Abstract
Degeneration of lumbar facet joints (FJs) has been implicated in lower back pain. To verify the biological links between cellular and structural alterations within FJ components and development of symptomatic chronic back pain, we generated an animal model for FJ degeneration by intra-articular injection of monosodium iodoacetate (MIA) in FJs (L3/L4, L4/L5, L5/L6) of Sprague Dawley rats followed by behavioral pain tests. The degree of primary hyperalgesia was assessed by measuring pain sensation due to pressure using an algometer, which mimics a mechanical stimulus for FJ injury. Biochemical assessments and µCT imaging revealed severely damaged FJ cartilage, proteoglycan loss and alterations of subchondral bone structure by MIA injection. The µCT analyses further suggested that the behavioral hyperalgesia from FJ degeneration is not associated with foramina stenosis. These biological and structural changes in FJs are closely related to sustained and robust chronic pain. Therapeutic modulation of chronic pain using pharmaceutical drugs was investigated in the facet joint osteoarthritis animal model. Morphine and pregabalin markedly alleviate pressure hyperalgesia while celecoxib (selective inhibitor of COX-2) and ketorolac (inhibitor of COX-1 and -2) demonstrate moderate to negligible anti-hyperalgesic effects, respectively.
Keywords: Lumbar facet joint, osteoarthritis, chronic pain animal model, monosodium iodoacetate, low back pain, pressure hyperalgesia
INTRODUCTION
Lower back pain (LBP) is a prevalent neuroskeletal condition and an estimated 80% of the population will suffer from LBP at some period in their lives (1–3). LBP frequently recurs, and chronic LBP is a main factor that limits activity in young adults under the age of 45, interfering with the daily lives of patients and eventually deteriorating their quality of life. The financial burden associated with this condition is formidable and includes both direct medical expenses and indirect costs due to decreased productivity in the workplace. Thus, studies on the etiology of LBP may have broad impact and could ultimately resolve both the health and socio-economic issues associated with this affliction. The functional spinal unit consists of two vertebrae, the intervertebral disc and the posterior elements (the facet or zygapophysial joints and ligaments). Degeneration of intervertebral disc and/or facet joint osteoarthritis (FJOA) is considered a clinically important source of chronic low-back pain (1). Induction of FJOA in an animal model by intra-articular injection of collagenase in the FJ has been reported (4). Nevertheless, there are no in vivo behavioral animal studies which provide direct evidence for the postulated link between FJ degeneration and chronic back pain. Therefore, it is necessary to establish an animal model for OA-like degeneration of FJ that permits correlations with the development of chronic pain behavior as an essential step that may lead to the development of therapeutic agents that can ameliorate pain associated with FJ degeneration.
FJs are the only synovial joints in the spine, with hyaline articular cartilage overlying subchondral bone, a synovial membrane, and a joint capsule which resemble knee joints. Monosodium iodoacetate (MIA), which is an inhibitor of glycolysis that disrupts metabolism in chondrocytes, has been widely used for several OA animal models, including knee joint OA (5) or disc degeneration animal model (6), and have provided biological insights into OA-induced progressive pathological changes in knee joint structures and OA-associated behavioral pain analyses (5, 7). Although comparative gene array data demonstrate substantial difference between the MIA-induced OA animal model and human OA (8), many other investigators have shown that intra-articular injection of MIA causes joint tissue damage that may mimic clinical OA in patients (5, 7). The main objective of this study is to establish links between FJ degeneration and the development of chronic back pain by examining the pathological lesions in cartilage and structural changes in subchondral bone in FJ upon intra-articular MIA administration. In addition, this study explores therapeutic modulation of chronic pain in a facet joint OA model by testing selective drugs for analgesic effects on back pain relief.
