Abstract
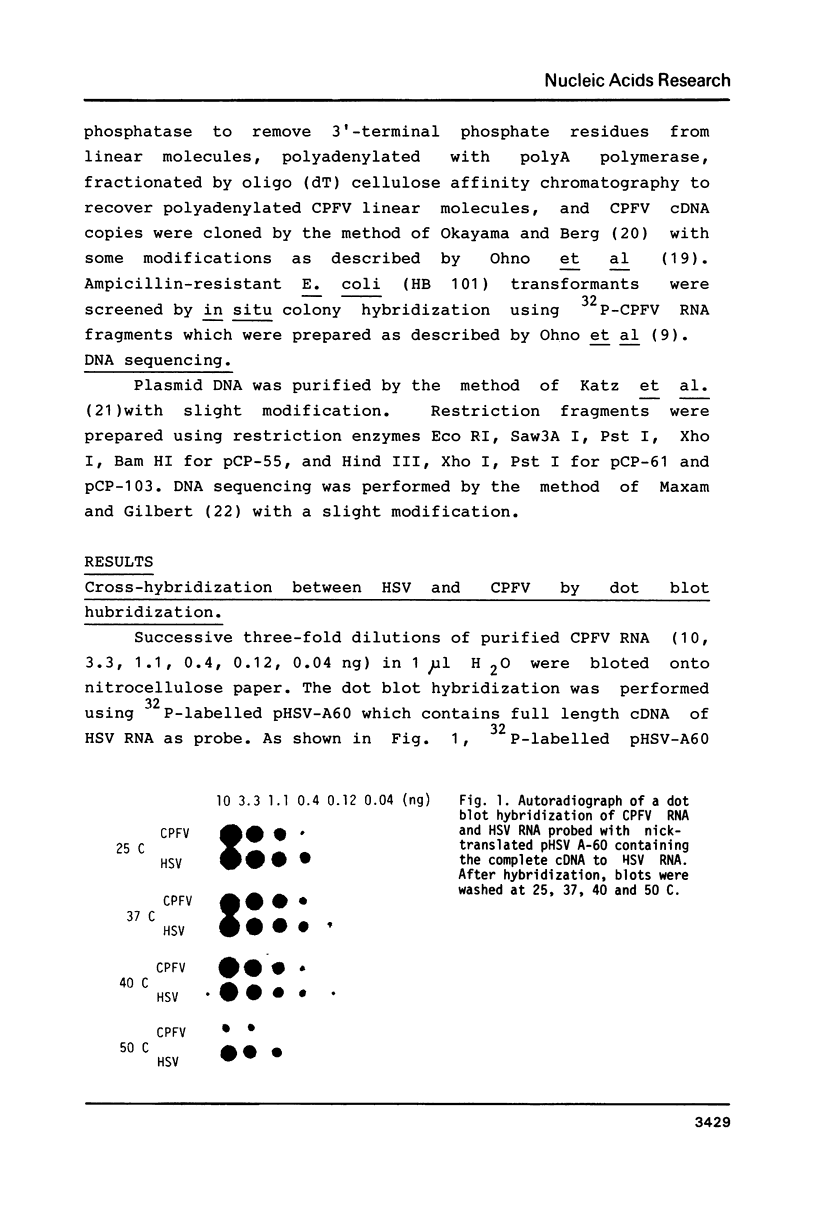
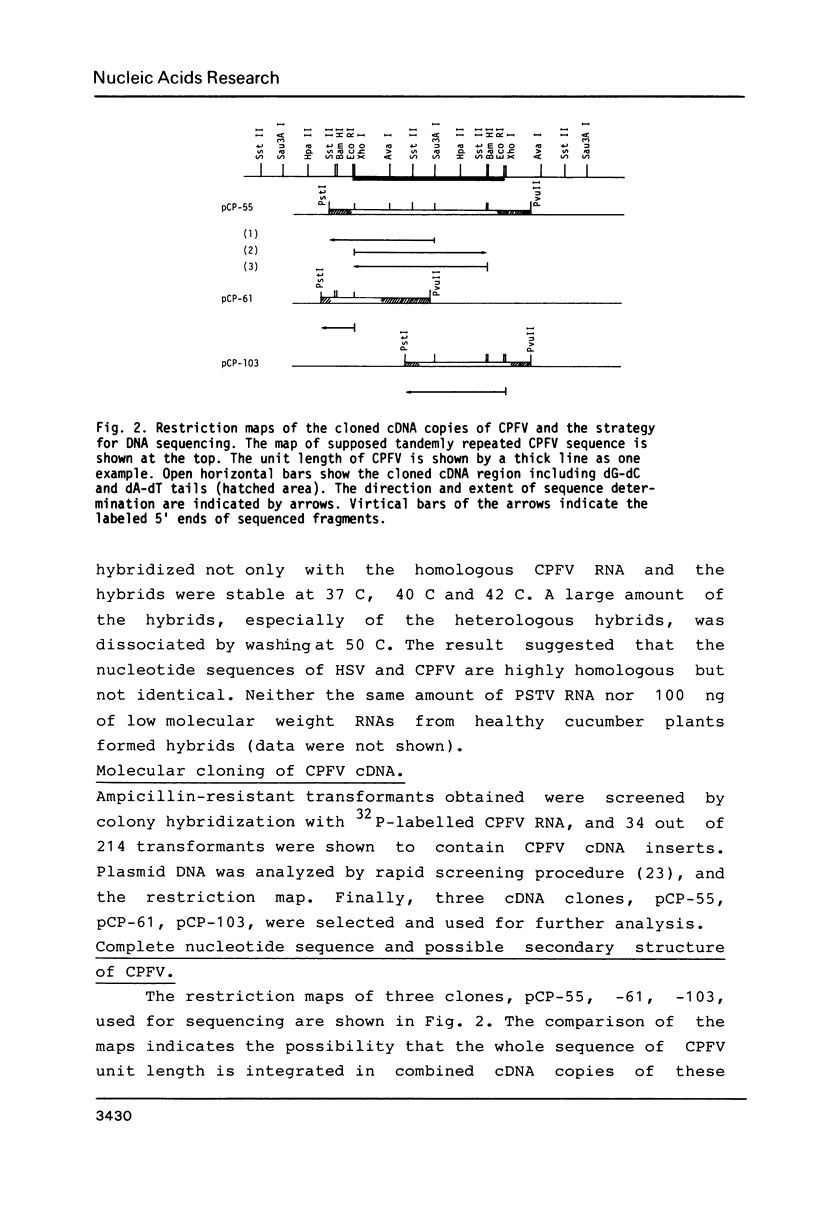
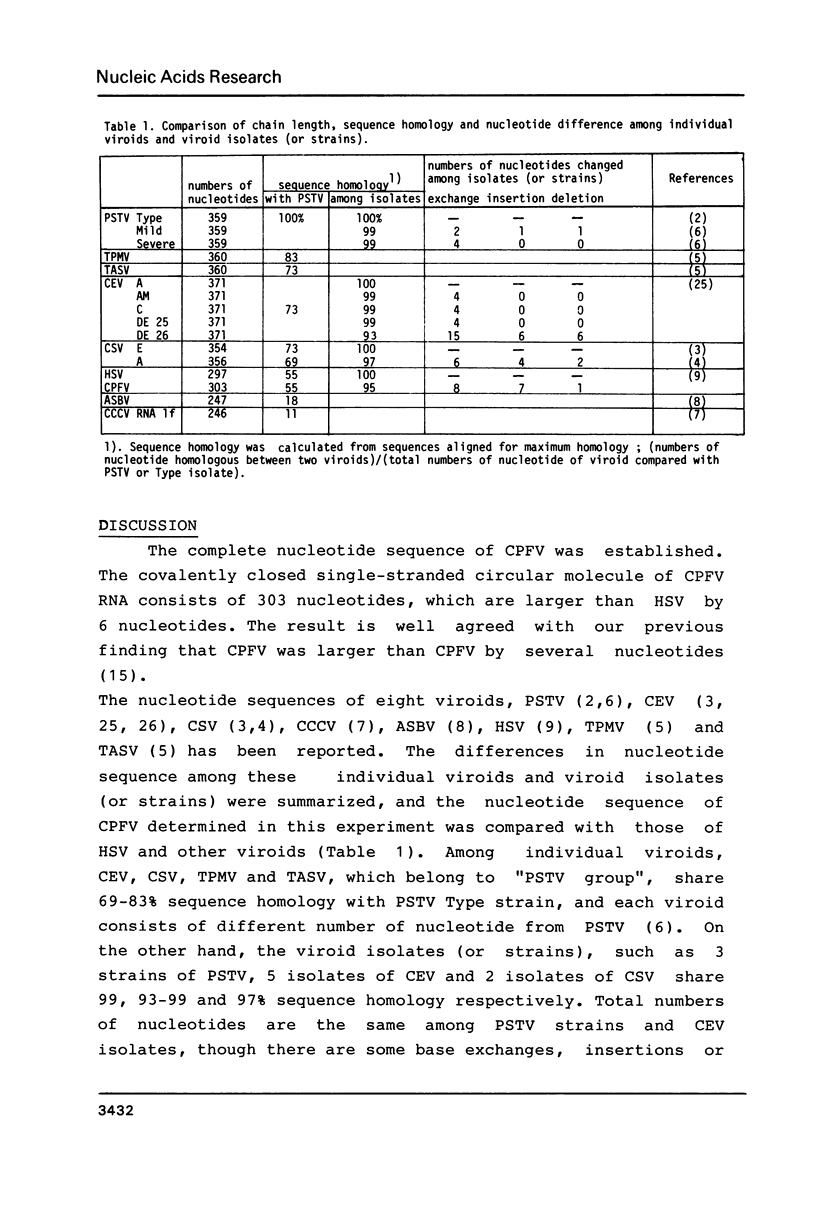
Double stranded cDNA of cucumber pale fruit viroid ( CPFV ) has been cloned by the method of Okayama and Berg (Mol.Cell.Biol.2,161-170 (1982] and the complete nucleotide sequence was established. The covalently closed circular molecules of single-stranded CPFV RNA consists of 303 nucleotides. The nucleotide sequence of CPFV was compared with the previously established sequence of hop stunt viroid (HSV), which consists of 297 nucleotides ( Ohno et al. Nucleic Acid Res.11,6185-6197 (1983]. CPFV differs from HSV in the nucleotide sequence at 16 positions which include 8 exchanges, 7 insertions and 1 deletion. Both viroids share about 95% sequence homology. Considering the pathogenic properties of both viroids together, it is concluded that CPFV is a cucumber isolate of HSV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]