Abstract
Non-enveloped viruses such as members of Picornaviridae and Reoviridae are assembled in the cytoplasm and are generally released by cell lysis. However, recent evidence suggests that some non-enveloped viruses exit from infected cells without lysis, indicating that these viruses may also utilize alternate means for egress. Moreover, it appears that complex, non-enveloped viruses such as bluetongue virus (BTV) and rotavirus interact with lipids during their entry process as well as with lipid rafts during the trafficking of newly synthesized progeny viruses. This review will discuss the role of lipids in the entry, maturation and release of non-enveloped viruses, focusing mainly on BTV.
Keywords: BTV, non enveloped, lipid rafts, entry, exit
1. Introduction
The replication cycle of viruses involves entry into host cells, synthesis of viral genes and proteins, assembly of progeny virus particles and their subsequent egress or release. Along with the plasma membrane, viruses also have to interact with the endosomal and vesicular membranes during their replication in host cells. All cellular membranes are composed of lipids and proteins that are usually arranged in various micro domains. During infection of cells by enveloped viruses, the lipids present in both viral and cellular membranes mediate fusion and fission reactions to facilitate virus entry and egress. Since non-enveloped viruses do not have a lipid envelope, it is generally believed that their entry mechanism does not involve membrane fusion activity and that these viruses are mainly released by cell lysis. Usually, non-enveloped viruses enter the cells by penetrating the membrane barrier, either via the endocytic pathway using clathrin-coated vesicles or caveolae, or by the formation of a pore at the cell surface [1–4]. Recent data obtained from biochemical and structural studies indicate that the overall mechanisms of both entry (Reoviridae) and release of certain non-enveloped viruses (e.g., members of the Picornaviridae and Reoviridae) are analogous to that of enveloped viruses, and that the capsid proteins can function in these activities in a similar manner to the membrane viral proteins. As an example, in this report we will discuss mainly the interaction of Bluetongue virus (BTV) proteins that form a separate genus in the non-enveloped Reoviridae family with membrane lipids both during the virus entry and the exit.
2. BTV morphology and its entry process into the host cells
Orbiviruses (some 140 members) which form a genus within the Reoviridae family, infect animals, plants and insects, and are transmitted by arthropod vectors. An important factor in the distribution of BTV worldwide is the availability of suitable vectors, usually biting midges (gnats) of the Culicoides genus. BTV infects wild and domestic ruminants, particularly sheep often with high morbidity and mortality. Bluetongue disease (BT) was first observed in domestic animals in Africa in the late 18th century. Currently, BTV (24 serotypes) is endemic in many parts of the world, particularly in tropical and subtropical countries. As a result of its economic significance, BTV has been the subject of extensive molecular, genetic and structural studies and is now the most well characterized orbivirus.
The virion particle is composed of seven discrete proteins (VP1-VP7) that are organized into two concentric shells (capsids); an outer shell and an inner shell or ‘core’ and a genome of 10 dsRNA segments [5]. The outer capsid, consisting of two major structural proteins, VP2 and VP5, forms a continuous layer that covers the inner core that is composed of two major proteins (VP3 and VP7) and three minor enzymatic proteins (VP1, VP4, and VP6). Shortly after infection, BTV is uncoated (removal of VP2 and VP5) to release the transcriptionally active core particles into the cytoplasm. The structure of the core is well characterized both by cryo-electron microscopy analysis and X-ray crystallography [6–9]. Hence, much is known about the core proteins and their structure-function relationship. In contrast, the atomic structure of neither the complete virion particle nor the outer capsid proteins is available to date. Until recently, the only structural information available for the outer capsid proteins and the whole virion were generated from two different cryo-EM studies, one at very low resolution and the other at a relatively higher resolution [10,11]. These data gave limited understanding of how the two proteins may function during virus entry into cells. However, very recent high resolution cryo-EM studies have thrown some new light on the structure of the two outer capsid proteins and how they may take part in virus entry.
In mammalian cells, BTV entry proceeds via virus attachment to the cell, followed by endocytosis and release of a transcriptionally active core particle into the cytoplasm. Of the two outer capsid proteins, the larger VP2 (110 kDa) is the most variable and is the serotype determinant of the virus. Indeed, VP2 is responsible for eliciting neutralizing antibodies and possesses haemagglutination activity of BTV. Further, VP2, which oligomerizes as a trimer, binds to receptor(s) on the plasma membrane of mammalian host cells and is also responsible for virus internalization [12].
Some limited information regarding BTV entry was initially generated based on ultra-structural electron microscopy studies, which indicated that BTV entry utilizes clathrin coated vesicles [13]. Therefore, it was necessary to undertake a more detailed analysis at a molecular level that combined both confocal microscopy and biochemical studies [14,15]. Initially, the adoption of the clathrin-mediated pathway was investigated by using a siRNA specifically designed to target the protein μ2, which is a subunit of the AP2 complex [16]. The main function of this protein complex is to recruit clathrin proteins from the cytoplasm and to bring them to the plasma membrane to form the clathrin pits [17,18]. The effect of μ2 depletion on BTV entry was independently monitored by immunofluorescence and biochemical studies. Data obtained from both investigations clearly indicated a direct correlation between the restriction of the clathrin pathway and BTV entry. Confocal microscopy revealed that depletion of transferrin uptake in HeLa cells by μ2 siRNA also resulted in a similar reduction of BTV uptake. The retention of BTV particles on the plasma membrane of cells lacking the endosomal vesicles proved that BTV internalization is dependent on clathrin. Further evidence regarding the role of clathrin in BTV entry was provided by measuring the virus replication of the cells that were infected with BTV after siRNA transfection. The results from these experiments demonstrated reduction of BTV replication was directly associated with the absence of the clathrin-pathway. A different approach involved the use of chlorpromazine (CPZ) to block the formation of the clathrin vesicles. This antibiotic directly interferes with the generation of the clathrin pits and hence its effect is very specific. Different sets of experiments have revealed that increasing amounts of CPZ during BTV infection resulted in inhibition of virus replication. Confocal microscopy analysis of the infected cells supported further that the reduction of BTV infectivity was not due to chemical toxicity. The data acquired by μ2 siRNA and CPZ treatments clearly demonstrated that BTV enters the cells using the clathrin-mediated pathway [15]. This particular system of endocytosis usually proceeds with the delivery of the cargo contained in the clathrin vesicles to the early and the late endosomes. While influenza virus uses the acidic environment of the late endosomes to become fusion competent, for the non-enveloped adenoviruses, the low pH of the early endosomes triggers conformational changes of a viral protein required for cell permeabilization ability [19]. For both viruses, the final aim of the mechanism is to release the virion into the cytoplasm. The relevance of the acidic pH in BTV entry was initially suggested by Hyatt et al. [20], who showed that the presence of ammonium chloride (NH4Cl) inhibited BTV replication. The pH-dependent character of BTV entry was further examined more recently by monitoring the effect of bafilomycin A1 in BTV infectivity [15]. This chemical has been classified as a vacuolar type H+-ATPase inhibitor, and is responsible for the specific inhibition of endosomal acidification, blocking endosomal traffic during endocytosis. From our studies, it was clear that the effect of bafilomycin A1 in BTV replication was strictly correlated with the reduction of virus infection in a dose-dependent manner. In parallel, confocal microscopy supported the hypothesis that the endosomes are the perfect location for BTV activation leading to virus uncoating. To further prove that BTV is internalized by the endosomal vesicles and to understand the internalization route followed by the virus, specific endosomal markers were used to investigate the presence of the virus in the early and late endosomes. The results obtained from various studies demonstrated that in the early stages of the infection BTV is present in the early endosomes for at least 30 minutes before further progression of the virion to the late endosomes [15]. Since the assay monitored the virus by labeling VP5 the second outer capsid protein, it is possible to speculate that, due to the membrane binding activity of the protein, it remains attached to the membrane of the early endosomes while the core is released into the cytoplasm. This pathway of virus entry probably relies on specific conformational changes that occur in the BTV outer capsid in response to the changing environment of the virion as it enters the endocytic pathway.