MATERIALS AND METHODS
Induction of osteoarthritis in FJ
Sprague Dawley rats (N=32; weight 200–220g) were housed under standard laboratory conditions (in a temperature-controlled (21±1°C) room with a normal 12-h light/dark cycle). The procedures used in this study were in agreement with the guidelines of the Rush Institutional Animal Care and Use Committee (IACUC). For induction of MIA-induced FJ degeneration, rats were anesthetized with 1.5 % isoflurane (Abbott Laboratories, North Chicago, IL, USA) in oxygen in a prone position. Following a 2-cm midline skin incision, the left paraspinal muscles were retracted exposing the left L3/L4, L4/L5, and L5/L6 FJs. Using a 26 gauge (26-G) needle attached to a 10 µL syringe (Hamilton model 701N, Reno, NV, USA) and a plastic sleeve around the needle to limit penetration to 2 mm, animals were given a single intra-articular injection of 0.02 mg of monosodium iodoacetate (MIA; Sigma, St. Louis, MO, USA; cat #I2512) through the FJ capsular tissue into each of the 3 FJs (N=22 animals) (see supplement Material and Methods, Figure 1). MIA was freshly dissolved in physiologic saline (0.9 % sodium chloride) and administered in a volume of 1 µL using a Hamilton microsyringe. A surgical sham group of animals (N=10) was generated in which all aspects of the MIA injection surgery were duplicated, except that the tip of the 26-G needle was firmly placed against the surface of the FJ, but did not puncture it.
Animal behavioral tests
Vocalization threshold in algometer test
Vocalization threshold to the force (g) of an applied force gauge was measured by pressing the 0.5 cm2 device tip directly on the skin over the ipsilateral facet joint region (left side) and comparing it with the force threshold on the the contralateral control side (right side) (see supplement Material and Methods, Figure 2).
Straight leg raising test
The hind leg was stretched out (knee at full extension) and lifted (hip flexion) for two seconds and the number of vocalizations in response to five leg lifts (five seconds apart) was recorded as previously described (see supplement Material and Methods, Figure 3). A negative straight leg raising test in our study would suggest that the hyperalgesia that develops after MIA injection does not involve nerve root compression.
Drug Treatment
For drug administration experiments, at 4 weeks after induction of OA in FJs, MIA injected rats (N=12) were gently restrained and given 0.5 mL drug solution either intraperitoneally (i.p.) or by oral gavage (p.o.). Drugs tested systemically were the: α2δ calcium channel blocker pregabalin 5–20 mg/kg p.o. (Pfizer, Piscataway, NJ), COX-2 selective inhibitor celecoxib 50 mg/kg p.o. (Pfizer), mixed cyclooxygenase (COX-1/COX-2) inhibitor ketorolac tromethamine 20.0 mg/kg i.p., µ-opioid agonist morphine sulfate 6.7 mg/kg i.p., and drug vehicle. The drug vehicle for i.p. delivery was saline (0.9% sodium chloride injection), and the drug-suspending vehicle Ora-Plus (Paddock Labs, Minneapolis, MN) was used as a control for p.o. administration. Pressure hyperalgesia was performed at a maximum of 4 time points after drug injection to limit local tissue hypersensitivity due to the repeated noxious stimulus itself.
Histology
The vertebral lumbar motion segments were fixed in 4% paraformalin and decalcified in EDTA solution (solution changed every 5 days) and then was cut in the transverse plane and paraffin-embedded. Serial FJ sections of exact 5 µm thickness were obtained to prepare slides. Safranin-O Fast Green stain was performed to assess general morphology and the loss of proteoglycan in cartilage ground substance. Hematoxylin and eosin (H&E) staining was performed to assess general morphology and immunohistochemistry (IHC) staining of CD11b was performed to detect the inflammatory cells using anti-CD11b antibody (Cell Signaling) (9).
µCT
Structural alterations of FJ cartilage surface and subchondral bone architecture were evaluated by microscopic examination and µCT scanning. Freshly dissected lumbar motion segments were immediately fixed in 10 % formalin followed by µCT imaging analyses in the Rush Imaging Core Facility using a Scanco Model 40 Desktop µCT. Microscopic analyses were performed to examine structural alterations in the FJ region using a Nikon SMZ1000 (Model #3.2.0, Diagnostic Instrument, Inc. Sterling Heights, MI). L4 lumbar FJs were scanned in a 10 mm region of the intact rat vertebral column at high resolution (20 mm tube, 10 µm resolution, 55 kVP, 145 µA, 300 ms integration time. Foraminal stenosis was evaluated by determining potential size alterations of intervertebral foramen. Briefly, the intact spinal lumbar segments were dissected and imaged by µCT with proper orientation so that the spinal processes were parallel to the x-ray path using 36 mm istotropic voxels with the same parameters that we described above. The images were filtered using sigma of 0.8 and support of 1.0, and segmented at a threshold of 270 (Scanco Evaluation Program V6.0). Three-dimensional renderings were generated. Using the “subdim” function in the manufacturer’s software, para-sagittal slabs which were 20 slices thick (i.e., 720 µm thick) were examined until the intervertebral foramen of interest was centered in the image. The caudal-cranial (foraminal width) and ventral-dorsal (foraminal height) dimensions were then determined by blinded tests. Foraminal height and width were calculated, and T-tests were performed.
