabolic cause remains possible, future studies in patients with Dravet syndrome may elucidate if this is a feature specific to Dravet syndrome. It is of interest that ataxia can be observed in Dravet syndrome. More data are required to establish whether the vacuolar myelopathy is its basis and whether such myelopathy will respond to, or be prevented by, better seizure control or modulation of Nav1.1 function; we note in passing that Nav1.1 channels are expressed in white matter astrocytes (Black et al., 1994) in close relationship with oligodendrocytes (Waxman and Black, 1984).
We found no significant alterations in the distribution and morphology of inhibitory interneuronal subsets in cortex, cerebellum, brainstem or hippocampus in adult Dravet syndrome, even with quantitative analysis. The prevalence of small, intensely-labelled Nav1.1-immunopositive cells was not different in adult post-mortem cases with Dravet syndrome and post-mortem controls with no known neurological disease. This, of course, does not exclude putative functional abnormalities in any of these cell types, as reported for the mouse models (Yu et al., 2006; Ogiwara et al., 2007).
The clinical association between seizures and febrile episodes was not underpinned by any evidence of persistent excessive neuroinflammatory pathology or microglia in cases with Dravet syndrome. Cx43, GFAP and HLA-DR immunoreactivities in the frontal cortex were not different between adult post-mortem cases with Dravet syndrome and controls. In the hippocampus, higher numbers of Cx43-immunopositive cells in adult post-mortem cases with Dravet syndrome and hippocampal sclerosis post-mortem controls were observed, compared with post-mortem controls with no neurological disease, where no Cx43-immunolabelling was seen in the hippocampus. Previous studies have suggested that the upregulation of Cx43 in medial temporal lobe epilepsy with hippocampal sclerosis may facilitate seizure propagation (Fonseca et al., 2002; Kielian, 2008). GFAP and HLA-DR immunoreactivities were similar between adult post-mortem cases with Dravet syndrome and controls with no neurological disease, in contrast with a greater expression in hippocampal sclerosis post-mortem controls. In the cerebellum, Cx43 immunoreactivity was similar between adult post-mortem cases with Dravet syndrome and controls (low immunoreactivity). The immunoreactivity of GFAP and HLA-DR is mainly observed in the granule cell layer and white matter of adult post-mortem cases with Dravet syndrome and controls, with higher immunoreactivity in the molecular layer of cases, with loss of Purkinje cell and processes.
Overall, we have not identified any histopathological hallmark of Dravet syndrome. In fact, a striking feature was the conspicuous preservation of neurons and interneurons, including within the hippocampus, and cortex in the frontal, temporal and occipital regions, despite decades of poorly controlled seizures. Where extensive changes were seen, in the paediatric post-mortem cases, these were compatible with their agonal states; in the paediatric sudden unexplained death in epilepsy cases, there were no neuropathological abnormalities beyond changes common to chronic epilepsy. Therefore, in neither paediatric nor adult post-mortem cases, at the levels we examined the blocks available for study, were there any pathological changes to explain the observed cognitive/developmental arrest or decline.
Seizure freedom was not attained in any of our adult patients, but seizure control was significantly improved in the three cases with sufficient follow-up after specific post-diagnosis anti-epileptic drug changes, with use of appropriate drugs and withdrawal of others previously described to worsen control, such as lamotrigine, carbamazepine, vigabatrin (Guerrini et al., 1998; Perucca et al., 1998), phenytoin and oxcarbazepine (Table 2), which may have different effects on different seizure types in Dravet syndrome. Even if the patient had had drug-resistant seizures for many years, the suppression of at least one seizure type was possible for at least several months, as also shown in a recent report (Akiyama et al., 2010). For our oldest living patient, at 60 years, rational drug changes proved possible once the clinical diagnosis, with confirmation from molecular genetics (which was important in this case given the lack of literature on long-term features of Dravet syndrome), gave carers confidence in such anti-epileptic drug changes. A previous anti-epileptic drug change had led to status epilepticus and strong reluctance to entertain further changes. Subsequent drug changes led to significant benefits, even after 60 years of drug-resistant seizures: convulsive seizures were controlled and the patient began speaking again for the first time for over 5 years (Video in Supplementary material).
