Abstract
γδ T cells mediate rapid tissue responses in murine skin and participate in cutaneous immune regulation including protection against cancer. The role of human γδ cells in cutaneous homeostasis and pathology is poorly characterized.
In this study we show in vivo evidence that human blood contains a distinct subset of pro-inflammatory cutaneous lymphocyte antigen (CLA) and C-C chemokine receptor (CCR) 6 positive Vγ9Vδ2 T cells, which is rapidly recruited into perturbed human skin. Vγ9Vδ2 T cells produced an array of pro-inflammatory mediators including IL-17A and activated keratinocytes in a TNF-α and IFN-γ dependent manner.
Examination of the common inflammatory skin disease psoriasis revealed a striking reduction of circulating Vγ9Vδ2 T cells in psoriasis patients compared to healthy controls and atopic dermatitis patients. Decreased numbers of circulating Vγ9Vδ2 T cells normalized after successful treatment with psoriasis-targeted therapy. Together with the increased presence of Vγ9Vδ2 T cells in psoriatic skin, this data indicates redistribution of Vγ9Vδ2 T cells from the blood to the skin compartment in psoriasis.
In summary, we report a novel human pro-inflammatory γδ T cell involved in skin immune surveillance with immediate response characteristics and with potential clinical relevance in inflammatory skin disease.
Introduction
In murine skin the function of gamma-delta (γδ) T cells has been extensively investigated. Mouse epidermis contains large numbers of dendritic Vγ5Vδ1+ T cells, appropriately termed dendritic epidermal T cells (DETC) (1). DETC have been shown to participate in immunoregulation of the skin (2, 3) and to protect against epithelial malignancies (4, 5). Furthermore, γδ T cells in murine skin may produce growth factors that maintain epidermal integrity (6, 7). In contrast to mouse skin, γδ T cells are rare in healthy human skin and an equivalent of mouse DETC does not appear to exist. Approximately 2-9% of all dermal and 1-10% of all epidermal T cells are γδ T cells (8-14). Vδ1 T cells are considered to be the primary γδ T cell subset in skin playing a role in skin cancer immune surveillance (14) and wound healing (13) while a direct association of Vγ9Vδ2 T cells with cutaneous immunology has to date been lacking. As γδ+ T cells are less frequent in human skin compared to the frequency of DETC in murine skin, a key question is whether they perform a similar lymphoid stress-surveillance role as their murine counterpart (15).
Psoriasis is a common chronic inflammatory skin disease with a significant genetic disease susceptibility component (16, 17). While the contribution of effector cells of the adaptive immune system including T cells to disease pathogenesis is well established, recent interest has focused on effector cells of the innate immune system and their putative roles in the initiation and maintenance of psoriatic inflammation (18).
Here we characterize a novel pro-inflammatory human skin homing Vγ9Vδ2 T cell subset, which is characterized by early migration to perturbed human skin in vivo suggesting a role in tissue immunosurveillance. In plaque-type psoriasis, this subset is preferentially decreased in peripheral blood and increased in psoriatic skin indicating a potential clinical relevance in the pathogenesis of this major inflammatory skin disease.
Materials and Methods
Study population
66 patients (age 25-56 years) with plaque type psoriasis (Psoriasis Area and Severity Index, PASI 1-33) were enrolled in this study. The PASI measures extent and severity of skin inflammation. Venous blood and in some cases skin biopsies of patients were taken. 32 age-matched healthy volunteers (age 28-50) and 8 age-matched atopic dermatitis patients (26-65) were used as control. The patients were gender matched (male to female ratio healthy: 0.56, psoriasis: 0.52, atopic dermatitis: 0.33). Discarded healthy skin from female donors aged between 19-59 years was obtained after plastic surgery procedures. Human studies were conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board of Guy’s and St Thomas’ Hospital and informed consent was obtained from all patients and control individuals.