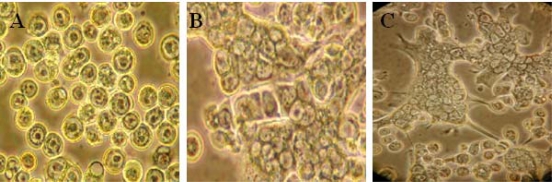
The 60 kDa protein, VP5, shares certain secondary structural features with the fusion proteins of enveloped viruses, indicating that it may be responsible for membrane penetration activity [21]. Two distinct biochemical studies support the membrane penetration activity of VP5; peptides representing the two amino terminal amphipathic helices cause leakiness of the membrane and the full length VP5 protein, when localized to the plasma membrane, triggered strong syncytia and formation of multinuclear cells (Figure 1) upon low pH treatment [14,21]. Thus, VP5 has the capacity to destabilize membranes and it does so after it has been activated by low pH conditions that are similar to the endosomal environment encountered during cell entry. In addition, VP5 also forms a trimer in solution when expressed as a recombinant protein, a feature shared by other fusion proteins. It is likely that upon receptor binding and entry, VP2 trimers undergo rearrangement or degradation allowing the exposure of VP5 trimers which subsequently changes to the functional conformation due to low pH. VP5 lacks the autocatalytic cleavage and N-terminal myristoyl group present in the entry proteins of reoviruses and rotaviruses and does not require proteolytic activation in contrast to some other viral fusion proteins [22]. How exactly VP2 and VP5 may interact to the lipid membrane during cell entry became clearer further from the structural data recently obtained from the whole virion particle.
Figure 1.
Fusogenic activity of VP5. Sf9 cells were infected for 48 hours and were then exposed to pH 5.0. Pictures were taken before (A) and after 4 hr (B) or 7 hr (C) pH shift.
3. BTV outer capsid structure
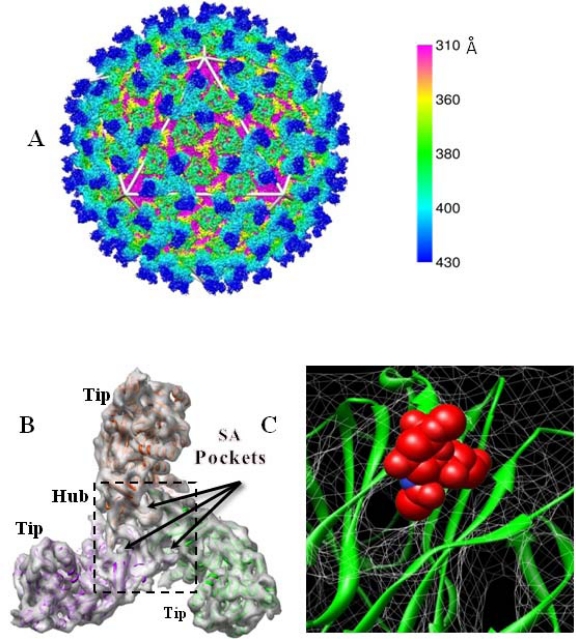
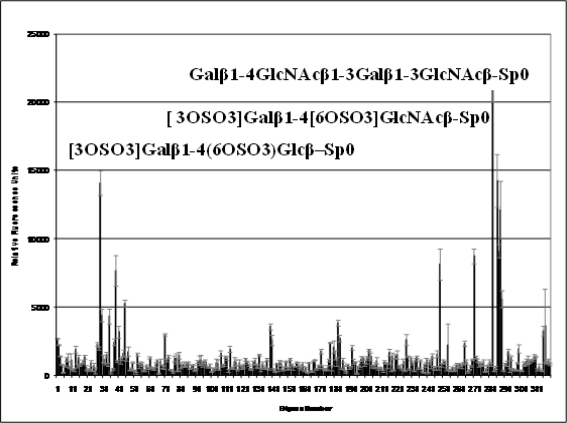
The BTV outer capsid has an icosahedral configuration with a diameter of ∼880 Å [11]. A series of three-dimensional structural studies, using cryo-EM (initially at 40 Å and subsequently at 25 Å resolution) have been undertaken. The reconstructed images revealed the overall organization of both the VP2 and VP5 trimers in the outer capsid, which consisted of a total of 60 triskelion spike-like structures formed by VP2 trimers and 120 globular VP5 trimers. Together, they covered most of the underlying core layer although both VP2 and VP5 trimers attach to the underlying core surface layer independently of each other [10,11,23]. However, a recent 7 Å resolution cryo-EM structure of BTV virions (Figure 2A) has identified the secondary structural elements and the topological arrangement of these two proteins in more details [24]. Each of the three monomers of VP2, which assembles as a triskelion structure, has two distinct domains: a tip domain and a distant lower region, three of which form a hub domain at the base. The top of the tip domain of each monomer is rich in β sheets and projects upward from the surface of the virion and its location implies that this region is responsible for the first encounter of the virus with the host cell membrane. The hub domain which is formed by all three monomers, on the other hand, has a distinct β-barrel fold (Figure 2B) that is similar to the sialic acid (SA) binding domain of the outermost region (VP8) of the rotavirus VP4 spike [25]. Further, when the ribbon model of the rotavirus SA-binding domain was docked into the VP2 density map it fitted perfectly into this hub region. The putative sialic acid binding region was further confirmed by fitting a SA into a pocket in this site (Figure 2C). Thus, even though there is no sequence homology between the rotavirus VP8 and the BTV VP2, these structural findings suggest that VP2 binds SA and this was further confirmed using biochemical approaches. Our recent data confirmed that VP2 binds type 2 oligosaccharide structures (Galβ1-4GlcNAcβ1-3) in glycan arrays (Figure 3). In BTV VP2 the SA binding domain is about 40 Å inward from the outer end of the tip domain, a distance well within the range of lengths of chains of sugars on glycoproteins. Hence after attachment of the tip domain to the host cell surface, the SA binding of VP2 might further stabilize the interaction, thus facilitating infection of host cells. This SA binding of VP2 has been recently confirmed biochemically and in