Abstract
Objective
Although previous neuroimaging studies suggest that adolescents with bipolar disorder exhibit smaller amygdala volumes compared with healthy adolescents, whether these abnormalities are present at illness onset or instead develop over time remains unclear. The aim of this study was to conduct a prospective longitudinal investigation comparing amygdala neurodevelopment among adolescents following their first manic episode, adolescents with attention deficit hyperactivity disorder (ADHD), and healthy adolescents.
Method
Thirty adolescents hospitalized for their first manic/mixed episode associated with bipolar disorder, twenty-nine adolescents with ADHD, and twenty-four demographically matched healthy teens underwent magnetic resonance imaging scans at index assessment and approximately 12 months later. Adolescents with bipolar disorder were prospectively evaluated using diagnostic interviews and symptom rating scales.
Results
Mixed models examining group-by-time effects for both left (p=0.005) and right (p=0.002) amygdala volumes were statistically significant. Change in left (p=0.01) and right (p=0.0008) amygdala volumes from baseline to 12 months were significantly different among groups. Specifically, left amygdala volumes increased over time in healthy adolescents (p=0.008) and adolescents with ADHD (p=0.0009), but not in adolescents with bipolar disorder (p=0.3). Right amygdala volume increased over time in adolescents with ADHD (p<0.001), but not in healthy adolescents nor in adolescents with bipolar disorder (p=0.1 and p=0.3, respectively). In adolescents with bipolar disorder, baseline total amygdala volume was significantly greater in those who subsequently achieved symptomatic recovery as compared to those who did not achieve recovery (p=0.02).
Conclusions
Adolescents with mania fail to exhibit normal increases in amygdala volume that occur during healthy adolescent neurodevelopment.
Keywords: amygdala, bipolar disorder, magnetic resonance imaging, adolescent, longitudinal
INTRODUCTION
The amygdala is involved in emotional processing and through inhibitory connections may also be instrumental in regulating emotion. Therefore, the amygdala may play an important role in the pathophysiology of bipolar disorder.1–4 Indeed, most magnetic resonance imaging (MRI) studies of adolescents with bipolar disorder report smaller amygdala volumes as compared to healthy subjects.5 Moreover, a recent meta-analysis revealed that smaller amygdala volume may be a marker of early- (vs. late) onset bipolar disorder.5 However, most MRI studies of adolescents with bipolar disorder are cross-sectional and include adolescents with bipolar disorder who have been ill for several years, making it difficult to determine whether structural amygdala abnormalities are present at illness onset or develop during the course of illness. Understanding the timeline of structural amygdala changes in bipolar disorder might identify whether this abnormality is a marker of illness onset, and/or a marker associated with illness progression. Additionally, the specificity of amygdala changes in adolescents with bipolar disorder remains unknown since few studies have included a psychiatric comparison group. Although a recent report provided preliminary evidence that volumetric reductions in the amygdala are stable over an interval of approximately two years in ten adolescents and young adults with bipolar disorder, the present report includes a larger and more homogeneous sample of inpatients with bipolar disorder. Additionally, we include a comparison group of adolescents with ADHD to determine the specificity of our findings.6
With these considerations in mind, we compared differences in amygdala volumes among adolescents with bipolar I disorder at their initial manic or mixed episode, adolescents with attention deficit hyperactivity disorder (ADHD), and a group of healthy adolescents at an initial, index assessment, and 12 months later, in order to clarify whether abnormalities in amygdala neurodevelopment occur early in the course of bipolar disorder. Based on previous studies and meta-analyses,5 we hypothesized that amygdala volumes would be smaller in adolescents with bipolar disorder at baseline, as well as at one year following their initial manic episode, as compared to the other two groups.
METHOD
Subjects
Adolescents 12 through 17 years of age, experiencing their first mixed or manic episode associated with bipolar disorder (n=30) were recruited from the inpatient units of Cincinnati Children’s Hospital Medical Center (CCHMC). Participants met DSM-IV-TR criteria for a manic or mixed episode associated with bipolar I disorder. Demographically-matched adolescents with ADHD (n=29) and healthy adolescents (n=24) were recruited from community advertisements and were excluded for history of any DSM-IV-TR Axis I disorder (other than ADHD in the former group) in themselves or their first-degree relatives. Psychopathology was assessed using the Family History Research Diagnostic Criteria.7 Study participants were group-matched for age, race, sex, socioeconomic status, and Tanner stage (Table 1). We matched for Tanner stage based on data which suggest that sex steroid hormones exert effects on brain and behavior at puberty and are involved in the reorganization of sensory and association regions of the brain including the visual cortex, amygdala, and hippocampus.8–11
Table 1.