Patients included in the study received different treatment regimes at the time of sampling: 32 had not received any systemic treatment for at least four weeks prior to the time the blood sample was taken (“no systemic treatment”). 34 patients received systemic therapies: 16 patients were treated with different biologic therapies such as etanercept (n=12), adalimumab (n=3), and efalizumab (n=1), 4 patients received biologic therapies in combination with methotrexate (methotrexate + infliximab n=2, methotraxate + etanercept n=2), 9 patients were treated with methotrexate only and 5 patients received other therapies such as fumaric esters (n=2) and acitretin (n=3). Skin biopsies were taken exclusively from patients who had not received any systemic treatment for at least 4 weeks prior. Patients who were evaluated before and 4 weeks after treatment initiation received etanercept (Patient C and E) and adalimumab (Patient D), respectively. For clinical correlation, only patients not receiving systemic treatment were used.
Cell isolation and Culture
PBMCs separated by density gradient centrifugation using lymphoprep (PAA) according to the manufacturer’s instruction. Vγ9Vδ2 T cells were expanded by culturing PBMCs with 1 nM 4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP, kindly provided to ACH by Hassan Jomaa), together with 60 IU/ml IL-2 (R&D Systems) and 12 ng/ml IL-15 (R&D Systems) in X-Vivo 15 medium (Lonza) supplemented with 10% human serum (HS) (PAA). For generation of skin T cell lines, skin explant cultures were performed as described by Clark et al (19). For isolation of primary human keratinocytes, skin was incubated overnight in dispase (Roche) on 4 °C. Then, epidermis and dermis were separated and epidermal sheets were immersed in trypsin for 10 minutes on 37 °C. Resulting cell suspension was seeded in 75 cm2 flasks pre-coated with coating matrix (Gibco) in Epilife keratinocyte medium (Gibco) supplemented with HKGS (Gibco).
Flow Cytometry
Analysis of fresh PBMCs, expanded Vγ9Vδ2 T cell lines and keratinocytes was performed with combinations of monoclonal antibodies directed against various surface markers. For intracellular staining, cells were activated with 50 ng/ml PMA and 1 μM Ionomycin for 5 hours in the presence of Golgiplug (Brefeldin A, BD Bioscience). After surface staining, cells were fixed (IC Fixation Buffer, Ebioscience) and permeabilised (Permeabilisation Buffer, Ebioscience) according to manufacturer’s instructions before staining for intracellular proteins. Dead cells were excluded after staining with Aqua dye (Live/Dead cell kit, Invitrogen). Acquisition of cells was performed using a Becton Dickinson FACSCalibur, FACSCanto or FACSAria and data were analyzed using FlowJo software (TreeStar). Directly conjugated antibodies used were: CD3 APC (Ebioscience), CD3 PB (Ebioscience), Vγ9 Cy5 (Beckman Coulter), Vδ2 FITC (Beckman Coulter), Vδ1 FITC (Endogen), CCR4 PE (BD Bioscience), CCR4 PE (BD Bioscience), CCR6 PE (BD Bioscience), CCR10 PE (R&D Systems), CLA FITC + PE (Miltenyi Biotech) CD4 PECy5 (Dako), CD8 FITC (Dako), CD56 FITC + PE (BD Bioscience), CD16 PE (BD Bioscience), 6B11 (BD Bioscience), CD161 PE (Beckman Coulter), ICAM APC (BD Bioscience), HLA-DR PECy7 (Biolegend) and HLA-ABC PE (BD Bioscience). Antibodies used for intracellular staining were goat polyclonal IGF-1 (Santa Cruz Biotechnology), TNF-α PE (BD Bioscience), IFN-γ FITC (BD Bioscience) and IL-17A PE (eBioscience). Appropriate isotype controls were used. Gates for specific staining on Vγ9Vδ2 T cells were set according to isotype staining gated on CD3+Vδ2+ cells. Procedure of staining and gating was identical for each specimen.