Dravet syndrome has been considered an ‘epileptic encephalopathy’ in the International League Against Epilepsy classification (Engel et al., 2001) and a syndrome carrying higher risk of epileptic encephalopathy in the recent reorganization (Berg et al., 2010), but controversy exists as to whether the seizures and interictal discharges themselves are responsible for the cognitive decline (Dravet et al., 2005). Our data show Dravet syndrome is indeed at least partly an epileptic encephalopathy: extensive neuropathology has not shown any consistent cerebral structural abnormalities, cell loss or other neurodegeneration, and clinically, even after many decades of drug-resistant seizures, medication changes may improve seizure control, and be associated with cognitive improvement. Necessarily, the pathological components of our study are cross-sectional, not longitudinal. We must therefore await long-term follow-up of newly diagnosed infants and children with Dravet syndrome, who are appropriately treated, to determine formally whether effective control of seizures and interictal discharges prevents encephalopathy and other co-morbidities (Scheffer et al., 2009)—not only cognitive decline, but also the additional features that we and others have reported. However, we have shown that the neurological substrate, at least at the levels we have examined, appears largely intact and therefore potentially capable of maintaining normal function—if seizures at least can be controlled. That unexpected longevity is possible further mandates efforts at earlier diagnosis and prompt effective treatment. We also conclude that Dravet syndrome may be found in older and younger adults and is a diagnosis that needs consideration in this group, because it has management implications. Dravet syndrome is an important example of the value of study of an apparently rare epilepsy, and the value of clinical acumen in syndrome discovery and clinical diagnosis.
Funding
This work was supported by the Medical Research Council (grant G0600934); the Wellcome Trust (grant 084730). This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. National Health and Medical Research Council of Australia; Great Ormond Street Hospital Children's Charity (to T.S.J.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We are indebted to the patients and their relatives. We would like to acknowledge Dr Daniel G. du Plessis, FRCPath (Neuropathology Unit, Department of Cellular Pathology and Greater Manchester Neurosciences Centre, Salford Royal Hospital, Stott Lane, Salford M6 8HD, UK), and Dr C.W. Chow, MBBS, FRCPA (Royal Children's Hospital, University of Melbourne, Victoria, Australia). We are grateful to Tharnitha Vasavan (UCL Institute of Neurology, London WC1N 3BG, UK) for her technical assistance. Finally, we would like to thank the anonymous reviewers for detailed and constructive comments.
Glossary
Abbreviations
- Cx43
connexin 43
- GFAP
glial fibrillary acidic protein
- HLA
human leucocyte antigen
- Nav1.1
voltage-gated sodium channel type 1.1
References
- Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51:1043–52. doi: 10.1111/j.1528-1167.2009.02466.x. [DOI] [PubMed] [Google Scholar]
- Auvin S, Dulac O, Vallée L. Do SCN1A mutations protect from hippocampal sclerosis? Epilepsia. 2008;49:1107–8. doi: 10.1111/j.1528-1167.2008.01549_3.x. [DOI] [PubMed] [Google Scholar]
- Baulac S, Gourfinkel-An I, Nabbout R, Huberfeld G, Serratosa J, Leguern E, et al. Fever, genes, and epilepsy. Lancet Neurol. 2004;3:421–30. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, et al. De novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–92. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Waxman SG, Oh Y, Zur KB, Sontheimer H, et al. Sodium channel mRNAs in cultured spinal cord astrocytes: in situ hybridization in identified cell types. Brain Res Mol Brain Res. 1994;23:235–45. doi: 10.1016/0169-328x(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Vanbogaert E, Narahari P, Switala A. A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol. 2010;20:451–8. doi: 10.1111/j.1750-3639.2009.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–77. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipaux M, Villeneuve N, Sabouraud P, Desguerre I, Boddaert N, Depienne C, et al. Unusual consequences of status epilepticus in Dravet syndrome. Seizure. 2010;19:190–4. doi: 10.1016/j.seizure.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res. 2000;41:63–73. doi: 10.1016/s0920-1211(00)00133-9. [DOI] [PubMed] [Google Scholar]