Demographic and clinical characteristics of adolescents with bipolar disorder, adolescents with attention deficit hyperactivity disorder (ADHD), and healthy adolescents
| Baseline | 12 Months | |||||
|---|---|---|---|---|---|---|
| Bipolar (n=30) |
ADHD (n=29) |
Healthy (n=24) |
Bipolar (n=17) |
ADHD (n=24) |
Healthy (n=23) |
|
| Age, mean (SD) year | 15.0 (1.4) | 15.3 (1.8) | 15.2 (1.7) | 15.8 (1.8) | 16.3 (1.7) | 16.3 (1.8) |
| Sex, n (%) female | 14 (47) | 14 (48) | 11 (46) | 11 (65) | 11 (46) | 10 (43) |
| Race, n (%) African American | 11 (37) | 3 (10) | 6 (25) | 5 (29) | 3 (13) | 6 (26) |
| YMRS, mean (SD)a | 29 (9) | 5 (4) | 1 (1) | 9 (7) | 4 (3) | 1 (1) |
| HAM-D, mean (SD)a | 14 (7) | 3 (2) | 1 (1) | 5 (4) | 1 (1) | 0 (0) |
| Scanner at UC-CIR, n (%) | 21 (70) | 19 (66) | 11 (46) | 10 (59) | 14 (58) | 11 (48) |
| IQ, mean (SD)b | 99 (9) | 105 (13) | 110 (10) | 101 (8) | 106 (14) | 110 (10) |
| Δt, mean (SD) days | N/A | N/A | N/A | 389 (34) | 404 (41) | 408 (47) |
Note: Δt = time between scans; HAM-D = The Hamilton Rating Scale for Depression; UC-CIR = University of Cincinnati Center for Imaging Research; YMRS = Young Mania Rating Scale.
p < 0.0001 among groups at baseline and among groups at 12 months
p = 0.001 for baseline; p=0.06 at 12 months
DSM-IV-TR diagnoses were confirmed by trained raters (kappa > 0.9) who administered the Washington University in St. Louis Kiddie Schedule of Affective Disorders and Schizophrenia (WASH-UKSADS). 12 Study participants were excluded by having a history of substance abuse within three months of the index assessment, a lifetime diagnosis of substance dependence, an IQ < 70 on the Wechsler Abbreviated Scales of Intelligence, any contraindication to undergoing an MRI scan, an unstable medical or neurological disorder, a history of head trauma resulting in a loss of consciousness for greater than five minutes, or Tanner stage < 3.13 Adolescents with bipolar disorder were excluded by having exposure to active psychotropic medications (other than antidepressants and psychostimulants) greater than one month cumulative or having a prior hospitalization for a mood episode.
All study participants provided written assent and their legal guardians provided written informed consent after study procedures were fully explained. This study was approved by the University of Cincinnati and the Cincinnati Children’s Hospital Medical Center Institutional Review Boards.
MRI procedures
Study participants completed two structural MRI scans, approximately 12 months apart. At the baseline scan, thirty-two subjects (9 bipolar, 10 ADHD, and 13 healthy) underwent an MRI scan at the Cincinnati Children’s Hospital Medical Center’s Imaging Research Center (CCHMC-IRC) on a Bruker Biospec 30/60 3-Tesla MRI scanner (Bruker Medizintechnik, Karisruhe, Germany) equipped with a head gradient radio frequency (RF) coil (Bruker NMR Instruments Inc., Fremont, California). The remaining fifty-one subjects (21 bipolar, 19 ADHD, and 11 healthy ) were scanned at the University of Cincinnati’s Center for Imaging Research (UC-CIR) using a 4-Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian Inc., Palo Alto, CA) equipped with a head gradient RF coil (MR Instruments Inc., Minneapolis, MN). Twelve months following their initial scan, 29 subjects (7 bipolar, 10 ADHD, and 12 healthy) had their second scan using the CCHMC-IRC MR scanner and 35 subjects (10 bipolar, 14 ADHD, and 11 healthy) had a second scan using the UC-CIR MR scanner. Each subject had a baseline and follow-up scan on the same MRI system.
At each MR session, a high-resolution T1-weighted, 3-D whole brain scan modified driven equilibrium Fourier transform (MDEFT) scan was acquired (CCHMC-IRC parameters: TR/TE=15/4.3 ms, flip angle=20°, FOV=25.6×19.2×19.2 cm and UC-CIR parameters: TR/TE=13/6 ms, flip angle=20°, FOV=25.6×19.2×19.2 cm) 14–15. The voxel size for each scanner was 1mm × 1mm × 1mm. To minimize subject motion during scanning procedures, all subjects were instructed to remain still and foam packing was inserted around their head.
The CCHMC-IRC MRI raw FID data were reconstructed using the Cincinnati Children's Hospital Imaging Processing Software (CCHIPS®), which is specifically tailored to reconstruct Bruker scanner images.16–20 Images were then converted to AFNI format (Analysis of Functional NeuroImages; http://afni.nimh.nih.gov/afni).21 The UC-CIR MRI raw FID data were reconstructed using in-house software developed in IDL (Interactive Data Language), which converts raw FID files into AFNI format. Each MDEFT image was reformatted into the same 3-D space, independent of scanner type, which allowed for consistent viewing of the amygdala boundaries between subjects.
Specifically, the amygdala was measured by identifying the sagittal plane that bisected the left and right cerebral hemispheres in both axial and coronal views in which the posterior commissure was most clearly visualized and the cerebral aqueduct was most patent. The anterior commissure was then identified where the white matter tract was most apparent, just before the fornix. Once these two points were located, AFNI aligned the brain by running a line through the marked points. This line was made parallel to the horizon by rotating the brain to a uniform position from which tracing could better take place. Finally, manual ROI tracing of the amygdala was performed on the reoriented coronal plane by trained raters (MPD, NM) with established high inter- and intra- rater reliability (ICC>0.90) and who were blind to subject identity and group. The left and right amygdalae were traced, separately, based on previously published ROI boundaries.22 Once defined, the volume of each individual ROI was determined through AFNI, by quantifying how many voxels comprised that ROI. ROI volume in cm3 was obtained by multiplying one voxel’s dimensions by the number of voxels in that given ROI. Total brain volume was obtained using standard procedures in AFNI.
