Abstract
Mice and rats are often used interchangeably in neuroscience research. However, species differences in brain structure and connectivity exist within the medial temporal lobe circuits that contribute to learning and memory. The hippocampus in particular contributes to both spatial learning and recognition memory, but the extent to which rats and mice are comparable in these two cognitive domains remains unclear. To evaluate potential species differences in spatial memory and object recognition, young adult male Sprague-Dawley rats and male C57Bl/6J mice were tested in the water maze and novel object recognition tasks. Following six days of training, with four trials per day, there was no difference in the ability of rats and mice to learn the location of a hidden platform. However, rats performed better than mice on the probe trial, indicative of superior retention. In the novel object preference test, no species differences in recognition memory were detected, although rats spent more time exploring the arena and took longer to approach the objects. These observations suggest that while species differences in spatial memory retention are present, they do not correlate with differences in object recognition memory.
Keywords: species difference, hippocampus, water maze, object recognition
Rats and mice are frequently used in neurobiological research to model the changes that underlie learning and memory. Data from these two species is presumed to be comparable with respect to the neuronal mechanisms that support cognition. However, species differences in behavioral performance have been reported, particularly in the water maze, the most commonly used behavioral assessment of hippocampal function. Rats learn the location of the hidden platform more quickly than mice when trained using four trials per day over three days (Whishaw, 1995; Whishaw and Tomie, 1996). However, rats also swim more quickly than mice, and earlier work by Whishaw and colleagues did not account for this external variable. In a task with high working memory load, using twelve trials presented on a single day, rats and mice performed comparably during acquisition training, but rats were superior to mice during retention testing (Frick et al., 2000). For the purpose of generalizing between rats and mice, it would be useful to identify a training protocol in which both species are able to acquire and retain the task.
Rats and mice exhibit species differences in some water maze testing paradigms, but perform comparably on dry land mazes, such as the radial arm maze (Whishaw and Tomie, 1996). The radial arm maze involves a recognition memory element, but direct comparisons of recognition memory between mice and rats have not previously been made. Because object recognition memory is increasingly being used to screen for behavioral deficits in transgenic mouse models (Winters et al., 2008), it is worthwhile to generate a detailed analysis of similarities and differences in recognition memory between mice and rats. Rats are phylogenetically closer to primates than mice (Gibbs et al., 2004), and understanding parallels between mice and rats could shed light on the evolutionary conservation of mechanisms underlying recognition memory, with potential relevance to primate species.
To compare spatial and recognition memory across rats and mice, young male Sprague-Dawley rats and C57Bl/6J mice were tested in the water maze and novel object preference tasks. Following training with four trials per day for six days, mice and rats performed indistinguishably over all days of training, after accounting for differences in swim speed. Although mice and rats spent similar proportions of time in the platform quadrant during the probe trial, rats exhibit superior spatial memory retention, as indicated by reduced mean distances from the target location. By contrast, performance in the novel object preference paradigm was similar between mice and rats, although rats waited longer to approach the objects and spent more time engaging in non-object oriented locomotor activity. The current report describes a water maze testing paradigm in which mice and rats perform comparably during acquisition training, and identifies novel parallels in recognition memory across rats and mice.
Materials and Methods
Animals and Housing Conditions
Two month old male Sprague-Dawley rats (N=8) were purchased from Harlan for use in these studies. Six week old male C57Bl/6J mice (N=8) were purchased from Jackson Laboratories. All rats and mice were housed individually with food and water available ad libitum on a 12 hour light-dark schedule for a minimum of three weeks prior to behavioral testing. The behavioral testing procedures and housing conditions following NIH guidelines and were approved by the National Institute on Aging Animal Care and Use Committee.