- Davidsson J, Collin A, Olsson ME, Lundgren J, Soller M. Deletion of the SCN gene cluster on 2q24.4 is associated with severe epilepsy: an array-based genotype-phenotype correlation and a comprehensive review of previously published cases. Epilepsy Res. 2008;81:69–79. doi: 10.1016/j.eplepsyres.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Deng YH, Berkovic SF, Scheffer IE. GEFS+ where focal seizures evolve from generalized spike wave: video-EEG study of two children. Epileptic Disord. 2007;9:307–14. doi: 10.1684/epd.2007.0126. [DOI] [PubMed] [Google Scholar]
- Depienne C, Arzimanoglou A, Trouillard O, Fedirko E, Baulac S, Saint-Martin C, et al. Parental mosaicism can cause recurrent transmission of SCN1A mutations associated with severe myoclonic epilepsy of infancy. Hum Mutat. 2006;27:389. doi: 10.1002/humu.9419. [DOI] [PubMed] [Google Scholar]
- Depienne C, Bouteiller D, Keren B, Cheuret E, Poirier K, Trouillard O, et al. Sporadic Infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet Syndrome but mainly affects females. PLoS Genet. 2009a;5:e1000381. doi: 10.1371/journal.pgen.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009b;46:183–91. doi: 10.1136/jmg.2008.062323. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat Genet. 2008;40:776–81. doi: 10.1038/ng.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C. Les epilepsies graves de l'enfant. Vie Med. 1978;8:543–8. [Google Scholar]
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy (Dravet syndrome) In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolff P, editors. Epileptic syndromes in infancy, childhood and adolescence. 4th edn. France: John Libbey Eurotext; 2005. pp. 89–113. [Google Scholar]
- Duvernoy HM. The human hippocampus: an atlas of applied anatomy. München: Springer; 1988. [Google Scholar]
- Eldridge R, Iivanainen M, Stern R, Koerber T, Wilder BJ. “Baltic” myoclonus epilepsy: hereditary disorder of childhood made worse by phenytoin. Lancet. 1983;2:838–42. doi: 10.1016/s0140-6736(83)90749-3. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr International League Against Epilepsy (ILAE) A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929:105–16. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara T. Clinical spectrum of mutations in SCN1A gene: severe myoclonic epilepsy in infancy and related epilepsies. Epilepsy Res. 2006;70(Suppl 1):S223–30. doi: 10.1016/j.eplepsyres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39:508–12. doi: 10.1111/j.1528-1157.1998.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–6. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin LA, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–52. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–17. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sugai K, Kurihara E, Tamagawa K. An autopsy case of severe myoclonic epilepsy of infancy, who died of acute encephalopathy associated with influenza infection. Epilepsia. 2004;45(Suppl 8):65. [Abstract] [Google Scholar]
- Hurst DL. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia. 1990;31:397–400. doi: 10.1111/j.1528-1157.1990.tb05494.x. [DOI] [PubMed] [Google Scholar]
- Jansen FE, Sadleir LG, Harkin LA, Vadlamudi L, McMahon JM, Mulley JC, et al. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006;67:2224–6. doi: 10.1212/01.wnl.0000249312.73155.7d. [DOI] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–74. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalviainen R, Khyuppenen J, Koskenkorva P, Eriksson K, Vanninen R, Mervaala E. Clinical Picture of EPM1-Unverricht-Lundborg disease. Epilepsia. 2008;49:549–56. doi: 10.1111/j.1528-1167.2008.01546.x. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Kaneda M, Sugawara T, Mazaki E, Okamura N, Montal M, et al. A nonsense mutation of the sodium channel gene SCN2A in a patient with intractable epilepsy and mental decline. J Neurosci. 2004;24:2690–8. doi: 10.1523/JNEUROSCI.3089-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K, Yoshida S, Hirose S, Oguni H, Kuwabara S, Sawai S, et al. Physicochemical property changes of amino acid residues that accompany missense mutations in SCN1A affect epilepsy phenotype severity. J Med Genet. 2009;46:671–9. doi: 10.1136/jmg.2008.060897. [DOI] [PubMed] [Google Scholar]
- Kearney JA, Wiste AK, Stephani U, Trudeau MM, Siegel A, RamanchandranNair R, et al. Recurrent de novo mutations of SCN1A in severe myoclonic epilepsy of infancy. Pediatr Neurol. 2006;34:116–20. doi: 10.1016/j.pediatrneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kielian T. Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem. 2008;106:1000–16. doi: 10.1111/j.1471-4159.2008.05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal F, Korff CM, Monso-Hinard C, Mund MT, et al. A case of SUDEP in a patient with Dravet syndrome with SCN1A mutation. Epilepsia. 2010;51:1915–8. doi: 10.1111/j.1528-1167.2010.02691.x. [DOI] [PubMed] [Google Scholar]