Measurements for each ROI (i.e. amygdala and total brain volume) were compared between subjects scanned on each scanner within each group, as well as the entire sample, at each time point. T-tests revealed no statistically significant differences between the region-of-interest (ROI) volume measurements from the two different scanners at each time point (p=0.22 at baseline and p=0.5 at 12 months). Additionally, three (2 bipolar, and 1 healthy) subjects who were scanned at the CCHMC-IRC and sixteen (11 bipolar, and 5 ADHD) subjects who were scanned at the UC-CIR did not complete the second scan; however chi-squared tests revealed no statistically significant differences between the number of subjects who completed both MR scans on the CCHMC scanner versus the CIR scanner within each subject group at twelve months (p=0.7). However, since two different scanners were used, we adjusted for scanner-type in all analyses in order to control for potential scanner effects.
Outcome Assessments
Clinical outcome assessments were administered to participants with bipolar disorder at 1, 4, 8, and 12 months following their index assessment. The index and 12 month clinical assessments were completed within 24 hours of the baseline and follow-up scans.23 At each follow-up visit, trained raters reviewed affective symptoms during the prior interval, week-by-week, with the adolescent and their primary caregiver. Particular attention was given to periods of changing affective symptoms, using the Modified Longitudinal Interval Follow-up Examination (LIFE).24 Each follow-up interview included weekly scores for each item of the symptom ratings scales including: the Young Mania Rating Scale (YMRS) and the Hamilton Rating Scale for Depression (HAM-D).25–27 In addition, an overall rating of symptom severity was made each week based on the scores of the rating and diagnostic instruments using a 1- to 6-point scale (Kappa = 0.92), as previously described.28 LIFE ratings were used to determine symptomatic recovery for each bipolar participant. Symptomatic recovery was defined a priori by at least 8 contiguous weeks with a LIFE overall score of ≤ 2.28–30
Data Analyses
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, Cary, NC, USA, 2002–2008). Analyses of variance (ANOVA) and chi-square tests were used to examine group differences in demographic characteristics at each time point. IQ was significantly different among groups at baseline (p=0.001), and was adjusted for in analyses. Total brain volume was not significantly different among groups at baseline nor at 12 months. However, change in total brain volume was significantly different among groups, and therefore, was also used as a covariate to ensure that the changes reported were in amygdala volume, and not the result of total brain volume changes. Finally, scanner-type was adjusted for to minimize potential scanner differences. Group differences in amygdala volumes were analyzed using the MIXED procedure (PROC MIXED) with group, time, and a group-by-time interaction term as the independent variables. Post-hoc analyses examining for differences in amygdala volumes within each group over time as well as among groups at each time point were conducted. Analyses of Covariance (ANCOVA) were used to determine if change in amygdala volume (the difference between the 12 month and baseline volumes) was significantly different among groups.
Information about current and previous psychotropic medications was collected by interviewing the participants and their legal guardians as well as by reviewing medical records. Medication exposure was examined using baseline medications and baseline volume measurements for baseline analyses and medications during the initial 12 months for analyses including change and 12 month measurements. Psychotropic medications were categorized into: psychostimulants, antidepressants, mood stabilizers, and antipsychotics. Analyses of covariance (ANCOVA) were performed to determine if medication exposure was associated with changes in amygdala volume using the ROI as the dependent variable, medication exposure as the independent variable, and IQ and scanner type as covariates.
ANCOVA were used to determine if left and right amygdala volumes at baseline, volumes at 12 months, and change over time were associated with symptomatic recovery using ROI volume as the dependent variable, the presence or absence of symptomatic recovery as the independent variable, and total brain volume as the covariate. Total amygdala volume (the sum of right and left amygdala volumes) was used in order to minimize the number of analyses. Effect sizes were calculated using the formula d = (M1 – M2)/spooled, where d = effect size, M1 – M2 is the difference between mean baseline amygdale volumes or change in amygdala volumes of the subjects within each of the groups (i.e. those with and without symptomatic recovery), and spooled = the pooled standard deviations (SD) of the two groups.31
RESULTS
Clinical Characteristics
There were no statistically significant group differences in age, sex, or race among groups at baseline or at 12 months, despite attrition (Table 1). Additionally, there were no statistically significant group differences in time between scans (Table 1). Statistically significant differences in IQ were observed among groups at baseline (p=0.001), but not at 12 months (p=0.06). However, IQ was not correlated with left or right amygdala volume change (R=0.17 and p=0.4; R=0.14 and p=0.5, respectfully). Additionally, there were no differences in findings of volumetric changes with and without controlling for group differences in IQ. Nineteen study participants did not complete the second scan (13 bipolar, 5 ADHD, and 1 healthy; Table 1). There were no statistically significant baseline differences in demographic or clinical variables between subjects who completed 12 months of study participation and subjects who discontinued prior to 12 months. At the baseline scan, 6 (20%) adolescents with bipolar disorder were experiencing a manic episode and 24 (80%) adolescents with bipolar disorder were experiencing a mixed episode. At the second scan, 2 (12%) adolescents with bipolar disorder were experiencing a manic episode and the remaining 15 (88%) adolescents with bipolar disorder were euthymic. Additionally, four of the adolescents with bipolar disorder experienced a second manic episode between their two scans, and one adolescent with bipolar disorder experienced a depressed episode between their scans. However, ANCOVA revealed no significant differences in baseline, 12 month, or change in amygdala volume between those that experienced an additional mood episode and those that did not.