Statistical analysis
To characterize the pain model, algometer pressure threshold measures over the 7-wk post-operative period were compared between different FJ surgical groups using a general linear model for repeated measures and a post-hoc Tukey-B test (SPSS 17.0 software). Behavioral pain measures after drug injection were compared among different drugs using a general linear model for repeated measures and a post-hoc Tukey-B test. For drug-response analysis, data was converted to a percent maximum possible effect (%MPE), and the ED50 computed with probit analysis
RESULTS
Intra-articular injection of MIA in to FJs causes OA-like joint degeneration with alteration of subchondral bone structure
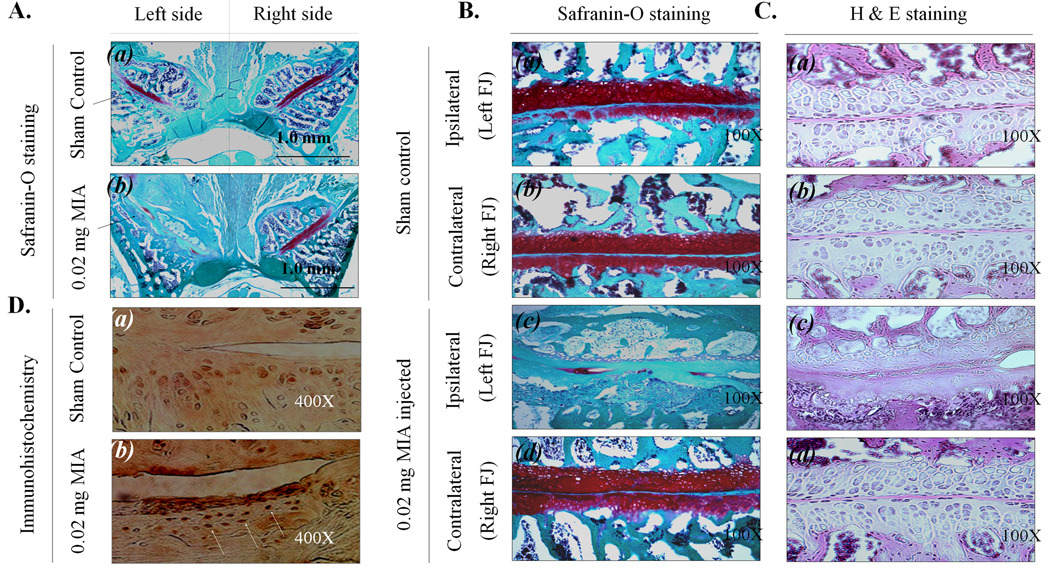
We initially performed a set of experiments for titrations to assess the minimal concentration and volume of MIA required for induction of OA (i.e., injections of 1 mg, 0.5 mg, 0.1 mg, 0.05 mg or 0.02 mg MIA per FJ) (data not shown). We determined that a dose of 0.02 mg MIA per FJ in 1 µl volume is optimal, because this amount causes slower destruction of FJ structural components, but produces sufficient behavioral pain responses. The histology of FJ cartilage in the sham surgery group [Fig. 1A (a)] and the experimental group with MIA injections (0.02 mg into the left side of FJ) [Fig. 1A (b)] was compared in equivalent sections for each sample. Tissue structures observed in sham surgery animals and MIA-injected rats are shown in Fig. 1B & C (a)–(d). Histological examination of articular cartilage in the sham surgery group reveals moderate proteoglycan depletion [Fig. 1B&C (a)] compared to FJ cartilage on the contralateral side Fig. 1B&C (b) or the contralateral FJ in the MIA-injected group Fig. 1B & C (d), which showed no sign of tissue degeneration during any time interval. The articular surface of each control FJ was smooth and the matrix was densely stained (red) with Safranin O. In the group injected with 0.02 mg MIA per FJ, all rats showed severe cartilage degeneration, proteoglycan loss and structural changes in the ipsilateral FJ components as reflected by surface irregularities and denudation at week 7 Fig 1B &C (c). As shown in Fig. 1D, CD11b immunoreactivity of cells on the MIA-injected side was increased, suggesting that MIA-induced L4/L5 FJ degeneration is accompanied by local inflammatory response.