confocal studies by infecting cells with BTV in the presence of Wheat Germ Agglutinin (WGA), a SA binding protein. This study revealed that not only was there a decrease in the total titer but also that the cells were comparably less infected. However, as the infection was not completely abolished in the presence of WGA, other receptors might also be influencing BTV entry. Since it is known that BTV particles agglutinate erythrocytes of ruminants [26] and VP2 alone is responsible for this activity [21], it can be postulated that SA binding may promote adsorption of the virus onto the surface of erythrocytes, thereby increasing the probability of ingestion by the blood-feeding Culicoides vector.
Figure 2.
Cryo-EM analysis of BTV particle at 7 Å. (A) Cryo-EM structure of complete BTV particle at 7 Å resolution, color coded by radial position: VP2 (blue) and VP5 (green) of outer capsid; VP7 (pink) of core. (B) Top view of VP2 triskelion formed by three VP2 monomers, each consisting of a tip domain and a part of the hub domain as indicated. A putative SA-binding pocket is located at the hub domain. (C) Fitting of SA into the pocket. Courtesy X. Zhang et al. [24]
Figure 3.
Glycan array analysis of VP2 binding. Version 3.0 of the printed array of the Consortium for Functional Glycomics was probed with VP2 (200 μg/ml). Error bars represent the mean ± standard error of four replicates. Glycans yielding significant binding are shown with their numbers and names.
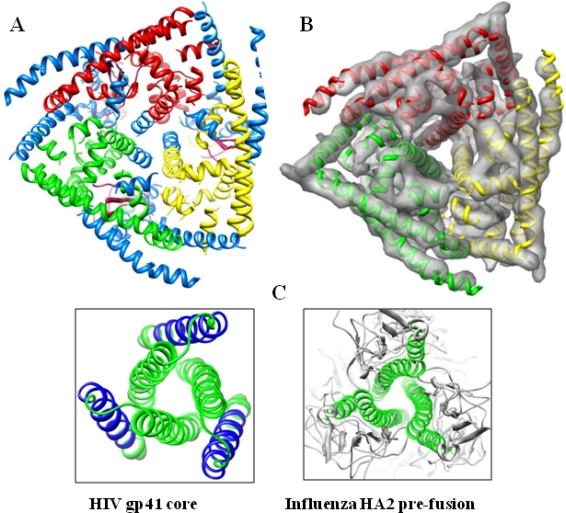
The structural data revealed that the globular VP5 is very rich in helices and possesses only one β sheet (Figure 4A). The N-terminal amphipathic α helical region is positioned on the exposed surface of VP5 and there are four additional amphipathic α-helical regions that are also located on the exterior surface of the protein [24]. In particular, three copies of one of the four amphipathic helices present on the top surface are well positioned to expose their hydrophobic undersides to endosomal membrane (Figure 4B). In addition, the other three amphipathic helices are similarly located on the exposed surface. Interestingly, similar peripheral triplet of α helices is present in HIV trimeric fusion protein [27]. Another trimer of α helices in the VP5 trimer that forms a coiled-coil helix bundle and runs up the center of the trimer is analogous to what is commonly observed in the fusion proteins of the enveloped HIV (Figure 4C) [28], influenza virus (Figure 4D) [29], herpesvirus [30] and VSV [31], as well as in the penetration protein of the non-enveloped rotavirus [32]. The gaps in the VP2 triskelion are filled by the three VP5 trimers.
Figure 4.
Cryo-EM analysis of VP5 trimer at 7Å resolution reveals it as a helical globular complex. (A) The top view of a VP5 trimer showing the amphipathic helices (blue) and three non-amphipathic helices (red, green, and yellow). (B) Six-fold average density map of the same with an embedded ribbon model. The density map reveals secondary structures demonstrating that VP5 contains many helices but only one β-sheet. (C) Ribbon diagrams of the central coiled-coil helix bundle and a triplet of peripheral helices in fusion proteins gp41 of HIV and HA2 of influenza. Blue ribbons represent amphipathic regions of α-helices; green ribbons represent non amphipathic regions of α-helices. Courtesy X. Zhang et al. [24].
Density maps have also revealed that the VP2 trimers interact (only via hub domains, not tip domains) with VP5 trimer very weakly, and similarly the interactions between VP5 and VP7 trimers are also very weak. These weak interactions would permit conformational changes of VP5 during the penetration process and during shedding of the outer coat. In comparison, both the VP2 tip and hub domains connect to their underlying VP7 trimers by stronger forces.
4. BTV replication and assembly
Removal of the outer capsid proteins activates the virion transcriptase [33,34] in the virus cores and results in the synthesis of capped mRNAs of each genome segment that are then extruded from the cores into the cytoplasm where they serve as templates for translation of viral proteins [33]. Thus, the core structure consisting of all the necessary enzymes (provided by the three minor proteins) is responsible for synthesizing mRNAs [33–39].