Volumetric Analyses
ANCOVA, using all subjects, revealed no statistically significant differences in total brain volume among groups at baseline (F=0.33, df=79, p=0.7) nor at 12 months (F=1.28, df=60, p=0.3; Table 2). There were statistically significant differences in change in total brain volume among groups (F=4.23, df=60, p=0.02). Post-hoc analyses revealed that adolescents with ADHD exhibited a greater increase in total brain volume as compared to adolescents with bipolar disorder (p=0.005). However, change in total brain volume was not significantly different between the healthy adolescents and adolescents with bipolar disorder (p=0.15), nor between the healthy adolescents and adolescents with ADHD (p=0.14).
Table 2.
Magnetic resonance imaging volumetric measurements of adolescents with bipolar disorder, adolescents with attention deficit hyperactivity disorder (ADHD), and healthy adolescents at baseline and 12 months later, adjusted for IQ, scanner type, and total brain volume
| Baseline | 12 months | |||||
|---|---|---|---|---|---|---|
| ROI volume (cm3) | Bipolar (n=30) |
ADHD (n=29) |
Healthy (n=24) |
Bipolar (n=17) |
ADHD (n=24) |
Healthy (n=23) |
| Amygdala, mean (SD) | ||||||
| Lefta | 3.3 (0.5) | 3.3 (0.6) | 3.4 (0.3) | 3.0 (0.5) | 3.8 (0.9) | 3.8 (0.7) |
| Rightb | 3.6 (0.5) | 3.5 (0.6) | 3.7 (0.3) | 3.4 (0.4) | 4.2 (0.8) | 4.1 (0.6) |
| Total Brain | 1575 (170) | 1569 (146) | 1548 (184) | 1543 (162) | 1640 (177) | 1581(208) |
Note: ROI = region of interest
p=0.005 for the mixed model group-by-time effect
p=0.002 for the mixed model group-by-time effect
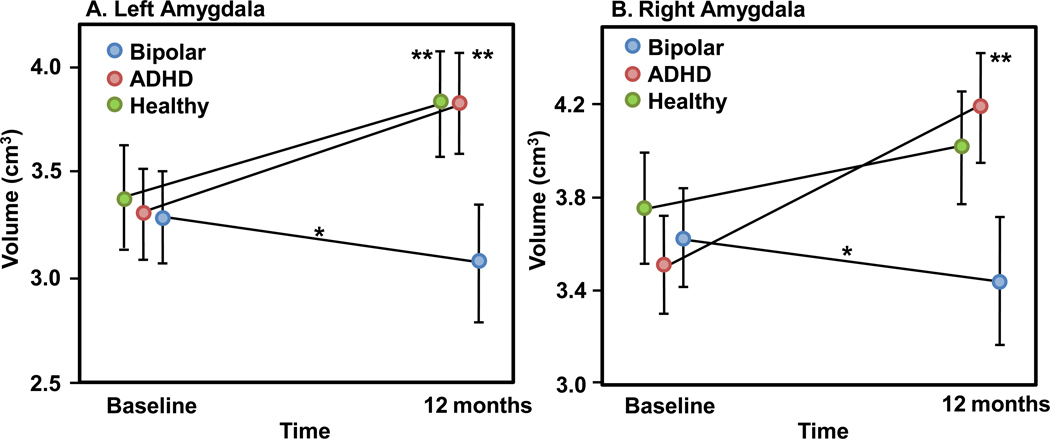
Mixed models examining group-by-time effects for both left (F=5.7, df=78, p=0.005) and right (F=6.7, df=78, p=0.002) amygdala volumes were statistically significant. Specifically, left amygdala volumes significantly increased over time in the healthy adolescents (12% increase, p=0.008) and adolescents with ADHD (13% increase, p=0.0009), but not in the adolescents with bipolar disorder (p=0.3). Right amygdala volume significantly increased over time in the adolescents with ADHD (16% increase, p<0.001), but not in the healthy comparison group nor in the adolescents with bipolar disorder (p=0.1 and p=0.3, respectively). Therefore, the group-by-time effects in the right amygdala are primarily driven by the significant increase in the adolescents with ADHD and the trend towards an increase in the healthy adolescent group (Figure 1 and Table 2).
Figure 1.
Adjusted volumetric changes from baseline to 12 months in adolescents with bipolar disorder (n=30), adolescents with attention deficit hyperactivity disorder (ADHD; n=29), and healthy (n=24) adolescents in A. left amygdala (mixed model group*time effect, p=0.005) and B. right amygdala (mixed model group*time effect, p=0.002).
* At 12 months, adolescents with bipolar disorder exhibit statistically significantly differences in A. left amygdala volume compared with adolescents with attention deficit hyperactivity disorder (ADHD; p=0.0002) and healthy adolescents (p=0.0002) and B. in right amygdala volume compared with adolescents with attention deficit hyperactivity disorder (ADHD; p=0.0002) and healthy adolescents (p=0.003)
** Volume change from baseline to 12 months is significant for: A. left amygdala in healthy adolescents (p=0.008) and adolescents with attention deficit hyperactivity disorder (ADHD; p=0.0009) and B. right amygdala in adolescents with attention deficit hyperactivity disorder (ADHD; p<0.0001).