Water Maze
After acclimating to the facility for three weeks, rats and mice were tested in the hidden platform version of the water maze between 7-11AM (lights on at 6AM). Testing took place in a circular water maze tank (162cm diameter) filled to a depth of 75 cm with water made opaque with white, nontoxic paint. The pool was surrounded by a clear plexiglass wall that extended 50 cm above the surface of the water. Cues were hung at four locations at the north, west, south, and east corners of the plexiglass wall. Water temperature was maintained at 28°C, and the circular escape platform (15 cm diameter) was submerged 1–2 cm below the water surface. Animals received six days of acquisition training, consisting of four trials per day, with an intertrial interval of approximately ten minutes. Each trial lasted until the animal found the platform, or for a maximum of sixty seconds, and animals that failed to find the platform within sixty seconds were guided there by the experimenter. On each trial they were placed into the pool, facing the wall, starting from one of four potential locations (north, south, east, or west), with start locations varied over each of the four trials. One day after the last acquisition training session, animals were tested in a single sixty–second probe trial without the platform. The following day, the cues were removed and animals received four trials in the visible platform version of the water maze. Data were acquired and analyzed using the HVS2020 automated tracking system (HVS Image, UK).
Novel Object Preference Testing
The same animals used for spatial learning experiments were also tested for object recognition memory. The objects were presented in the home cage (19.1cm × 29.2cm × 12.7cm cage dimensions) and the position of the objects was varied across trials as shown in Supplementary Figure 1. Duplicate objects were used to avoid olfactory cues (for images of objects used in this study, see Supplementary Figure 1) and testing took place at the onset of the dark phase under red-light illumination (18:00). On day 1, animals were videotaped for ten minutes as an index of baseline motor activity. On day 2, animals were videotaped while exploring a pair of identical objects (recognition training; objects were two identical plastic barbells, shown in Supplementary Figure 1), and thirty minutes later the animals were videotaped for five minutes while interacting with 1 familiar and 1 novel object (objects consisted of a plastic barbell and a plastic ball, shown in Supplementary Figure 1). One hour later the animals were again videotaped for five minutes as they explored a familiar and a novel object (objects consisted of a plastic barbell and a plastic jack, shown in Supplementary Figure 1). The following day, animals were again videotaped while exploring 1 familiar and 1 novel object for five minutes (objects consisted of a plastic barbell and a square made of dominoes which were glued together as shown in Supplementary Figure 1). The position of the novel and familiar objects within the home cage was rotated between trials as shown in Supplementary Figure 1. In a separate group of experimentally naïve animals, we also tested for baseline preference rates among the objects, and observed no significant differences in exploration.
Videotapes were viewed at half-normal frame rates to time the exploratory intervals. An ‘exploratory interval’ was initiated when the animal's nose was within 5mm of the object and terminated when the nose was moved away. Climbing on the objects without nose contact was not counted as an exploratory interval. Freely available Etholog software was used to time the exploratory intervals (http://www.ip.usp.br/ebottoni/EthoLog/ethohome.html). The three retention intervals were selected on the basis of previous studies demonstrating sensitivity to genetic manipulations using a similar testing paradigm in mice (Rampon et al., 2000; Stranahan et al., 2008).
Statistics
Water maze data (latencies, swim speeds, and path lengths) were analyzed using repeated measures ANOVA with Bonferroni's post hoc. Retention data from the water maze (percent of time in target, adjacent, and opposite quadrants) were also compared across rats and mice using one-way repeated measures ANOVA with Bonferroni's post hoc. Average distance from the target location during the probe trial and path lengths during the visible platform test were compared across rats and mice using bidirectional student's t-tests. For the novel object recognition tests, ‘percent novel’ was calculated based on the amount of time spent exploring the novel object relative to the total time spent exploring both objects. This percent novel was judged to be greater than 50% (chance) following a one-sample t-test, which was conducted separately for each species at each time point. Percent novel object exploration was then compared between mice and rats over the different post-training intervals using repeated measures ANOVA with Bonferroni's post hoc. A repeated measures ANOVA design was also used to compare latency to explore and total time spent exploring both objects between mice and rats, again with Bonferroni's post hoc. Baseline motor activity (percent of time spent moving) was compared between mice and rats using bidirectional student's t-tests. All analyses were conducted using Graphpad Prism software and statistical significance was set at p <0.05.