Figure 1.
The histological analysis of MIA-injected L4/L5 FJ (7 wk post-surgery). A. L4/L5 FJ was sectioned horizontally and stained with Safranin-O. Bar is 1.0 mm. B & C. Magnified L4/L5 FJ stained with Safranin-O (100X) (B) and stained with H & E (100X) (C). D. Immunohistological analysis with CD11b.
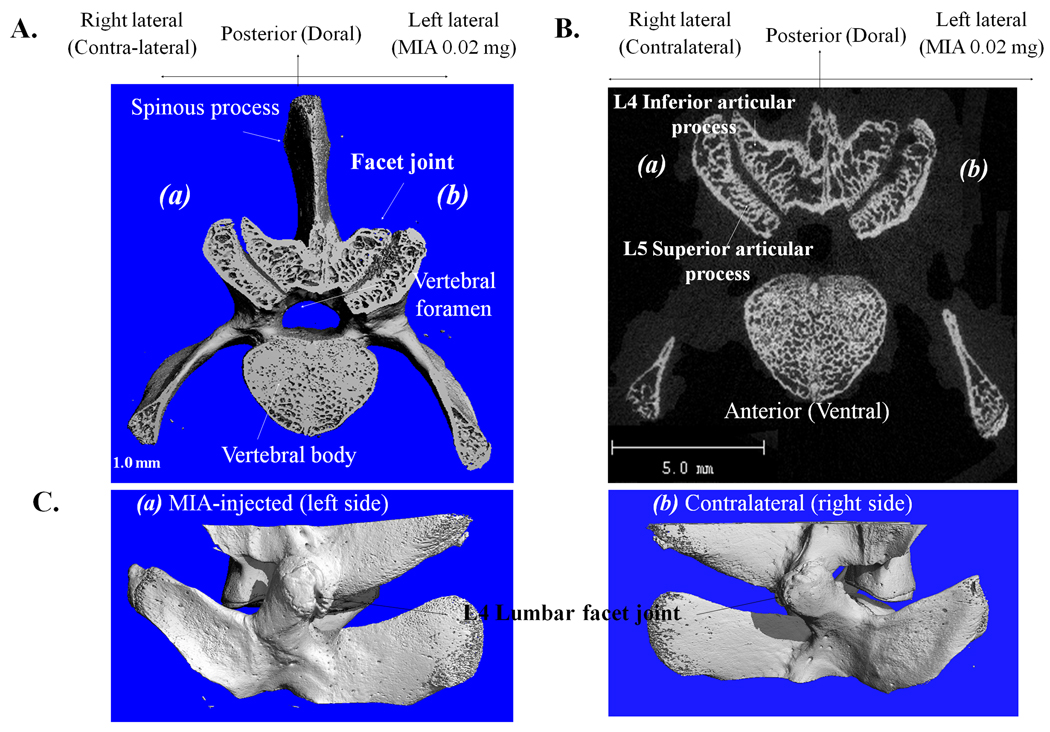
Three-dimensional µCT scans of subchondral bone of MIA-induced FJs revealed structural modifications that are similar to those observed in MIA-induced rat knee joint OA (5) and human OA pathology (10). As evidenced by cranial views (Fig. 2A) and cross-sectional images (Fig. 2B), the surface rendering of the subchondral plate indicates that FJ surface topography in MIA-injected specimens is considerably altered as evidenced by severely elevated (‘heaved’) and depressed (‘sunken’) surfaces [Fig. 2A&B (b)] compared to the contralateral side [Fig. 2A&B (a)]. In the transverse plane of the ipsilateral FJs, pathological changes are evident in large regions beneath the subchondral plates, reflected by the changes in bone structure. Three-D renderings of lateral views of FJs suggest that there are small osteophyes on the left FJ margins [Fig. 2C (a)] compared to the contralateral control (right side) [Fig. 2C (b)].
Figure 2.
Three-dimensional µCT scans of subchondral bone of MIA-induced FJs. A. Cranial view and B. Cross-sectional image of µCT (a) contralateral intact control and (b) MIA-induced FJ L4/L5. C. Lateral view of FJ (a) MIA (0.02mg)-injected and (b) contralateral intact normal.