- Livingston JH, Cross JH, Mclellan A, Birch R, Zuberi SM. A novel inherited mutation in the voltage sensor region of SCN1A is associated with Panayiotopoulos syndrome in siblings and generalized epilepsy with febrile seizures plus. J Child Neurol. 2009;24:503–8. doi: 10.1177/0883073808324537. [DOI] [PubMed] [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain Dev. 2009;31:114–30. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Mancardi MM, Striano P, Gennaro E, Madia F, Paravidino R, Scapolan S, et al. Familial occurrence of febrile seizures and epilepsy in severe myoclonic epilepsy in infancy (SMEI) patients with SCN1A mutations. Epilepsia. 2006;47:1629–35. doi: 10.1111/j.1528-1167.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Mei D, Cox K, Dibbens LM, et al. SCN1A duplications and deletions detected in Dravet syndrome: implications for molecular diagnosis. Epilepsia. 2009;50:1670–8. doi: 10.1111/j.1528-1167.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, et al. Altered function of the SCN1A voltage-gated sodium channel leads to GABAergic interneuron abnormalities. J Biol Chem. 2010;285:9823–34. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle EJ, Kunic JD, George AL., Jr Novel SCN1A frameshift mutation with absence of truncated Nav1.1 protein in severe myoclonic epilepsy of infancy. Am J Med Genet A. 2008;146A:2421–3. doi: 10.1002/ajmg.a.32448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, McMahon J, Dibbens LM, Iona X, Scheffer IE, et al. Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. Lancet Neurol. 2010;9:592–8. doi: 10.1016/S1474-4422(10)70107-1. [DOI] [PubMed] [Google Scholar]
- Meisler MH, O'Brien JE, Sharkey LM. The sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588:1841–8. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen SA, Scheffer IE. Translational research in epilepsy genetics – sodium channels in man to interneuronopathy in mouse. Arch Neurol. 2009;66:21–6. doi: 10.1001/archneurol.2008.559. [DOI] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. PNAS. 2009;106:3994–9. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Kanemoto K, Koyama M, Shirasaka Y, Kawasaki J, Yamasaki S. Dysphagia and long-term follow-up of Lennox-Gastaut. Seizure. 2001;10:197–202. doi: 10.1053/seiz.2000.0483. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Nav1.1 Localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–14. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LRF, Lopez-Santiago LF, Slat EA, Dondeti RSR, Chen C, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–78. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S, Vieira JP, Barroca F, Roll P, Carvalhas R, Cau P, et al. Severe epilepsy, retardation, and dysmorphic features with a 2q deletion including SCN1A and SCN2A. Neurology. 2004;63:191–2. doi: 10.1212/01.wnl.0000132844.20654.c1. [DOI] [PubMed] [Google Scholar]
- Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia. 1998;39:5–17. doi: 10.1111/j.1528-1157.1998.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Ragona F, Brazzo D, De Giorgi I, Morbi M, Freri E, Teutonico F, et al. Dravet syndrome: early clinical manifestations and cognitive outcome in 37 Italian patients. Brain Dev. 2010;32:71–7. doi: 10.1016/j.braindev.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Ragona F, Granata T, Bernardina BD, Offredi F, Darra F, Battaglia D, et al. Cognitive development in Dravet syndrome: A retrospective, multicenter study of 26 patients. Epilepsia. 2011;52:386–92. doi: 10.1111/j.1528-1167.2010.02925.x. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–60. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Renier WO, Renkawek K. Clinical and neuropathological findings in a case of severe myoclonic epilepsy of infancy. Epilepsia. 1990;31:287–91. doi: 10.1111/j.1528-1157.1990.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Saemundsen E, Ludvingsson P, Rafnsson V. Risk of autism spectrum disorders after infantile spasms: a population-base study nested in a cohort with seizures in the first year of life. Epilepsia. 2008;49:1865–70. doi: 10.1111/j.1528-1167.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- Sakakibara T, Nakagawa E, Saito Y, Sakuma H, Komaki H, Sugai K, et al. Hemiconvulsion-hemiplegia syndrome in a patient with severe myoclonic epilepsy in infancy. Epilepsia. 2009;50:2158–62. doi: 10.1111/j.1528-1167.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- Sakauchi M, Oguni H, Kato I, Osawa M, Hirose S, Kaneko S, et al. Retrospective multiinstitutional study of the prevalence of early death in Dravet syndrome. Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03053.x. Advance Access published on April 14th, 2011, doi:10.1111/j.1528-1167.2011.03053.x. [DOI] [PubMed] [Google Scholar]