Results
Spatial learning in the water maze is comparable between mice and rats
Following six days of training with four trials per day, mice consistently exhibit longer latencies to find the hidden platform than rats (Figure 1A; F(1,14)=21.01, p=0.003). However, this was probably not due to impaired learning, as mice also swim more slowly than rats (Figure 1B; F(1,14)=40.65, p=0.001). Escape latency was correlated with swim speed, such that faster swimmers found the platform more quickly (Pearson's r = -0.55, p = 0.03). By contrast, path lengths were not correlated with swim speed (p > 0.05 following Pearson's correlation). Based on the comparable path lengths taken during spatial navigation (Figure 1C; F(1,14)=0.72, p=0.41), it is likely that, under these training conditions, mice and rats perform similarly with regard to spatial memory acquisition.
Figure 1. Differences in swim speed and spatial memory retention between mice and rats in the water maze paradigm.
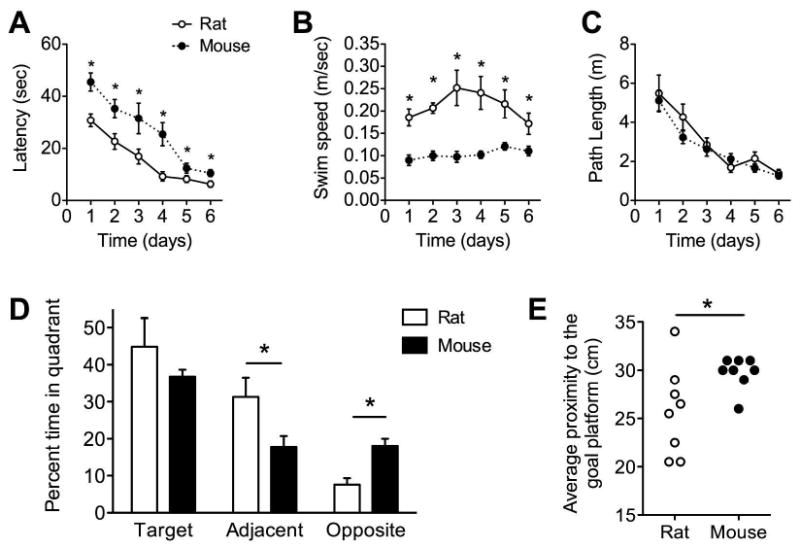
(A), Mice required more time to locate the hidden platform in the water maze test of hippocampal function. (B), Mice swim slower than rats during water maze training. (C), Path lengths are comparable between mice and rats, suggesting that both species are similarly able to navigate effectively to the hidden platfom. (D), Although mice and rats spend comparable proportions of time searching in the platform quadrant during the probe trial, mice spend less time in the adjacent quadrants, and more time in the opposite quadrant, relative to rats. Data in panels (A-C) were analyzed using one-way ANOVA with Bonferroni's post hoc. Asterisk (*) indicates significance at p<0.05 and error bars represent s.e.m. (E), Rats searched more accurately than mice during the probe trial, indicated by shorter average distances to the platform location. Asterisk (*) indicates statistical significance at p<0.05 following bidirectional t-test with correction for heterogeneity of variance.
Mice and rats exhibit different patterns of spatial bias during retention testing (F(1,14)=5.49, p=0.03; Figure 1D). Although rats and mice spend comparable proportions of time searching in the target quadrant, rats spent more time than mice in the adjacent quadrants (t(14)=2.27, p=0.04), and rats spent less time than mice searching in the opposite quadrant (t(14)=4.03, p=0.001). Rats also spent more time searching in the vicinity of the target, as indicated by shorter average distances to the platform location during the probe trial (Figure 1E; t(14)=2.31, p=0.03). Species differences in the mean distance from the platform location remained statistically significant after correcting for heterogeneity of variance (Welch's corrected t(8)=2.31, p=0.04). Path lengths to find a visible platform were comparable across rats and mice (t(14)=1.62, p=0.13).