ANCOVA revealed that there were statistically significant group differences in volume change from baseline to 12 months, using volume change as the dependent variable, in left (F=5.03, df=59, p=0.01) and right (F=8.07, df=59, p=0.0008) amygdala. Specifically, change in left amygdala volume was significantly different between the adolescents with ADHD and adolescents with bipolar disorder (p=0.007) and the healthy adolescents and adolescents with bipolar disorder (p=0.006). Right amygdala volume change was significantly different between the adolescents with ADHD and adolescents with bipolar disorder (p=0.0002) and healthy adolescents and adolescents with bipolar disorder (p=0.01).
At baseline, ANCOVA revealed no statistically significant differences among groups in left (F=0.23, df=78, p=0.8) nor right (F=1.23, df=78, p=0.3) amygdala volumes. However, at 12 months, there were statistically significant differences among groups in left (F=7.54, df=59, p=0.001) and right (F=7.8, df=59, p=0.001) amygdala volumes. Specifically, left and right amygdala volumes were significantly larger in the healthy adolescents (p=0.0002, p=0.003, respectively) and adolescents with ADHD (p=0.0002, p=0.0002, respectively) than in adolescents with bipolar disorder.
At baseline, there were no statistically significant differences in the left or right amygdala volumes between those subjects who completed both scans, and those who did not (left amygdala, p=0.9 and effect size, Cohen’s d=0.03; right amygdala, p=0.2 and effect size, Cohen’s d=0.2).
Effects of Clinical and Outcome Variables
Rating Scales
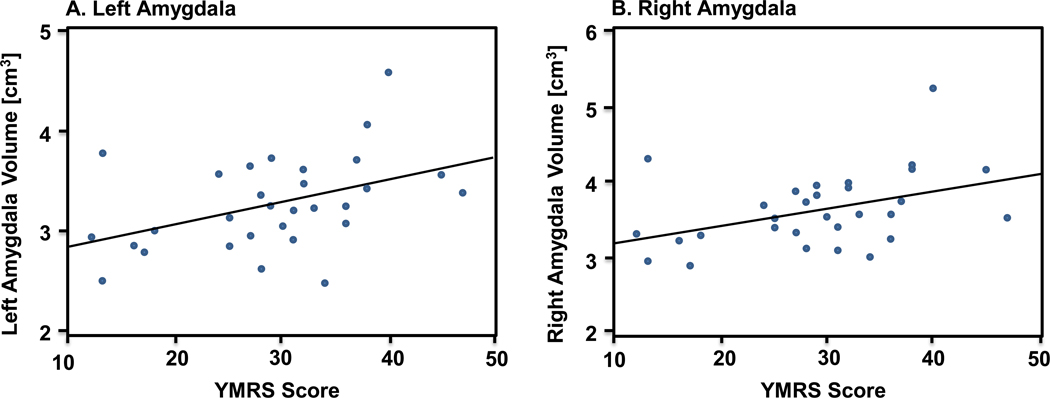
Within the adolescents with bipolar disorder, baseline left and right amygdala volumes were significantly correlated with baseline YMRS score (p=0.02, R=0.43 and p=0.02, R=0.41, respectively; Figure 2). Eight of the 30 adolescents with bipolar disorder (27%) had psychosis with their index manic/mixed episode. Although limited due to small sample size, t-tests comparing baseline, follow-up and change in left amygdala, right amygdala, and total brain volumes between those with and without psychosis revealed no statistically significant differences (smallest p value=0.54).
Figure 2.
Left and right amygdala volume and symptom severity (YMRS) at baseline for adolescents with bipolar disorder (n=30) Note: Baseline amygdala volume is significantly correlated with baseline YMRS scores. A. Left amygdala (p=0.02, R=0.43). B. Right amygdala (p=0.02, R=0.41).
Medication Effects
Additionally, at the baseline scan, 15 (50%) adolescents with bipolar disorder were taking an antipsychotic, eight (27%) were taking a mood stabilizer, and one (3%) was taking an antidepressant. Antipsychotics and mood stabilizers had only been taken for less than one month to be included in the study. At the 12 month scan, seven (41%) adolescents with bipolar disorder were on antipsychotics, six (35%) were on mood stabilizers, three (18%) were on psychostimulants, and three (18%) were on antidepressants.
At the baseline scan, 16 (55%) of the adolescents with ADHD were taking a psychostimulant, three (10%) were on an antidepressants, one (3%) was taking an antipsychotic, and one (3%) was taking a mood stabilizer. Two adolescents with ADHD had taken an antipsychotic or a mood stabilizer for less than 1 month. At the 12 month scan, 13 (54%) of the adolescents with ADHD were on psychostimulants, five (21%) were on antidepressants, and one (4%) was on a mood stabilizer.
ANCOVA revealed that medication exposure had no statistically significant effect on amygdala volumes at baseline, 12 months, or volume change over time in adolescents with bipolar disorder. Medication exposure; however, did have a significant effect on volume in adolescents with ADHD. Psychostimulants taken at baseline had a significant effect on right amygdala volume change (p=0.04). In addition, psychostimulants taken at 12-months had a significant effect on right amygdala volume change (p=0.01). In both cases, adolescents with ADHD had a smaller right amygdala volume change if they were taking psychostimulants. Furthermore, adolescents with ADHD taking psychostimulants at 12-months had a significantly smaller left amygdala volume at that scan (p=0.046).