- Sander JW, Barclay J, Shorvon SD. The neurological founding fathers of the National Society for Epilepsy and of the Chalfont Centre for Epilepsy. J Neurol Neurosurg Psychiatry. 1993;56:599–604. doi: 10.1136/jnnp.56.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE. Does genotype determine phenotype? Sodium channel mutations in Dravet syndrome and GEFS+ Neurology. 2011;76:588–9. doi: 10.1212/WNL.0b013e31820d8b51. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Zhang YH, Janssen FE, Dibbens L. Dravet syndrome or genetic (generalized) epilepsy with febrile seizures plus? Brain Dev. 2009;31:394–400. doi: 10.1016/j.braindev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Shi X, Yasumoto S, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. Missense mutation of the sodium channel gene SCN2A causes Dravet syndrome. Brain Dev. 2009;31:758–62. doi: 10.1016/j.braindev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Siegler Z, Barsi P, Neuwirth M, Jerney J, Kassay M, Janszky J, et al. Hippocampal sclerosis in severe myoclonic epilepsy in infancy: a retrospective MRI study. Epilepsia. 2005;46:704–8. doi: 10.1111/j.1528-1167.2005.41604.x. [DOI] [PubMed] [Google Scholar]
- Singh NA, Pappas C, Dahle EJ, Claes LR, Pruess TH, De Jonghe P, et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genetics. 2009;5:e1000649. doi: 10.1371/journal.pgen.1000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano P, Coppola A, Pezzella M, Ciampa C, Specchio N, Ragona F, et al. An open-label trial of levetiracetam in severe myoclonic epilepsy of infancy. Neurology. 2007b;69:250–4. doi: 10.1212/01.wnl.0000265222.24102.db. [DOI] [PubMed] [Google Scholar]
- Striano P, Mancardi MM, Biancheri R, Madia F, Gennaro E, Paravidino R, et al. Brain MRI findings in severe myoclonic epilepsy in infancy and genotype-phenotype correlations. Epilepsia. 2007a;48:1092–6. doi: 10.1111/j.1528-1167.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Tang B, Dutt K, Papale L, Rusconi R, Shankar A, Hunter J, et al. A BAC transgenic mouse model reveals neuron subtype-specific effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) mutation. Neurobiol Dis. 2009;35:91–102. doi: 10.1016/j.nbd.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Lin JP, Hughes E, Siddiqui A, Lim M, Lascelles K. Encephalopathy and SCN1A mutations. Epilepsia. 2011;52:e26–30. doi: 10.1111/j.1528-1167.2011.03019.x. [DOI] [PubMed] [Google Scholar]
- Thom M, Martinian L, Catarino C, Yogarajah M, Koepp M, Caboclo L, et al. Bilateral synaptic reorganisation of the dentate gyrus in hippocampal sclerosis: a post-mortem study. Neurology. 2009;73:1033–40. doi: 10.1212/WNL.0b013e3181b99a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Liagkouras I, Elliot KJ, Martinian L, Harkness W, McEvoy A, et al. Reliability of patterns of hippocampal sclerosis as predictors of postsurgical outcome. Epilepsia. 2010;51:1801–8. doi: 10.1111/j.1528-1167.2010.02681.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balomo S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–43. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Hodgson BL, Grinton BE, Gardiner RM, Robinson R, Rodriguez-Casero V, et al. Sodium channel α1-subunit mutations in severe myoclonic epilepsy of infancy and infantile spasms. Neurology. 2003;61:765–9. doi: 10.1212/01.wnl.0000086379.71183.78. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA. Freeze-fracture ultrastructure of the perinodal astrocyte and associated glial junctions. Brain Res. 1984;308:77–87. doi: 10.1016/0006-8993(84)90919-3. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–52. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Wolff M, Cassé-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl 2):45–8. doi: 10.1111/j.1528-1167.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Zuberi SM, Brunklaus A, Birch R, Reavey E, Duncan J, Forbes GH. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology. 2011;76:594–600. doi: 10.1212/WNL.0b013e31820c309b. [DOI] [PubMed] [Google Scholar]
- Zucca C, Redaelli F, Epifanio R, Zanotta N, Romeo A, Lodi M, et al. Cryptogenic epileptic syndromes related to SCN1A. Twelve novel mutations identified. Arch Neurol. 2008;65:489–94. doi: 10.1001/archneur.65.4.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.