Novel object recognition is similar in mice and rats
Mice and rats exhibit a bias in favor of novel stimuli. This bias was detected at all post-training time points, indicated by greater than fifty percent preference for the novel object following one-sample t-tests (Figure 2A; 30min posttraining: for rat, t(7)=3.31, p=0.01, for mouse, t(7)=6.32, p=0.004; 60min posttraining, for rat, t(7)=7.73, p=0.001, for mouse t(7)=8.76, p=0.001; 24hr posttraining, for rat, t(7)=3.85, p=0.006, for mouse, t(7)=3.20, p=0.01). The degree of preference for the novel object was indistinguishable between mice and rats (F(1,14)=0.95, p=0.34). Moreover, the amount of time spent exploring the objects (novel + familiar) was similar between mice and rats (Figure 2B; F(1,14)=2.86, p=0.11). Differences emerged when we examined exploratory patterns. Rats showed no difference relative to mice in their latency to explore the testing space when objects were not present; however, when objects were present in the arena, rats waited longer than mice to approach either object (Figure 2C; F(1,14)=4.58, p=0.002). This could not be attributed to an overall suppression of locomotor activity in rats, because rats spent more time moving than mice during the habituation period when no objects were present (Figure 2D; t(14)=4.05, p=0.001).
Figure 2. Comparable cognitive capacity, but distinct patterns of locomotor activity, during novel object preference testing in mice and rats.
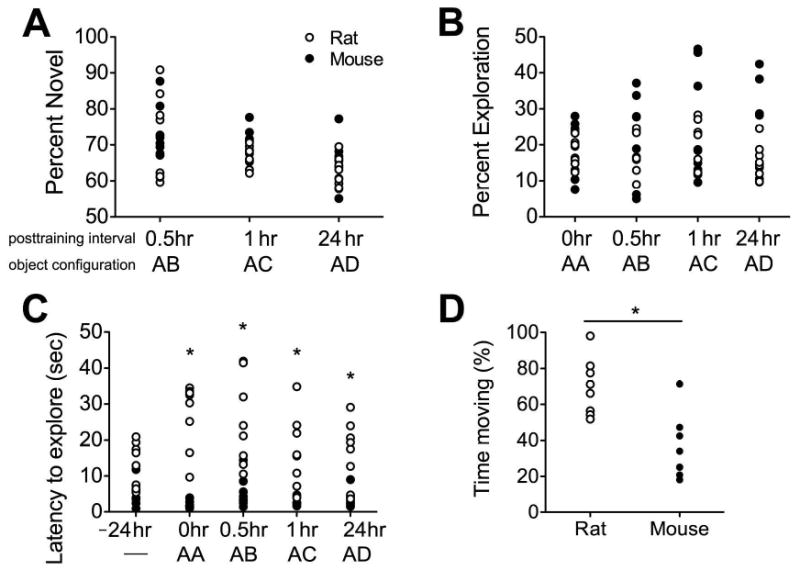
(A), Bias in favor of the novel object was similar in mice and rats across all post-training time points. (B), The amount of time spent exploring both objects was indistinguishable between mice and rats during both the training trial with two identical objects, and the test trials with one novel and one familiar object. (C), Latency to approach the objects was significantly greater in rats, relative to mice. Asterisk (*) indicates statistical significance at p<0.05 following repeated measures ANOVA with Bonferroni's post hoc. For panels (A-C), the posttraining interval is shown relative to time of training with two identical objects. Object configurations are represented alphabetically; — = no objects; AA = two identical objects; AB, AC, AD = familiar+novel pairings (see Supplementary Figure 1 for images of objects and their placement). (D), Rats spend more time engaging in locomotor activity than mice when no objects are present. Asterisk (*) indicates statistical significance at p<0.05 following bidirectional student's t-test.