Immunohistochemistry and Immunofluorescence
Frozen 5 μm thick sections of skin punch biopsies were fixed in ice cold acetone for 10 min. For immunofluorescence staining, sections were incubated with 5% normal goat serum (DAKO) for 20 min and were then immersed in antibody diluent (DAKO) containing the primary antibodies for one hour followed by extensive washing in PBS. Antibodies used for the identification of Vγ9Vδ2 T cells were polyclonal rabbit CD3 (1:100, Dako) and monoclonal mouse Vδ2 (1:50, Beckman Coulter). The secondary antibodies (goat α-mouse 488, goat α-rabbit 555) at a concentration of 1:200 were applied to the sections for 30 min. For nuclear staining Topro-3 (1:500) was added to the secondary antibody solution. Immunofluorescence pictures were taken using a Zeiss LSM Confocal microscope and analyzed with LSM image browser software. For immunohistochemical staining with the Vγ9 antibody (Beckman Coulter, 1:50), LSAB kit (DAKO) was used according to the manufacturer’s instructions.
Determination of chemokine, cytokine and growth factor levels
HMB-PP expanded CLA+Vγ9Vδ2 T cells were sorted, seeded at 1.5*105 cells per well in 96 well plates and cultured for 72 hours with respective stimuli for analysis of supernatant. 5 - 7.5*105 cells/well in 48 well plates were cultured for 24 hours for subsequent RNA isolation. The following stimuli were used: 10 nM HMB-PP + 60 IU/ml IL-2, 10 μl/ml anti-CD3/CD28 coated beads + 60 IU/ml IL-2, 1000 IU/ml IFN-α or 5 ng/ml PMA and 1μM Ionomycin. Medium only served as a negative control. Keratinocytes were activated as described in Keratinocyte Activation Assay. Supernatants were assayed using the Milliplex Map Human Cytokine/Chemokine kit (Millipore) and acquired on a Luminex 100 flow-based sorting and detection analyzer (Luminex Corporation). Cytokines in the panel were IFN-γ, TNF-α, IL-4, IL-6, IL-10, CCL20, CCL3, CCL4, CCL5, CXCL9, CXCL10, VEGF, FGF-2, EGF, KGF, IL-8, and IL-17A. IL-22 cytokine levels were assayed using a commercially available IL-22 ELISA kit (R&D Systems) according to manufacturers’ instructions.
Keratinocyte activation assays
Primary human keratinocytes at passage 2-4 were incubated with a 1:1 mixture of Epilife medium and supernatant of resting or activated CLA+ Vγ9Vδ2 T cells. Epilife medium and RPMI at a ratio of 1:1 were used as a negative control, whereas addition of IFN-γ (200 U/ml) and TNF-α (50 ng/ml) to the culture served as a positive control. Activation was blocked with 5 μg/ml anti-IFN-γ and α-TNF-α antibodies (R&D). For RNA extraction, keratinocytes were harvested after 24 hours and for flow cytometry and analysis of supernatant, keratinocytes were cultured for 48 hours.
Skin blisters
Skin suction blisters were induced applying negative pressure of 25–40 kPa (200–300 mmHg) below atmospheric pressure via a suction chamber (Medical Engineering, Royal Free Hospital, UK) for 2–4 h using a clinical suction pump (VP25; Eschmann) until a unilocular blister measuring 10–15 mm in diameter was formed between dermis and epidermis at the level of the lamina lucida. Blisters were performed either on unperturbed skin or after skin perturbation (injection of 100 μl sterile saline 24 hours prior to blister). Blister fluid was aspirated after 16-20 hours using a sterile syringe. The amount of blister fluid was measured and then microcentrifuged at 650 x g for 4 min. Cells were stained for flow cytometry.
CLA regulation
Experiments were performed on fresh PBMCs and HMB-PP expanded Vγ9Vδ2 T cell lines. 1*105 cells/96 well plate were cultured in 200 ul of X-Vivo medium + 10 % of HS and stimulated with either 10 μl/ml anti-CD3/CD28 coated beads (Invitrogen) or 10 nM HMB-PP + 60 IU/ml IL-2 or 100 ng/ml of superantigen staphylococcal enterotoxin B (SEB) as well as medium only as control. Every condition was performed with or without IL-12 (10 ng/ml) (R&D Systems). After three days cells were harvested, stained with directly labeled antibodies directed against CD3, Vγ9, and CLA and analyzed by flow cytometry.