Primary degeneration of FJs is associated with pressure hyperalgesia and development of chronic pain
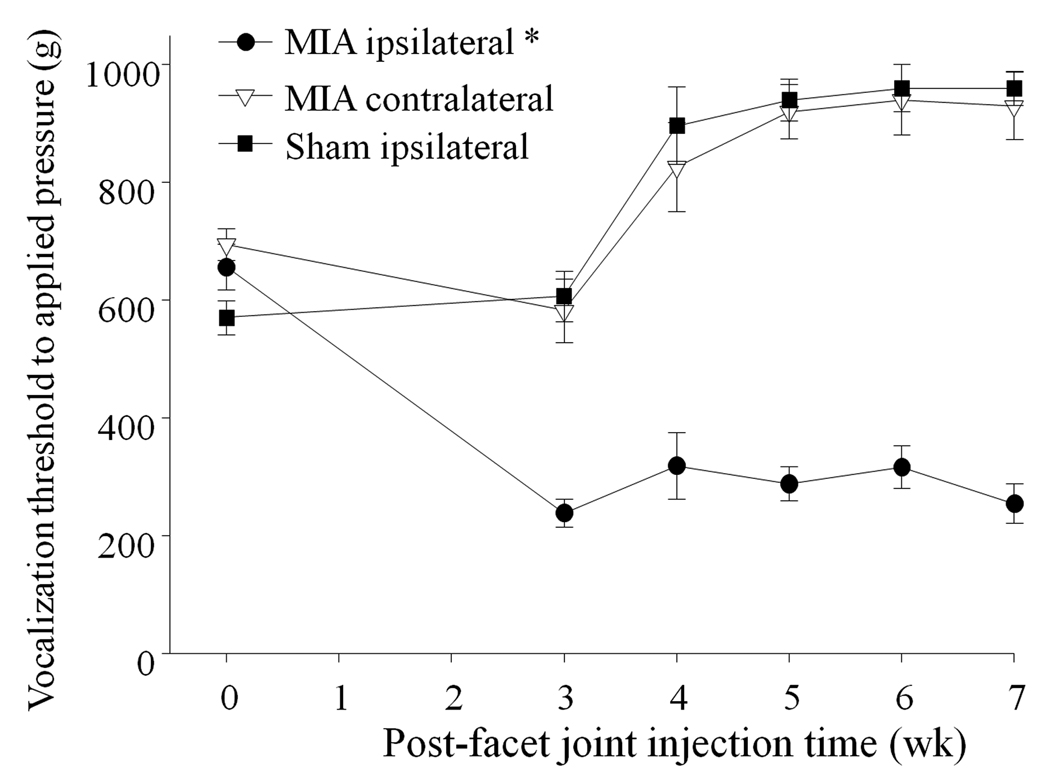
We performed behavioral pain assessments to investigate whether the biochemical changes in FJs induced by MIA injection (e.g., cartilage loss, structural alterations in FJ components and the subchondral bone region) are accompanied by back pain. Primary mechanical hyperalgesia was determined by comparing the vocalization threshold (g) upon stimulation of either the MIA-treated degenerated FJs (ipsilateral, left side) or the contralateral joint (right side) in an algometer test. We also assessed the pressure-induced vocalization threshold of a surgical sham control group (ipsilateral, left side FJs) to be compared with the experimental group. A difference among groups was seen over the 7-wk post-MIA injection period (F=88, p<0.001). The vocalization pressure threshold on the MIA-injected ipsilateral side of the lower back was less than the ipsilateral side of surgical sham animals or the contralateral side of MIA-injected animals (Fig. 3), indicating ipsilateral primary hyperalgesia and the development of chronic pain. In surgical sham rats, the vocalization pressure threshold on the ipsilateral side was no different from the contralateral side (e.g., 896± 66 vs. 898± 64 g at week 4; P=0.983), and just below the protective cut-off of 1000 g pressure (data not shown).
Figure 3.
Behavioral pain assessment over a 7-wk time course by measurement of the vocalization threshold using a pressure algometer. MIA-injected ipsilateral FJ animals had greater ipsilateral pain sensitivity (*) than on the contralateral side, and more pain sensitivity than animals with sham surgery FJs. Data are presented as mean ±SEM.