Discussion
In the current report, mice and rats exhibit differences in spatial memory retention, similarities in object recognition memory, and distinct patterns of exploratory behavior. Rats swam faster and engaged in more locomotor activity relative to mice, but they also waited longer before exploring the test objects, indicative of possible differences in neophobia. Mice swam more slowly, but the efficiency of their spatial navigation (as measured by path length during acquisition trials) was comparable to that of the Sprague-Dawley rats. Despite attaining comparable levels of performance during acquisition training, rats perform better during probe trials, indicative of superior retention. Taken together, these observations support the existence of species differences in spatial memory and exploratory behavior between mice and rats.
This comparative analysis was conducted using pigmented C57Bl/6J mice and albino Sprague-Dawley rats. Because albino rodents are known to have poor visual acuity relative to pigmented rodents, one alternative interpretation could involve differences in visual acuity, which might handicap the albino rats, thereby obscuring any potential superiority in their learning ability. However, mice and rats in the current study were equally capable of swimming towards a visible platform, indicated by similar path lengths during cued training in the water maze. Therefore, based on their performance in the visual platform version of the maze, it is unlikely that differences in visual acuity can account for differences in performance of mice and rats in the water maze and object recognition tasks.
The tasks used here recruit the hippocampus and associated cortical structures (Morris et al., 2003; Broadbent et al., 2009). Clear anatomical differences in hippocampal volume distinguish between mice and rats, and more subtle differences in the extent of intracortical connectivity have also been demonstrated (van Groen et al., 2002). However, the dendritic length and spine density of hippocampal CA1 neurons are comparable in Sprague-Dawley rats and C57Bl/6J mice (Routh et al., 2009). Taken together with similarities in their cognitive abilities during acquisition training in the water maze and in the object recognition testing paradigm, similarities in dendritic structure suggest that neuronal morphology, as opposed to regional volume, is more closely associated with learning capacity. In this regard, mouse strains with fewer hippocampal spines relative to rats and C57Bl/6J mice, such as the 129/Sv (Routh et al., 2009), would be predicted to learn less efficiently. This is indeed the case, as the 129/Sv strain performs poorly relative to the C57Bl/6J strain across a number of hippocampus-dependent learning paradigms (Motkowski et al., 1997; Smith et al., 2007). While mice and rats are clearly different with regard to their gross anatomy, the cognitive capacities of Sprague-Dawley rats and C57Bl/6J mice are similar during acquisition training in the water maze, and during object recognition testing. Comparable performance of rats and mice during acquisition training differs from a previous report (Frick et al., 2000), but spacing of acquisition trials over six days in the current study, as opposed to the massed training protocol used by Frick and colleagues (2000), could account for differences in the results. Additional studies designed to directly compare cognition across a larger number of species and strains are warranted to determine whether interspecies variability is greater or smaller than interstrain variability in learning capacity.
Supplementary Material
Acknowledgments
A.M.S. is currently supported by start-up funds from the Physiology Department at Georgia Health Sciences University. Over the course of the project, A.M.S. was funded by a grant from the National Institute on Aging (F31 AG024690).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2009;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–5. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Montkowski A, Poettig M, Mederer A, Holsboer F. Behavioural performance in three substrains of mouse strain 129. Brain Res. 1997;762:12–8. doi: 10.1016/s0006-8993(97)00370-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–86. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–44. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Harris K, Chitwood RA. Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol. 2009;102:2288–302. doi: 10.1152/jn.00082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Lee K, Cutler RG, Egan JP, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature Neuroscience. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav. 1995;58:687–93. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996;60:1191–7. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–70. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Species differences in the projections from the entorhinal cortex to the hippocampus. Brain Res Bull. 2002;57:553–6. doi: 10.1016/s0361-9230(01)00683-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