RNA extraction, cDNA generation and real-time PCR analysis
Total RNA was obtained using the RNAeasy Plus Mini Kit (Qiagen) according to the manufacturers’ instructions and reverse transcribed into cDNA (Superscript II, Invitrogen). TNF, IFN-γ, IL-17A, IL-6, CXCL9, CXCL-10, CCL3, CCL4, CCL5, IL-8, CCL20, VEGF, FGF, KGF, IL-22, IL-4, S100A7, S100A8, β-defensin and LL37 expression was assessed by multiplex real-time quantitative RT-PCR using Taqman assays (Applied Biosystems) according to the manufacturer’s instructions. For each sample, mRNA abundance was normalized to the amount of human GAPDH (Vγ9Vδ2 T cells) or cyclophilin (keratinocytes). Data analysis was performed using the ΔΔCt method: results are expressed as fold change.
Quantification
To calculate absolute numbers of CD3+ cells and Vγ9Vδ2 T cells in the blister we used Trucount tubes (BD Bioscience) together with BD Tritest (CD3/CD16/CD56/CD45) (BD Bioscience) according to the manufacturer’s instructions. For absolute quantification of T cells in skin the method published by Clark et al (20) was used. Pictures of 4 randomly selected fields (200 x magnification) were taken with a Zeiss LSM. Pictures were then analyzed with the LSM image browser software. For each picture, Vδ2 T cells were counted, the length of skin recorded and number of Vδ2 T cells in 1 cm skin calculated. For quantification of Vδ2 percentage of total CD3 T cells in skin, T cell numbers in skin were counted for CD3 and Vδ2/CD3 expressing cells using the ImageJ software.
Extrapolation of total absolute Vγ9Vδ2 T cell numbers in skin and blood
Absolute numbers of Vδ2 expressing cells in 1 cm skin were acquired as described above and the resulting number was multiplied by 2000 to calculate Vδ2 T cell numbers in 1 cm2 of skin. Patient body surface area (BSA) was calculated using the following formula by Mosteller: BSA (m2) = ([Height(cm) × Weight(kg) ]/ 3600 )½. Clinical examination revealed the approximate percentage of BSA affected by psoriasis from which we extrapolated absolute Vδ2 T cell in psoriatic skin. For analysis of absolute numbers in blood, the absolute number of lymphocytes present in 1 μl blood was performed in the hematology laboratory at Guy’s and St. Thomas’ Hospital. Flow cytometry staining revealed the percentage of CD3 T cells and Vδ2 T cells in the lymphocyte population. Patient blood volume was estimated using the following formula: 0.3669 * Height (m)3 + 0.03219 * Weight (kg) + 0.6041, from which the absolute Vδ2 T cells in peripheral circulation was calculated
Statistical analysis
Data was assayed for normal distribution by using the D’Agostino-Pearson test and analyzed using unpaired two tailed student t test for determination of significant differences between two unpaired groups normally distributed and one-way Anova for more than two unpaired normally distributed groups. For comparison of two paired groups with normal distribution paired t test was used and repeated measure one way Anova was used for more than 2 matched groups. To compare not normally distributed samples, we used Mann-Whitney test to compare 2 groups and Kruskal-Wallis test for comparison of 3 groups. As a non-parametric test for two paired groups we used Wilcoxon matched pair test. Pearson correlation was used to correlate clinical variables and percentage/absolute numbers of gamma-delta T cells while one-way exponential decay was applied for calculation of R2. For all statistical tests, we considered P values <0.05 to be statistically significant. Results are expressed as mean +/− standard error of the mean.