The straight leg raising test is clinically relevant and is a useful tool to separate radicular from axial pain (11–12). Our straight leg raising test results show no response (0/5 trials) from both experimental and sham control groups, suggesting that the behavioral changes in the experimental symptomatic pain group may not be due to radicular pain (data not shown).
FJ pain is not due to foraminal stenosis
Foraminal stenosis can induce severe radiculopathy because of nerve compression caused by the narrowing of intervertebral foramen. To ensure that the painful responses were not due to radiculopathy in our animal model, we performed µCT imaging analyses to determine possible size alterations of intervertebral foramen by measuring foraminal height (p=0.1823, n=8) on cranial view and width (p=0.4739, n=8) on both lateral views (Fig. 4A–D). Our data demonstrate that there is no significant difference in the sizes between the contralateral and ipsilateral foramina, suggesting that the FJ degeneration-induced back pain in our animal model is not due to foraminal stenosis.
Figure 4.
The analysis of foraminal stenosis on three-dimensional microCT images. A, foraminal height on transverse plan; B, foraminal width on coronal plan; C, foraminal width on lateral view; D, the measurement of foraminal size alteration between contralateral FJ and MIA-injected ipsilateal FJ (n=7).
Pregabalin and Morphine are highly analgesic for FJ degeneration-caused chronic pain
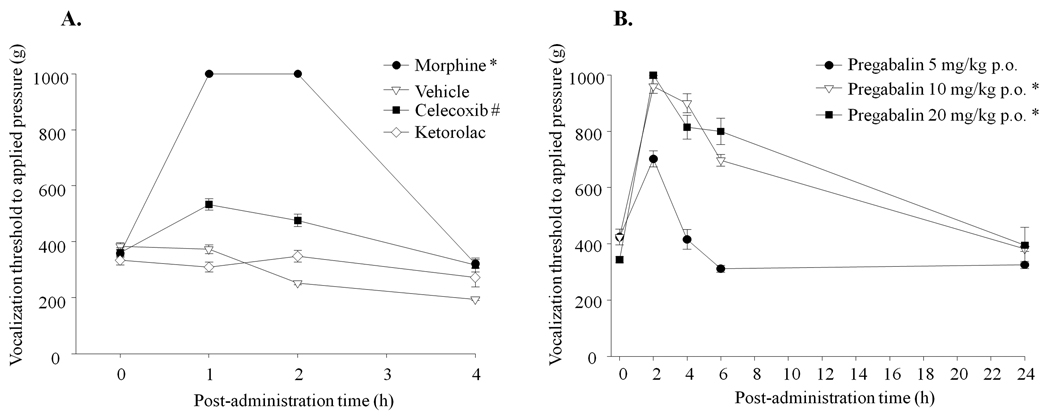
Selected clinically-used drugs for pain, such as pregabalin, morphine, celecoxib (COX-2 inhibitor) and ketorolac (mixed COX-1 and COX-2 inhibitor) were tested to examine the anti-hyperalgesic effects on primary hyperalgesia in the FJ OA model. A group of rats with systemic administration of vehicle was evaluated in parallel as a baseline for the vocalization threshold. There was a difference in anti-hyperalgesic effect among the drugs (F=381, p<0.001). Pain-related behavior to noxious physical stimuli was completely reversed by the administration of morphine sulfate (6.7 mg/kg) (Fig. 5A). The selective COX-2 inhibitor celecoxib (50 mg/kg) produced only moderate anti-hyperalgesic effects (% MPE = 27 % at 1 hr), while the NSAID ketorolac had no analgesic effect. For pregabalin, we performed a dose-response over the range 5–20 mg/kg and observed a difference in anti-hyperalgesic effects among the doses (F=76, p<0.001). Prominent anti-hyperalgesic effects were achieved by a single administration of the 10 mg/kg dose of pregabalin (Fig. 5B). The computed ED50, at the time of peak effect (120 min after administration) was 5.39 mg/kg. None of the drugs caused motor incoordination at the indicated doses as evaluated by the animal’s ability to remain on a rotating rod (rotarod) at 10 rpm for 300 sec (data not shown).
Figure 5.