EM analysis of thin sections of BTV infected cells have revealed the presence of small numbers of intracellular virus particles and large numbers of juxtanuclear fibrillar networks referred to as virus inclusion bodies (VIBs). These structures are composed predominantly of non-structural phosphoprotein NS2 that recruits all the components of subviral particles (subcore), i.e., the viral ssRNAs, transcriptase complex (TC) proteins (VP1, VP4 and VP6) as well as VP3 that encapsidates the TC and the viral genome [40–47]. The polymerase protein VP1 in the newly formed subcores is then believed to synthesize the negative strand RNAs on the ssRNA templates to produce genomic dsRNA [39]. The subcore in the VIBs serves as a scaffold for the addition of VP7 trimers, thereby giving rise to more rigid and stable cores [47–49]. Similar inclusion bodies are also present in rotavirus and reovirus infected cells [50–52]. However, the outer capsid proteins are not recruited by NS2 and are not assembled within the VIBs [45,47]. Both the proteins appear to be assembled onto the core in a different location than the VIBs.
5. Interaction of outer capsid proteins of BTV with lipid rafts
In infected cells at later times post-infections, both VP2 [53] and VP5 (Figure 5) [54] can be seen at the plasma membrane by confocal microscopy. Recent biochemical data also suggests that VP2 [53] and VP5 [54] interact with the raft domains in the cellular membrane. Lipid rafts are dynamic microdomains in cellular membranes and are formed by the segregation of membrane lipids like sphingolipids and cholesterol in a glycerophospholipid-rich environment [55,56]. Many viruses and other pathogens hijack lipid rafts to facilitate their successful infection [57–59]. Usually, proteins interact with rafts either via their transmembrane domains [60] or through covalent lipid modifications [61]. For enveloped viruses, generally fusion proteins associate with lipid rafts in cellular membranes. Thus, it is not surprising that VP5, which acts like a fusion protein, has this property. However, VP5 is not myristoylated although it contains a conserved glycine residue at a position suitable for modification [3].
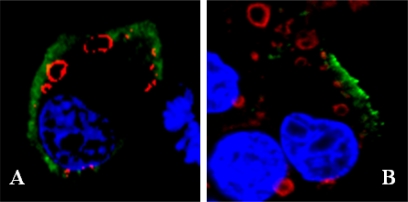
Figure 5.
Confocal microscopy shows VP5 at the plasma membrane of BTV infected cells. BSR cells were infected with BTV and analyzed at 12 (A) and 16 (B) hours post infection. VP5 was stained with anti-guinea pig FITC (green), BTV NS2 with anti-rabbit TRITC (red) and nuclear DNA with Hoechst stain (blue).
The association of VP5 with raft domains is quite convincing, since depletion of cellular cholesterol with methyl beta cyclodextrin not only altered the association of VP5 with raft domains, but also decreased the relative BTV viral titer, indicating the importance of raft domains in BTV replication [54]. In addition, VP5 possesses a WHXL motif, a highly conserved domain that is also present in a SNARE regulatory protein Synaptotagmin I (Syt1) [62]. This domain has been shown to be responsible for docking of the cellular protein in the plasma membrane. Interestingly, when the WHXL of VP5 was substituted with a series of alanines, the association of VP5 with lipid rafts was severely perturbed and it was no longer localized at the plasma membrane but was visible as patches in the cytoplasm [54]. In comparison to VP5, VP2 does not contain any obvious lipid raft binding domains, thus it is still not clear the exact mechanism of interaction between VP2 and lipid raft domains. However, VP2 interacts with vimentin [63] which itself interacts with both microtubules and lipid rafts. Thus it may be that the association of vimentin acts as a bridge in the association of VP2 with lipid rafts.
Similar interactions have been observed for rotavirus and lipid rafts during the later stages of virus replication. Although rotavirus specifically infects highly polarized intestinal cells in vivo [64], the majority of research in rotavirus assembly has been undertaken in non-differentiated MA 104 cells. Subsequent to the assembly of the double layered particles (DLPs) in inclusion bodies, termed as “viroplasm”, DLPs bud out from the viroplasm, (a process that is facilitated by a viral non-structural protein, NSP4) and acquire the outercapsid proteins VP4 and VP7. Both VP7 and NSP4 are inserted in the ER membrane co-translationally and recent data suggests that the assembly of VP7 layer onto the DLPs takes place in the ER [65]. NSP4 has also been localized with plasma membrane raft protein caveolae in some cells [66].
In contrast to VP7, the other outer capsid protein of Rotavirus, VP4, is cytosolic. However, it associates with lipid rafts in both MA 104 cells and Caco 2 cells but this association is sensitive to cholesterol extraction by methyl beta cyclodextrin only in one cell line but not the other [67]. Since VP4 does not exhibit an obvious transmembrane domain nor does it have a GPI (glycosylphosphatidylinositol) anchor and neither is it glycosylated, it has been speculated that its interaction with lipid rafts is most likely via an alternative method. VP4 possesses two potential lipid raft binding domains in its sequence, a caveolin binding consensus motif and a flotillin binding motif. Both caveolin and flotillin are lipid raft proteins. Although the caveolin binding domain of VP4 is a suitable candidate for raft interaction, the absence of caveolin in Caco-2 cells [68] raises questions regarding rotavirus-lipid raft interactions in polarized intestinal cells. In addition, there is no experimental data to date to support the activity of the flotillin binding motif present in VP4 although it has been proposed that it might be responsible for its interaction with rafts [69]. However, it has been proposed that since the amino terminal end of the VP4 has a galectin-like fold, this might interact with raft domains in cells thus behaving in analogous manner to cellular galectin [69].
In infected Caco-2 cells, VP4 associates with cellular lipid raft domains early in infection [70]. Since other rotaviral structural proteins also associates with rafts, it was proposed that the final step of rotavirus assembly is an extrareticular event. This was further confirmed by the interaction of rotaviruses (rhesus and murine strains) with lipid rafts during infection in vitro and in vivo [71]. Studies analyzing the effect of inhibitors of cholesterol biosynthesis also suggested that absence of cellular cholesterol results in poor incorporation of viral proteins into virions [72].