Outcome Characteristics
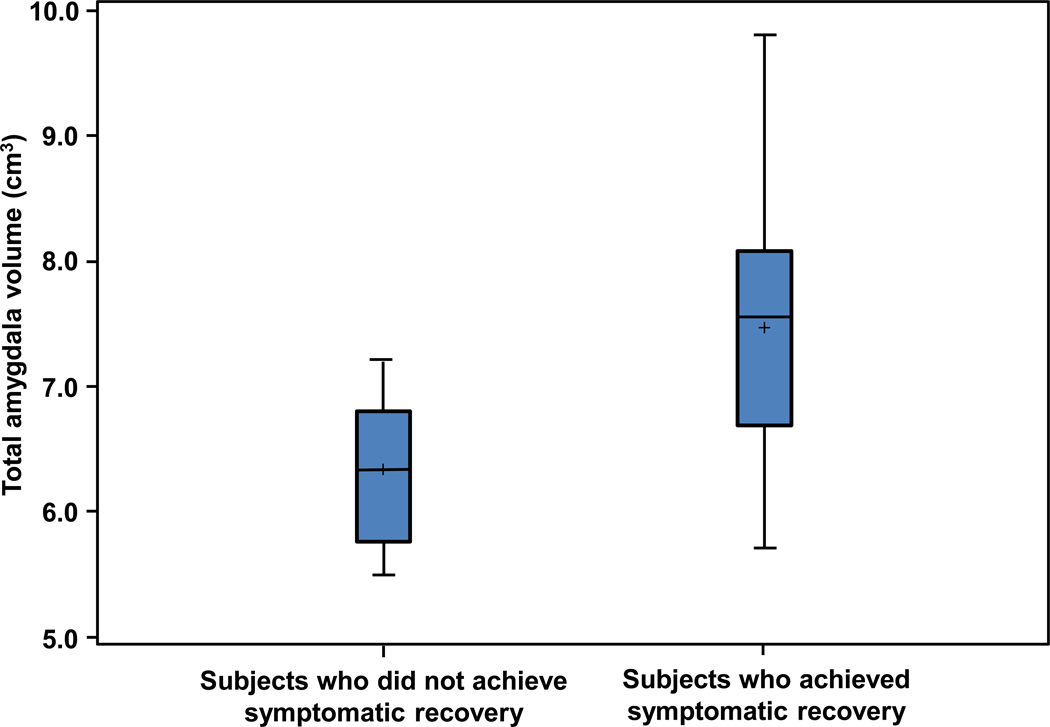
Nine adolescents with bipolar disorder who had a baseline scan achieved symptomatic recovery during follow-up. Eleven adolescents with bipolar disorder who had a baseline scan did not achieve symptomatic recovery during the 12 months of follow-up assessments. ANCOVA revealed that baseline total amygdala volume was significantly greater in those who subsequently achieved symptomatic recovery as compared to those who did not have symptomatic recovery (d=1.34, p=0.02; Figure 3). Post-hoc analyses revealed that both right and left baseline amygdala volumes were significantly greater in those who subsequently achieved symptomatic recovery (d=1.2, p=0.008 and d=1.5, p=0.04, respectively). Additionally, an ANCOVA, using total brain volume change as the covariate, revealed no significant differences between those adolescents with bipolar disorder who achieved symptomatic recovery and those who did not in left amygdala volume change (p=0.8) nor right amygdala volume change (p=0.7).
Figure 3.
Baseline total amygdala volume for adolescents with bipolar disorder who did (n=9) and did not (n=11) achieve symptomatic recovery. Note: Baseline total amygdala volume was significantly greater in those who subsequently achieved symptomatic recovery as compared to those who did not experience symptomatic recovery during the initial year following their first manic episode (d=1.34, p=0.02).
Six adolescents with bipolar disorder developed substance use disorders during the 12 month follow-up period. Although, there were no statistically significant differences in baseline, year, or change in amygdala volumes between those with and without substance use during follow-up (smallest p=0.2), these findings should be interpreted with caution due to the small sample size.