Measurement of the vocalization threshold using a pressure algometer test to examine pharmacological efficacy of drugs on FJ pain (up to 24 hr). A. Systemic administration of morphine, celecoxib and ketorolac by either oral gavage or intraperitoneal injection followed by primary pressure pain assessment by algometer. *Morphine provided more pain relief than all other COX inhibitor drugs tested; #celecoxib produced a moderate anti-hyperalgesic effect. B. Dose-dependent anti-hyperalgesic effects by oral administration of pregabalin (5, 10 and 20 mg/kg) followed by behavioral pain assessment by algometer. *Potent anti-hyperalgesic effect at the higher doses of pregabalin (10 and 20 mg/kg). Data are presented as mean ±SEM. N=12 for each group.
DISCUSSION
FJ degeneration is believed to be secondary to osteoarthritic changes followed by disc degeneration in experimental animal models (13–14). Nevertheless, advanced degenerative changes of the FJs that are primed by disc degeneration, cannot be observed in such in vivo models. We developed a new FJ animal model for OA to investigate whether the degenerative process in FJ may be a primary contributor to pain sensation. Indeed, using a microinjection approach that recapitulates primary OA of the FJ, we determined that FJ degeneration has a primary role in back pain. Our data demonstrate that intra-articular microinjection of MIA induces osteoarthritic modifications in FJ. These changes include degradation of cartilage, loss of proteogycan, degradation of cartilage, apoptosis, remodeling of subchondral bone and osteophyte formation. The current observations resemble those presented in previous reports from our group and others that were obtained using a knee joint OA model involving intra-articular injection of MIA (5). Importantly, these structural changes of FJs are accompanied by sustained and robust pain throughout the experimental time period of 7 weeks. Collectively, our data suggest that unilateral injection of MIA in FJs provides a novel model for local cartilage loss with structural changes in subchondral bone by increased sensitivity to regional mechanical stimuli.
Using H&E staining, Yeh and colleagues demonstrated time- and dose-dependent degradation of cartilage, and evidence of infiltration of inflammatory cells in an animal model based on intra-articular injection of collagenase into FJs (4). In our study, we also observed immune cell recruitment at the site of injury after intra-articular injection of MIA into the FJ at the chronic time point (after 7 weeks). Our results suggest that inflammatory components may be involved in the facet joint degeneration-related chronic pain. Furthermore, our µCT imaging analyses as well as straight leg raising test results evidence that facet joint degeneration-induced pain in the experimental group of animals is axial but not radicular pain.
One key innovative point of our study is that we have applied an animal model in which we associated FJ degeneration with behavioral pain responses. This animal model may prove to be a useful tool for studies on mechanisms of vertebral OA and for the development of new strategies that can prevent the pain-causing structural and functional changes that characterize FJ degeneration. We anticipate that our model will provide the translational basis for the evaluation of new pharmacological drugs that intervene with the common and debilitating conditions associated with back pain caused by osteoarthritic FJs. In the future, further development of our animal model will permit a non-invasive approach for FJ degeneration followed by regeneration. This concern is particularly important when primary mechanical hyperalgesia is determined [e.g., by an algometer test (g)] during a slow healing process (> 3 weeks). Scar formation of soft tissue may interfere with the behavioral and pre-clinical assessments of pain, thus significantly confounding determination of symptoms, especially during the early stages of FJ degeneration following open surgery.
Primary pressure hyperalgesia in a rat FJ degeneration model responds to therapeutic intervention with the opioid morphine at 28 days after induction. The potency of morphine in reducing primary pressure hyperalgesia may indicate that there is an ongoing nociceptive process at the injury site. A single dose of pregabalin, a drug of the gabapentoid class, was also efficacious in reversing the unilateral pressure hyperalgesia in the FJ degeneration model with an ED50 = 5.39 mg/kg in the current studies. Modest but significant anti-hyperalgesic effects are achieved with a selective COX-2 inhibitor and no response is observed with an NSAID.
Although NSAIDs are the most frequently prescribed medication worldwide, it has been reported that “NSAIDs may not be best bet for low back pain” and apparently NSAIDs are not the “magic bullets” to alleviate back pain – see the commentary which is quoted verbatim from http://cme.medscape.com/viewarticle/569247. Another feasible reason why NSAIDs do not work very well and have only a moderate effect by celecoxib (COX-2 inhibitor) on our animal model would be, perhaps due to the early- and advanced-stage of FJ degeneration. Patients who usually take NSAIDs for back pain treatment may be early-stage of the symptom. Because MIA injection produces relatively rapid joint cartilage loss, the pain syndrome observed at 28 days post MIA may be considered advanced stage of OA. We have not assessed early-treatment of back pain using NSAIDs in the current study, and it would be extremely interesting to conduct a time-course effect of NSAIDs on back pain using this animal model as a future study. Based on our theory, drugs widely used to treat patients with OA such as NSAIDs may be of limited use once joint destruction is complete.