Results
Identification of a subset of circulating Vγ9Vδ2 T cells expressing CLA and skin homing chemokine receptors
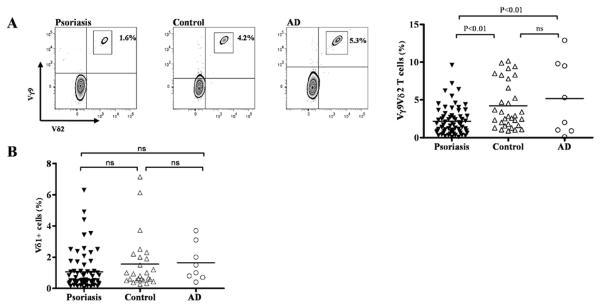
We performed extensive phenotypic screening of innate and adaptive lymphocytes in peripheral blood from healthy volunteers and patients with psoriasis. The percentage of CD4 T cells, CD8 T cells, invariant NKT cells, CD3+CD161+ NK-like T cells, CD56+CD16+ NK cells and CD56bright NK cells did not show any statistically significant difference between psoriasis patients and healthy individuals (Supplementary Figure 1, A-F). However, we found a significant decrease of circulating Vγ9Vδ2 T cells in psoriasis patients 2.16% (±0.23%, n=66) compared to healthy controls 4.21% (±0.55%, n=32), (p=0.01) (Figure 1A). To investigate whether this observation was restricted to psoriasis we assessed Vγ9Vδ2 T cells in atopic dermatitis, another common inflammatory skin disease with T cell involvement (21). Percentages of circulating Vγ9Vδ2 T cells in atopic dermatitis patients (n=8) were significantly higher than in psoriasis patients (5.18% (±1.75%), p<0.01) (Figure 1A) indicating that the reduction of Vγ9Vδ2 T cells in psoriasis was not due to unspecific skin inflammation. Vδ1 T cells, the other main γδ T cell subset in humans, was unchanged in the peripheral blood of psoriasis compared to healthy controls and AD (Figure 1B). We had absolute cell numbers for 44 out of 66 psoriasis patients. Mean absolute Vγ9Vδ2 T cell numbers in one μl peripheral psoriatic blood was substantially lower (mean 29 (± 4.7)/μl) compared to what has been published for healthy controls (49 cells/μl (22) and 68.6 cells/μl (23) (Supplementary Figure 1G) further supporting a reduction of circulating Vγ9Vδ2 T cells in psoriasis.
Figure 1. Vγ9Vδ2 T cells are decreased in peripheral blood of psoriasis patients.
Vγ9Vδ2 T cells in peripheral blood of age-matched psoriasis patients suffering from plaque type psoriasis (n=66), healthy controls (n=32) and atopic dermatitis (AD) patients (n=8) were stained for CD3, Vγ9 and Vδ2 and examined by flow cytometry. One representative staining for each group is shown in A. Vγ9Vδ2 T cells within the CD3 subset were significantly decreased in psoriasis patients (2.16% (+0.23%) compared to healthy controls (4.21% (±0.55%), p<0.01) and AD patients (5.18% (±1.75%), p<0.01) (A). Vδ1 T cells were not different between psoriasis patients, controls and AD patients (1.06% (±0.15) vs. 1.55% (±0.33%) vs. 1.65% (±0.42%) (B).
We then assessed the skin homing phenotype of circulating Vγ9Vδ2 T cells in psoriasis patients and healthy volunteers using flow cytometry analysis of fresh PBMCs. A proportion of Vγ9Vδ2 T cells in patients and controls both expressed CLA, a marker for skin homing T cells (Figure 2A). Only the CLA+Vγ9Vδ2 T cell subset was significantly decreased in patients (n=32) compared to controls (n=19) (0.94% (±0.11%) vs. 2.22% (±0.38%); p<0.001), while the CLA− subset was not significantly changed (1.74% (±0.29%) vs. 2.67% (±0.58%) (Figure 2B) demonstrating a preferential reduction of peripheral CLA+Vγ9Vδ2 T cells in psoriasis. CLA+ total T cells were comparable in patients and controls (16.75% (±1.39%) vs. 18.76% (±1.26%)) (data not shown).