6. Release of BTV from infected cells; role of NS3 protein
BTV infects and replicates successfully both in mammalian and insect cells. Although BTV is released from infected mammalian cells mainly by cell lysis, it also egresses either by budding or protrusion and the nature of release depends on the particular cell type (Figure 6). In infected cells, virus particles were observed along intermediate filaments by EM studies [73] and this phenomenon has been further confirmed more recently [63]. Interestingly, along with virion particles, the smallest non-structural protein NS3, the only glycosylated protein of BTV, has also been observed with cellular membranes together with VP2 and VP5, the two outer capsid proteins [54,74,75]. NS3 has also been localized to the ER, Golgi complex (Figure 7A) and to the actin [76,77]. Proteins that are synthesized in the ER are transported to the Golgi where they are sorted in the trans-Golgi network (TGN) for delivery to early/sorting endosomes. They may be then transported to late endosomes/ multi vesicular bodies (MVBs) that are important in the segregation of proteins and lipids destined for (a) lysosomal degradation (b) recycling to the Golgi, and (c) plasma membrane exocytosis. Among the cellular proteins NS3 directly interacts with Tsg101 (Figure 7B), a component of MVBs (the transport vesicles of late endosome), and annexin II complex involved in exocytosis, respectively [75,78–80]. By using recombinant proteins it was shown that Tsg101 interaction is mediated by a conserved PSAP motif in NS3 which appears to play a role in virus release. Depletion of Tsg101 with siRNA also inhibited the release of BTV [80].
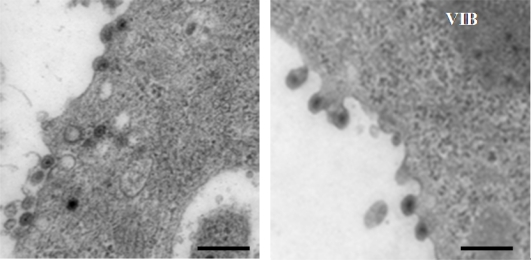
Figure 6.
Ultrastructural analysis of BTV infected cells. BSR cells were infected, fixed at 12 hours post infection, and processed for cell sectioning. Note: virus particles are underlying the plasma membrane (left panel) or budding from the cell membrane (right panel) of infected cells. Presence of virus inclusion bodies (VIBs) is indicated. Bar = 100 nm.
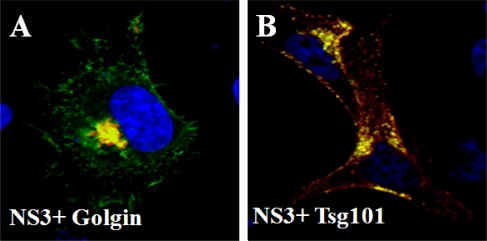
Figure 7.
Localization of NS3 with cellular markers. Co-localization (yellow) of NS3 with Golgi marker Golgin (A) and Tsg101 (B) in transfected cells. The proteins are indicated in each panel.
More recently using a reverse genetic system we confirmed that viruses containing mutations of the Tsg101 binding motif had altered patterns of virus egress and left nascent particles tethered to the cellular membrane, indicating that Tsg101 binding of NS3 is directly involved in pinching off the newly assembled viruses from the plasma membrane [81]. Further, cells infected with mutant viruses incapable of NS3-VP2 interaction not only perturb the budding process of the virion particles but also arrest all the particles in the cytosol [81].
In subcellular fractionation experiments, not only does NS3 co-fractionate with raft domains in virus infected cells but cholesterol-dependent raft integrity is also essential for NS3 segregation with rafts [54]. Since cholesterol is synthesized in the ER and Triton X-100 fractionation methods have identified cholesterol/sphingolipid-enriched structures in the trans-Golgi network and lysosomes [82,83], it can be postulated that NS3 uses these microdomains for transit from the ER to membranes. Lipid rafts are thought to arise from the Golgi apparatus, where sphingolipids are synthesized and to which they recruit proteins destined for apical trafficking in epithelial cells, as proposed by Simons and Ikonen in “the raft hypothesis” [84]. Although, NS3 does not have any raft interacting domain, it is glycosylated and has two transmembrane domains that might be guiding its interaction with the raft domains. In enveloped viruses, localization of the viral glycoprotein occurs at the site of virus budding. Although, for most enveloped viruses their glycoproteins contain specific signals that result in targeting, or retention, at the budding site, other viral factors also determine the budding of enveloped viruses such as VSV [85,86] and Measles virus [87,88].
Similar to BTV, rotavirus release also depends on cell type. While the virus is released by lysis in MA104 cells, it exits by non-lytic means from the apical surface of differentiated cells [89–91]. Since disruption of lipid raft integrity by cellular cholesterol depletion also decreased virus release from infected cells it was concluded that rotaviruses uses these domains to transport to the cell surface during replication [91].
7. Conclusions
Membranes provide a physical barrier both in cell entry and egress of viruses from infected cells. Compared to the enveloped viruses that have a lipid bilayer that enables fusion of virus and cellular membrane, the non-enveloped naked capsids must adopt alternate strategies to penetrate through the cellular membrane lipid bilayer. In BTV this function is achieved by VP2 and VP5, the outer most virus structural proteins
Structural studies of VP2 suggest that following the attachment of the tip domain to the cell surface, secondary binding to sialic acid via the hub domain might cement the interaction and lead to cell ingress [24]. BTV then enters the host cell by a clathrin-dependent endocytic pathway. Once within the cellular endosomes the adjacent globular VP5 trimer layer is exposed to the acidic pH and becomes fusiogenic. It has been proposed that the amphipathic helical regions present on the outer surface of VP5 could swing up to the membrane and subsequently roll to make extensive hydrophobic contact that perforates the membrane to release the transciptionally active cores into the cytoplasm. This unfurling of VP5 could detach it and the rest of the VP2 from the core [24]. The importance of amphipathic helices in insertion of proteins into the lipid membrane bilayer has also been revealed for the μ1 and VP4 proteins of reovirus and rotavirus, respectively. Recently it has been reported that the conformational changes of rotavirus VP4 influence its interaction with the lipid bilayer which in turn might influence subsequent stages of viral core penetration from the endosome into the cytoplasm [92]. The similarity of the location and the sequence of the key domain of the rotavirus fusion protein with a cluster of three hydrophobic loops of Semliki Forest virus has led to the hypothesis that the disruption of the endosomal membrane might involve insertion of the hydrophobic apex of this domain into the membrane bilayer [93].