These results are consistent with the concept that chronic pain at 28 days after MIA FJ injection is a primarily a neuropathic syndrome rather than an inflammatory process, because gabapentoids are useful in patients with neuropathic pain (and in animal models of neuropathic pain) (15). In contrast, the efficacy of NSAIDs in neuropathic pain patients is still unclear (16). Indeed, these findings corroborate our recent report that chronic knee joint OA-like pain, caused by intra-articular MIA injection (5), was not relieved by NSAIDs. Because MIA injection produces rapid joint cartilage loss, the pain syndrome observed at 28 days post MIA should be considered an advanced stage of OA. Consequently, drugs widely used to treat patients with OA such as NSAIDs may be of limited use once joint destruction is complete. We conclude that primary pressure hypersensitivity in the rat FJ degeneration model responds best to classes of drugs (i.e., opioids and gabapentoids) that are utilized for the treatment of moderate to severe chronic pain in patients. This finding should enable future research on new therapeutic interventions that can lead to improvement in the treatment of OA patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by University Anesthesiologists S.C., Chicago, IL and grants from NIH (R01AR053220 to HJI), the Arthritis Foundation (to HJI), and the National Arthritis Research Foundation (to HJI). We also appreciate helpful support from Rush Imaging Core Facility (Director, Dr. Rick D Sumner) for µCT analyses.
REFERENCES
- 1.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;165:110–123. [PubMed] [Google Scholar]
- 2.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976) 1994;19(10):1132–1137. doi: 10.1097/00007632-199405001-00006. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer AC, Wang SC, Bogduk N, McNaught PJ, Laurent R. Prevalence and clinical features of lumbar zygapophysial joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54(2):100–106. doi: 10.1136/ard.54.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh TT, Wen ZH, Lee HS, Lee CH, Yang Z, Jean YH, et al. Intra-articular injection of collagenase induced experimental osteoarthritis of the lumbar facet joint in rats. Eur Spine J. 2008;17(5):734–742. doi: 10.1007/s00586-008-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 62(10):2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JS, Kroin JS, Im HJ. Lumbar disc injury causes chronic discogenic back pain: a novel animal model for disc degeneration. 7th Combined ORS meeting. 2010 2010E-0223. [Google Scholar]
- 7.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain. 2009;5:45. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barve RA, Minnerly JC, Weiss DJ, Meyer DM, Aguiar DJ, Sullivan PM, et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage. 2007;15(10):1190–1198. doi: 10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz AR, Coxon A, Maurer M, Gurish MF, Austen KF, Friend DS, et al. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J Immunol. 1998;161(12):6463–6467. [PubMed] [Google Scholar]
- 10.Appleton CT, McErlain DD, Pitelka V, Schwartz N, Bernier SM, Henry JL, et al. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9(1):R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6(4):e1000047. doi: 10.1371/journal.pmed.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroin JS, Buvanendran A, Cochran E, Tuman KJ. Characterization of pain and pharmacologic responses in an animal model of lumbar adhesive arachnoiditis. Spine (Phila Pa 1976) 2005;30(16):1828–1831. doi: 10.1097/01.brs.0000174276.73908.f0. [DOI] [PubMed] [Google Scholar]
- 13.Gotfried Y, Bradford DS, Oegema TR., Jr Facet joint changes after chemonucleolysis-induced disc space narrowing. Spine (Phila Pa 1976) 1986;11(9):944–950. doi: 10.1097/00007632-198611000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Moore RJ, Crotti TN, Osti OL, Fraser RD, Vernon-Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine (Phila Pa 1976) 1999;24(6):519–525. doi: 10.1097/00007632-199903150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456–472. doi: 10.1097/ACO.0b013e3282effaa7. [DOI] [PubMed] [Google Scholar]
- 16.Vo T, Rice AS, Dworkin RH. Non-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use? Pain. 2009;143(3):169–171. doi: 10.1016/j.pain.2009.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.