DISCUSSION
Consistent with findings from Kalmar and colleagues, who reported abnormal changes in prefrontal regions over time in adolescents with bipolar disorder, our study suggests abnormalities in amygdala development during the first year following the initial manic episode in adolescents with bipolar I disorder.32 Specifically, our results indicate that adolescents with bipolar disorder do not exhibit abnormalities in amygdala volumes at the onset of manic illness. However, adolescents with bipolar disorder failed to exhibit increases in amygdala volumes over the first year following their initial manic episode that were observed in healthy adolescents and adolescents with ADHD during the same time period. Similar to healthy adolescents, adolescents with ADHD showed increases in amygdala volumes, suggesting that abnormal amygdala neurodevelopment may be useful as a marker associated with illness progression for adolescent bipolar disorder. During healthy adolescent brain development, there are visible patterns of growth that take place in the limbic system. For example, prior studies of healthy adolescent neurodevelopment indicate that the temporal lobe structures (amygdala and hippocampus) increase in volume with age.33 Our findings of decreased amygdala volume in adolescents with bipolar disorder at one year, but not at the time of the first manic episode, are consistent with cross-sectional reports of decreased amygdala volumes in adolescents with bipolar disorder, as well as reports indicating that these abnormalities are not present in adolescents at risk for developing bipolar disorder, prior to illness onset.34–41
Although the neurpathological basis for failure to exhibit healthy amygdala growth in bipolar disorder is unknown, it may result from failure to expand the neuronal tree within the amygdala during adolescence or disturbances in the growth of glial cells.41–44 As a part of the ventral-limbic pathway, the amygdala is central in emotional processing, so that incomplete amygdala development in adolescents with bipolar disorder may lead to increased problems for these individuals in adapting responses to emotional and social stimuli.45–51 Indeed, prior studies suggest that adolescents with bipolar disorder exhibit alterations in emotional regulation and in their ability to recognize facial affect.23,52–56 Dysfunction of this pathway, along with the dorsal-subcortical pathway, that modulates cognitive processes, is believed to underlie the neurophysiology of bipolar disorder.36,57–58
One explanation for the differences in amygdala volumes over time may be related to differences among groups in medication exposure. In our analyses, amygdala volumes of adolescents with bipolar disorder did not appear to be significantly affected with medication exposure. However, the amygdale volumes of adolescents with ADHD were significantly associated with psychostimulant use. In contrast to our findings, previous studies report that medication exposure can increase the volume of the hippocampus and the amygdala, and therefore, future medication studies are needed to further support this conclusion.59–61
Greater amygdala volumes at the onset of mania were found in those who subsequently achieved symptomatic recovery. This finding demonstrates that amygdala volume at initial manic episode may be useful as a potential marker of treatment response or a prognostic indicator of clinical outcome. The variability present in the baseline amygdala volumes of the adolescents with bipolar disorder who recovered compared with those who did not might be attributable to other factors including substance use and medication exposure. Future studies examining the impact of these variables on structural and functional brain changes in a larger sample of adolescents with bipolar disorder, over more time points, are required to determine whether specific interventions may influence these associations. Additionally, a larger study employing a receiver operating characteristic (ROC) analysis could help determine whether specific values of amygdala volume provide maximal sensitivity and specificity for predicting outcomes.
There are several limitations that should be considered when interpreting the results of our study. First, the use of two different scanners might have caused discrepancies in ROI volumes. However, multiple steps were taken to ensure comparability. These steps included maintaining the same scanner for each individual for both of that individual’s scans, co-varying for scanner-type during the analyses, and using change in ROI volume as a dependent variable. Additionally, no scanner specific effects were observed. Second, although our study is a prospective study, adolescents and their family members were interviewed retrospectively at each follow up visit, which might have limited their ability to accurately recall mood symptoms. However, in order to improve the accuracy of information obtained, subjects and their primary caregivers were each interviewed. These interviews were performed frequently (at 1, 4, 8, and 12 months) and all subjects were reminded of anchor time points (i.e., holidays, birthdays, and school events) that occurred during each follow up period. Third, IQ significantly differed among the groups, and therefore, was adjusted for in volumetric analyses. However, a correlation between IQ and amygdala volume did not reveal any significant associations, consistent with previously reported findings in healthy adults. 62 Fourth, clinical outcome measures revealed that by 12 months many of the adolescents with bipolar disorder who began the study manic were euthymic (Table 1). Associations revealed a significant correlation between baseline YMRS scores and amygdala volume in the bipolar group; however no correlation was found between 12 month YMRS scores and amygdala volume. Additionally, no correlations were found between HAM-D scores and amygdala volume. The percentage of subjects who did not complete 12 months was greater in adolescents with bipolar disorder (43%) than in adolescents with ADHD (17%) or healthy (4%) adolescents (p<0.0001). However, we examined differences in the demographic and clinical characteristics, as well as the amygdala volumes for those subjects who dropped out and those who completed the study and no statistically significant differences were found. Additionally, we did not systemically collect data regarding stressful life events or trauma that occurred between assessments. However, Post Traumatic Stress Disorder (PTSD) KSADS modules were repeated and no subject developed PTSD during the 12-month follow-up period. Nonetheless, studies examining whether high-stress or trauma impacts the neurodevelopment of bipolar disorder and/or symptomatic recovery are needed. Finally, comparisons made examining relationships between outcome measures and medication exposure with amygdala volumes were likely underpowered due to the small sample sizes.
Nonetheless, despite these limitations, to our knowledge this study is the first to demonstrate abnormal amygdala development in early course bipolar disorder compared with healthy adolescents and adolescents with ADHD. Although there were too few subjects in our sample to examine the impact of prior depressive episodes or co-occurring anxiety disorders, future studies assessing the effects of these factors, as well as family history of mood disorders on amygdala volumes are needed. Furthermore, studies examining whether amygdala volumes at illness onset or changes in amygdala volumes over time are predictive of clinical outcome are also needed. Similarly, long-term prospective studies that investigate whether amygdala changes may be modified with effective medications and whether adults with adolescent onset bipolar disorder exhibit similar amygdala abnormalities into adulthood are necessary to enhance our knowledge about the pathophysiology of bipolar disorder.
Acknowledgments
Support for this research was provided by the National Institute of Mental Health (NIMH) Grant number: K23 MH63373 (MPD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this manuscript were presented, in part, at the 48th Annual Meeting of the American College of Neuropsychopharmacology; 2009 December 6–10, Hollywood, Florida.