Traditionally, it has been believed that non-enveloped viruses are released by cell lysis. However, it has been shown that along with BTV, rotavirus, simian virus 40, poliovirus, and parvovirus can be released without lysis of the infected cells [ [92]. This indicates that naked capsid viruses can behave similarly to enveloped viruses and interact with cellular membranes during virus release.
During BTV assembly, recent studies have shown that along with VP2 and VP5, the non-structural protein NS3 also associates with lipid rafts. The fact that extraction of cellular cholesterol decreases viral titer is consistent with the hypothesis that this interaction is important for the assembly of the outer capsid proteins onto the core particles to form the mature virus particle.
The presence of a transient envelope like structure associated with BTV in EM sections of infected cells has led to the hypothesis that BTV may acquire an envelope via NS3 during virus release. It is noteworthy that BTV and related orbiviruses, seadornaviruses and coltiviruses are the only groups of arboviruses that lack a lipid envelope. It is possible that orbiviruses were originally also enveloped but lost their stable lipid membrane at some point during evolution.
Acknowledgments
We would like to thank Core H of The Consortium for Functional Glycomics, USA for providing us the glycan array data. We are grateful to Xing Zhang and Hong Zhou (UCLA, USA) for providing us structural figures of BTV particle, VP2 and VP5.
References and Notes
- 1.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 3.Iwata H, Hirasawa T, Roy P. Complete nucleotide sequence of segment 5 of epizootic haemorrhagic disease virus; the outer capsid protein VP5 is homologous to the VP5 protein of bluetongue virus. Virus Res. 1991;20:273–281. doi: 10.1016/0168-1702(91)90081-6. [DOI] [PubMed] [Google Scholar]
- 4.Hogle JM. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu Rev Microbiol. 2002;56:677–702. doi: 10.1146/annurev.micro.56.012302.160757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy P, Noad R. Bluetongue virus assembly and morphogenesis. Curr Top Microbiol Immunol. 2006;309:87–116. doi: 10.1007/3-540-30773-7_4. [DOI] [PubMed] [Google Scholar]
- 6.Hewat EA, Booth TF, Loudon PT, Roy P. Three-dimensional reconstruction of baculovirus expressed bluetongue virus core-like particles by cryo-electron microscopy. Virology. 1992;189:10–20. doi: 10.1016/0042-6822(92)90676-g. [DOI] [PubMed] [Google Scholar]
- 7.Prasad BVV, Yamaguchi S, Roy P. Three-dimensional structure of single-shelled bluetongue virus. J Virol. 1992;66:2135–2142. doi: 10.1128/jvi.66.4.2135-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimes JM, Jakana J, Ghosh M, Basak AK, Roy P, Chiu W, Stuart DI, Prasad BV. An atomic model of the outer layer of the bluetongue virus core derived from X-ray crystallography and electron cryomicroscopy. Structure. 1997;5:885–893. doi: 10.1016/s0969-2126(97)00243-8. [DOI] [PubMed] [Google Scholar]
- 9.Grimes JM, Burroughs JN, Gouet P, Diprose JM, Malby R, Zientara S, Mertens PPC, Stuart DI. The atomic structure of the bluetongue virus core. Nature. 1998;395:470–478. doi: 10.1038/26694. [DOI] [PubMed] [Google Scholar]
- 10.Hewat EA, Booth TF, Roy P. Structure of bluetongue virus particles by cryoelectron microscopy. J Struct Biol. 1992;109:61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- 11.Nason E, Rothnagel R, Muknerge SK, Kar AK, Forzan M, Prasad BVV, Roy P. Interactions between the Inner and Outer Capsids of Bluetongue Virus. J Virol. 2004;78:8059–8067. doi: 10.1128/JVI.78.15.8059-8067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan SH, Roy P. Expression and functional characterization of bluetongue virus VP2 protein: role in cell entry. J Virol. 1999;73:9832–9842. doi: 10.1128/jvi.73.12.9832-9842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton BT, Hyatt AD, Brookes SM. The replication of bluetongue virus. Curr Top Microbiol Immunol. 1990;162:89–118. doi: 10.1007/978-3-642-75247-6_4. [DOI] [PubMed] [Google Scholar]
- 14.Forzan M, Wirblich C, Roy P. A capsid protein of nonenveloped Bluetongue virus exhibits membrane fusion activity. Proc Natl Acad Sci USA. 2004;101:2100–2105. doi: 10.1073/pnas.0306448101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forzan M, Marsh M, Roy P. Bluetongue virus entry into cells. J Virol. 2007;81:4819–4827. doi: 10.1128/JVI.02284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraile-Ramos A, Kohout TA, Waldhoer M, Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4:243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 17.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 18.Smythe E. Clathrin-coated vesicle formation: a paradigm for coated-vesicle formation. Biochem Soc Trans. 2003;31:736–739. doi: 10.1042/bst0310736. [DOI] [PubMed] [Google Scholar]
- 19.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6:S152–163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 20.Hyatt AD, Eaton BT, Brookes SM. The release of bluetongue virus from infected cells and their superinfection by progeny virus. Virology. 1989;173:21–34. doi: 10.1016/0042-6822(89)90218-3. [DOI] [PubMed] [Google Scholar]
- 21.Hassan SH, Wirblich C, Forzan M, Roy P. Expression and functional characterization of bluetongue virus VP5 protein: role in cellular permeabilization. J Virol. 2001;75:8356–8367. doi: 10.1128/JVI.75.18.8356-8367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 23.Hewat EA, Booth TF, Roy P. Structure of correctly self-assembled bluetongue virus-like particles. J Struct Biol. 1994;112:183–191. doi: 10.1006/jsbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Boyce M, Bhattacharya B, Zhang X, Scheina S, Roy P, Zhou ZH. Bluetongue virus coat protein VP2 contains a sialic acid-binding domain and VP5 has similarities to enveloped virus fusion proteins. Proc Natl Academy Sci USA. 2010;107:6292–6297. doi: 10.1073/pnas.0913403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dormitzer PR, Sun Z, Wagner G, Harrison SC. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. Embo J. 2002;21:885–897. doi: 10.1093/emboj/21.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaton BT, Crameri GS. The site of bluetongue virus attachment to glycophorins from a number of animal erythrocytes. J Gen Virol. 1989;70:3347–3353. doi: 10.1099/0022-1317-70-12-3347. [DOI] [PubMed] [Google Scholar]
- 27.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 29.Sauter NK, Hanson JE, Glick GD, Brown JH, Crowther RL, Park SJ, Skehel JJ, Wiley DC. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry. 1992;31:9609–9621. doi: 10.1021/bi00155a013. [DOI] [PubMed] [Google Scholar]
- 30.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 31.Roche S, Rey FA, Gaudin Y, Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 32.Dormitzer PR, Nason EB, Prasad BV, Harrison SC. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature. 2004;430:1053–1058. doi: 10.1038/nature02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dijk AA, Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980;104:347–356. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- 34.Van Dijk AA, Huismans H. In vitro transcription and translation of bluetongue virus mRNA. J Gen Virol. 1988;69:573–581. doi: 10.1099/0022-1317-69-3-573. [DOI] [PubMed] [Google Scholar]
- 35.Stauber N, Martinez-Costas J, Sutton G, Monastyrskaya K, Roy P. Bluetongue virus VP6 protein binds ATP and exhibits an RNA-dependent ATPase function and a helicase activity that catalyze the unwinding of double-stranded RNA substrates. J Virol. 1997;71:7220–7226. doi: 10.1128/jvi.71.10.7220-7226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costas J, Sutton G, Roy P. Guanylyltransferase and RNA 5′-triphopatase activities of the purified expressed VP4 protein of bluetongue virus. J Mol Biol. 1998;280:859–866. doi: 10.1006/jmbi.1998.1926. [DOI] [PubMed] [Google Scholar]
- 37.Ramadevi N, Burroughs JN, Mertens PPC, Jones IM, Roy P. Capping and methylation of mRNA by purified recombinant VP4 protein of Bluetongue virus. Proc Natl Acad Sci. 1998;95:13537–13542. doi: 10.1073/pnas.95.23.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramadevi N, Roy P. Bluetongue virus core protein VP4 has nucleoside triphosphate phosphohydrolase activity. J Gen Virol. 1998;79:2475–2480. doi: 10.1099/0022-1317-79-10-2475. [DOI] [PubMed] [Google Scholar]
- 39.Boyce M, Wehrfritz J, Noad R, Roy P. Purified recombinant bluetongue virus VP1 exhibits RNA replicase activity. J Virol. 2004;78:3994–4002. doi: 10.1128/JVI.78.8.3994-4002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas CP, Booth TF, Roy P. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J Gen Virol. 1990;71:2073–2083. doi: 10.1099/0022-1317-71-9-2073. [DOI] [PubMed] [Google Scholar]
- 41.Loudon PT, Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991;180:798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- 42.Lymperopoulos K, Wirblich C, Brierley I, Roy P. Sequence specificity in the interaction of Bluetongue virus non-structural protein 2 (NS2) with viral RNA. J Biol Chem. 2003;278:31722–31730. doi: 10.1074/jbc.M301072200. [DOI] [PubMed] [Google Scholar]
- 43.Kar AK, Ghosh M, Roy P. Mapping the assembly of Bluetongue virus scaffolding protein VP3. Virology. 2004;324:387–399. doi: 10.1016/j.virol.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Kar AK, Iwatani N, Roy P. Assembly and intracellular localization of the bluetongue virus core protein VP3. J Virol. 2005;79:11487–11495. doi: 10.1128/JVI.79.17.11487-11495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modrof J, Lymperopoulos K, Roy P. Phosphorylation of Bluetongue Virus Nonstructural Protein 2 Is Essential for Formation of Viral Inclusion Bodies. J Virol. 2005;79:10023–10031. doi: 10.1128/JVI.79.15.10023-10031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lymperopoulos K, Noad R, Tosi S, Nethisinghe S, Brierley I, Roy P. Specific binding of Bluetongue virus NS2 to different viral plus-strand RNAs. Virology. 2006;353:17–26. doi: 10.1016/j.virol.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kar AK, Bhattacharya B, Roy P. Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly. BMC Mol Biol. 2007;8:4. doi: 10.1186/1471-2199-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka S, Roy P. Identification of domains in bluetongue virus VP3 molecules essential for the assembly of virus cores. J Virol. 1994;68:2795–2802. doi: 10.1128/jvi.68.5.2795-2802.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limn CH, Staeuber N, Monastyrskaya K, Gouet P, Roy P. Functional dissection of the major structural protein of bluetongue virus: identification of key residues within VP7 essential for capsid assembly. J Virol. 2000;74:8658–8669. doi: 10.1128/jvi.74.18.8658-8669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silvestri LS, Taraporewala ZF, Patton JT. Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms. J Virol. 2004;78:7763–7774. doi: 10.1128/JVI.78.14.7763-7774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broering TJ, Kim J, Miller CL, Piggott CD, Dinoso JB, Nibert ML, Parker JS. Reovirus nonstructural protein mu NS recruits viral core surface proteins and entering core particles to factory-like inclusions. J Virol. 2004;78:1882–1892. doi: 10.1128/JVI.78.4.1882-1892.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller CL, Arnold MM, Broering TJ, Hastings CE, Nibert ML. Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of microNS. J Virol. 2010;84:867–882. doi: 10.1128/JVI.01571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattacharya B, Roy P. 2008. London School of Hygiene and Tripical Medicine, London, UK. Unpublished work.