Disclosure: Dr. Adler has served on the speakers’ bureau for Merck, and Janssen. He has received research support from Eli Lilly and Co., Janssen, Pfizer, Forrest, AstraZeneca, Bristol-Myers Squibb, Repligen, and Johnson and Johnson. Dr. Strakowski has served as a consultant for Pfizer, Eli Lilly and Co., Tikva, and Dimedix. He has received research support from Pfizer, Eli Lilly and Co., Janssen, AstraZeneca, Martek Biosciences, Nutrition 21, Bristol-Myers Squibb, Somerset, Johnson and Johnson, Forest, Shire, and Repligen. Dr. DelBello has served on the speakers’ bureau or as a consultant for Bristol-Myers Squibb and Merck. She has received research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Co., Forrest, Amylin, GlaxoSmithKline, Pfizer, Janssen, Merck, and Johnson and Johnson, Otsuka and Somerset. Ms. Bitter and Mr. Mills report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.MacKinnon DF, Zamoiski R. Panic comorbidity with bipolar disorder: what is the manic-panic connection. Bipolar Disord. 2006;6:648–664. doi: 10.1111/j.1399-5618.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 2.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 3.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 4.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer JC, et al. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg HP, et al. Preliiminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Arch. Gen. Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 8.Blankemore S, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez JL, Huppenbauer CB, McAbee MD, Jurasaka JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- 10.Hebbard PC, King RR, Malsbury CW, Harley CW. Two organisational effects of pubertal testosterone in male rats: Transient social memory and a shift away from LTP following a tetanus in hippocampal CA1. Exp Neurol. 2003;182:470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- 11.Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- 12.Geller B, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc Dev. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 14.Wilke M, et al. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Strakowski SM, et al. MRI brain activation in first-episode bipolar mania during a response inhibition task. Early Interv Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. Functional magnetic resonance imaging of neuronal activation with symptom provocation in unmedicated patients with obsessive–compulsive disorder. J Psychiatry Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 17.Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg HL, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- 18.Adler CM, Holland SK, Enseleit S, Strakowski SM. Age-related changes in regional activation during working memory in young adults: an fMRI study. Synapse. 2001;42:252–257. doi: 10.1002/syn.1111. [DOI] [PubMed] [Google Scholar]
- 19.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:1–11. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 20.Holland S, Strawsburg RH, Weber AM, Dunn RS, Schmithorst V. SNR XVI/ASNR. Philadelphia, PA: American Society of Neuroradiology; 1998. fMRI of language distributions in pediatric epilepsy patients. [Google Scholar]
- 21.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Biophysics Research Institute, Medical College of Wisconsin, Milwaukee 53226-0509, USA. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 22.Strakowski SM, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 23.DelBello MP, et al. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 24.Keller MB, et al. The Longitudinal Interval Follow-Up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 25.Fristad MA, Weller EB, Weller RA. The Mania Rating Scale: can it be used in children? A preliminary report. J Am Acad Child Adolesc Psychiatry. 1992;31:252–257. doi: 10.1097/00004583-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Young RC, et al. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;25:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keck PE, et al. 12-Month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am J Psychiatry. 1998;155:646–652. doi: 10.1176/ajp.155.5.646. [DOI] [PubMed] [Google Scholar]
- 29.Strakowski SM, et al. Twelve-month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry. 1998;55:49–55. doi: 10.1001/archpsyc.55.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Strakowski SM, et al. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000;48:477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 32.Kalmar JH, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durston S, et al. Anatomical MRI of the developing human brain: What have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 34.DelBello MP, et al. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adoelscents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 35.Frazier JA, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 36.Strakowski SM, DelBello MP, Adler CM. The functional neuro-anatomy of bipolar disorder: a review of neuro-imaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 37.Steen RG, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 38.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 39.Frazier JA, et al. Brain anatomic magnetic resonance imaging in childhood- onset schizophrenia. Arch Gen Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 40.Blumberg HP, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 41.Bowley MP, et al. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2004;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 42.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Tkachev D, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 44.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 45.Bechara A, et al. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5781. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 47.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 48.Morris JS, et al. A differential neual response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 49.Anderson AK, Phelps EA. Expression without recognition: contributions of the human amygdala to emotional communication. Psychol Sci. 2000;11:106–111. doi: 10.1111/1467-9280.00224. [DOI] [PubMed] [Google Scholar]
- 50.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 51.Fudge JL, et al. Considering the role of the amygdala in psychotic illness: a clinicopatholgical correlation. J Neuropsychiatry Clin Neurosci. 1998;10:383–394. doi: 10.1176/jnp.10.4.383. [DOI] [PubMed] [Google Scholar]
- 52.Yurgelun-Todd DA, et al. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 53.George MS, et al. Abnormal facial emotion recognition in depression: serial testing in an ultra-rapid-cycling patient. Behav Modif. 1998;22:192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- 54.Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- 55.Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Development and Psychopathology. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- 56.Dickstein DP, Brazel AC, Goldberg LD, Hunt JI. Affect regulation in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Am. 2009;18:405–420. doi: 10.1016/j.chc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang K, et al. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 58.DelBello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11:298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- 59.Chang K, et al. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 60.Foland LC, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. NeuroReport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajek T, et al. Amygdala volumes in mood disorders--Meta-analysis of magnetic resonance volumetry studies. Journal of Affective Disorders. 2009;115:395–410. doi: 10.1016/j.jad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Amata JA, Bansala R, Whitemana R, Haggerty R, Royala J, Petersona BS. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain and Cognition. 2008;66:105–114. doi: 10.1016/j.bandc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]