- 54.Bhattacharya B, Roy P. Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain. J Virol. 2008;82:10600–10612. doi: 10.1128/JVI.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harder T, Simons K. Caveolae, DIGs and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 56.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 58.Briggs JA, Wilk T, Fuller SD. Do lipid rafts mediate virus assembly and pseudotyping. J Gen Virol. 2003;84:757–768. doi: 10.1099/vir.0.18779-0. [DOI] [PubMed] [Google Scholar]
- 59.Ono A, Freed EO. Role of lipid rafts in virus replication. Adv Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- 60.Kundu A, Avalos RT, Sanderson CM, Nayak DP. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin-Belmonte F, Lopez-Guerrero JA, Carrasco L, Alonso MA. The amino-terminal nine amino acid sequence of poliovirus capsid VP4 protein is sufficient to confer N-myristoylation and targeting to detergent-insoluble membranes. Biochemistry. 2000;39:1083–1090. doi: 10.1021/bi992132e. [DOI] [PubMed] [Google Scholar]
- 62.Fukuda M, Moreira JE, Liu V, Sugimori M, Mikoshiba K, Llinas RR. Role of the conserved WHXL motif in the C terminus of synaptotagmin in synaptic vesicle docking. Proc Natl Acad Sci USA. 2000;97:14715–14719. doi: 10.1073/pnas.260491197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhattacharya B, Noad RJ, Roy P. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. J Virol. 2007;4:7. doi: 10.1186/1743-422X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Starkey WG, Collins J, Wallis TS, Clarke GJ, Spencer AJ, Haddon SJ, Osborne MP, Candy DC, Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986;67:2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- 65.Pesavento JB, Crawford SE, Estes MK, Prasad BV. Rotavirus proteins: structure and assembly. Curr Top Microbiol Immunol. 2006;309:189–219. doi: 10.1007/3-540-30773-7_7. [DOI] [PubMed] [Google Scholar]
- 66.Storey SM, Gibbons TF, Williams CV, Parr RD, Schroeder F, Ball JM. Full-length, glycosylated NSP4 is localized to plasma membrane caveolae by a novel raft isolation technique. J Virol. 2007;81:5472–5483. doi: 10.1128/JVI.01862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delmas O, Breton M, Sapin C, Le Bivic A, Colard O, Trugnan G. Heterogeneity of Raft-type membrane microdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells. J Virol. 2007;81:1610–1618. doi: 10.1128/JVI.01433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirre C, Monlauzeur L, Garcia M, Delgrossi MH, Le Bivic A. Detergent-resistant membrane microdomains from Caco-2 cells do not contain caveolin. Am J Physiol. 1996;271:887–894. doi: 10.1152/ajpcell.1996.271.3.C887. [DOI] [PubMed] [Google Scholar]
- 69.Chwetzoff S, Trugnan G. Rotavirus assembly: an alternative model that utilizes an atypical trafficking pathway. Curr Top Microbiol Immunol. 2006;309:245–261. doi: 10.1007/3-540-30773-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sapin C, Colard O, Delmas O, Tessier C, Breton M, Enouf V, Chwetzoff S, Ouanich J, Cohen J, Wolf C, Trugnan G. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J Virol. 2002;76:4591–4602. doi: 10.1128/JVI.76.9.4591-4602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuadras MA, Greenberg HB. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology. 2003;313:308–321. doi: 10.1016/s0042-6822(03)00326-x. [DOI] [PubMed] [Google Scholar]
- 72.Mohan KV, Muller J, Atreya CD. Defective rotavirus particle assembly in lovastatin-treated MA104 cells. Arch Virol. 2008;153:2283–2290. doi: 10.1007/s00705-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eaton BT, Hyatt AD, White JR. Association of bluetongue virus with the cytoskeleton. Virology. 1987;157:107–116. doi: 10.1016/0042-6822(87)90319-9. [DOI] [PubMed] [Google Scholar]
- 74.Hyatt AD, Gould AR, Coupar B, Eaton BT. Localization of the non-structural protein NS3 in bluetongue virus-infected cells. J Gen Virol. 1991;72:2263–2267. doi: 10.1099/0022-1317-72-9-2263. [DOI] [PubMed] [Google Scholar]
- 75.Beaton AR, Rodriguez J, Reddy YK, Roy P. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc Natl Acad SciUSA. 2002;99:13154–13159. doi: 10.1073/pnas.192432299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Chen SY, Iwata H, Compans RW, Roy P. Multiple glycoproteins synthesized by the smallest RNA segment (S10) of bluetongue virus. J Virol. 1992;66:7104–7112. doi: 10.1128/jvi.66.12.7104-7112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Z, Harty RN. The NS3 protein of bluetongue virus exhibits viroporin-like properties. J Biol Chem. 2004;279:43092–43097. doi: 10.1074/jbc.M403663200. [DOI] [PubMed] [Google Scholar]
- 78.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 79.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 80.Wirblich C, Bhattacharya B, Roy P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J Virol. 2006;80:460–473. doi: 10.1128/JVI.80.1.460-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celma CC, Roy P. A viral nonstructural protein regulates bluetongue virus trafficking and release. J Virol. 2009;83:6806–6816. doi: 10.1128/JVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanchette-Mackie EJ, Dwyer NK, Amende LM, Kruth HS, Butler JD, Sokol J, Comly ME, Vanier MT, August JT, Brady RO, et al. Type-C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc Natl Acad Sci USA. 1988;85:8022–8026. doi: 10.1073/pnas.85.21.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coxey RA, Pentchev PG, Campbell G, Blanchette-Mackie EJ. Differential accumulation of cholesterol in Golgi compartments of normal and Niemann-Pick type C fibroblasts incubated with LDL: a cytochemical freeze-fracture study. J Lipid Res. 1993;34:1165–1176. [PubMed] [Google Scholar]
- 84.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 85.Zimmer G, Zimmer KP, Trotz I, Herrler G. Vesicular stomatitis virus glycoprotein does not determine the site of virus release in polarized epithelial cells. J Virol. 2002;76:4103–4107. doi: 10.1128/JVI.76.8.4103-4107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergmann JE, Fusco PJ. The M protein of vesicular stomatitis virus associates specifically with the basolateral membranes of polarized epithelial cells independently of the G protein. J Cell Biol. 1988;107:1707–1715. doi: 10.1083/jcb.107.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maisner A, Klenk H, Herrler G. Polarized budding of measles virus is not determined by viral surface glycoproteins. J Virol. 1998;72:5276–5278. doi: 10.1128/jvi.72.6.5276-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naim HY, Ehler E, Billeter MA. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. EMBO J. 2000;19:3576–3585. doi: 10.1093/emboj/19.14.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jourdan N, Maurice M, Delautier D, Quero AM, Servin AL, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sapin C, Colard O, Delmas O, Tessier C, Breton M, Enouf V, Chwetzoff S, Ouanich J, Cohen J, Wolf C, Trugnan G. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J Virol. 2002;76:4591–4602. doi: 10.1128/JVI.76.9.4591-4602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cuadras MA, Greenberg HB. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology. 2003;313:308–321. doi: 10.1016/s0042-6822(03)00326-x. [DOI] [PubMed] [Google Scholar]
- 92.Trask SD, Kim IS, Harrison SC, Dormitzer PR. A rotavirus spike protein conformational intermediate binds lipid bilayers. J Virol. 2010;84:1764–1770. doi: 10.1128/JVI.01682-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibbons DL, Vaney MC, Roussel A, Vigouroux A, Reilly B, Lepault J, Kielian M, Rey FA. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]