Figure 2. Identification of a novel subset of skin homing Vγ9Vδ2 T cells expressing CLA and skin homing chemokine receptors.
Psoriasis patients (n=32) and healthy controls (n=19) were analyzed for CLA expression on peripheral Vγ9Vδ2 T cells by flow cytometry. The isotype for CLA and chemokine receptors was set with specific isotype controls for each marker, gated on CD3 and Vδ2. A subset of Vγ9Vδ2 T cells in patients and controls both expressed CLA, a marker for skin homing T cells (A). Percentage of CLA+Vγ9Vδ2 T cells gated on CD3+ cells (blue border and symbols) were significantly lower in psoriasis patients compared to healthy controls (0.94% (±0.11%) vs. 2.22% (±0.38%); p=0.001) whereas the CLA- subset was not significantly changed in patients (1.74% (±0.29%) vs. 2.67% (±0.58%) indicating a selective decrease of CLA+Vγ9Vδ2 T cells in psoriasis patients (B). The skin associated chemokine receptors CCR4, CCR6 and CCR10 gated on peripheral blood Vγ9Vδ2 T cells were analyzed by flow cytometry in patients and controls (C). Vγ9Vδ2 T cells expressed high levels of CCR6 (purple border and symbols) (patients: 16.5% (+3.5%-73%) vs. controls: 30.61% (7.3%-59%); p<0.05) while expressing variably levels of CCR4 (green border and symbols) (patients: 2.75% (0%-14.9%) vs. controls: (2.8% (1%-17%), ns) and little CCR10 (turquoise border and symbols) (patients: 1.6% (0%-15%) vs. controls: 5% (1%-10%), p<0.05) (D). Staining of one representative patient (Patient F) and one representative control (Control A) is shown for the expression of CLA, CCR4, CCR6 and CCR10.
Next, we investigated the expression of skin homing chemokine receptors. CCR6 expressing cells were the most represented within peripheral Vγ9Vδ2 T cells of patients and controls while a variable Vγ9Vδ2 T cell subset expressed CCR4 and a small subset CCR10 (Figure 2C). Comparing psoriasis patients and controls we found that Vγ9Vδ2 T cells of patients showed decreased expression of CCR6 (16.5% (3.5%-73%) (n=24) vs. 30.61% (7.3%-59%) (n=22), p<0.05) and CCR10 (1.6% (0%-15%) (n=22) vs. 5% (0%-10%) (n=17), p<0.05) compared to healthy controls (Figure 2D) while there was no difference in CCR4 expression (2.75% (0%-14.9%) (n=22) vs. 2.8% (1%-17%) (n=17), ns) (Figure 2D). Vγ9Vδ2 T cells did not express CD103 or CCR9 associated with gut homing (Supplementary Figure 2A+B).
As Vγ9Vδ2 T cells are rare in peripheral blood we generated Vγ9Vδ2 T cell lines by culturing PBMCs with the Vγ9Vδ2 T cell-specific antigen 4-Hydroxy-3-methyl-but-2-enyl pyrophosphate HMB-PP and IL-2 as described previously (24). Using these Vγ9Vδ2 T cell lines we examined the regulation of CLA on Vγ9Vδ2 T cells. Activation with HMB-PP or the super-antigen staphylococcal enterotoxin B (SEB) did not up-regulate CLA expression on peripheral Vγ9Vδ2 T cells. However, incubation with the cytokine IL-12, which has been shown to induce CLA on αβ T cells (25), induced CLA expression also on Vγ9Vδ2 T cells independent of activation (n=3) (Supplementary Figure 2C). IL-12 dependent CLA up-regulation was confirmed on fresh peripheral Vγ9Vδ2 T cells (n=4) (Supplementary Figure 2D).
Vγ9Vδ2 T cells are recruited to perturbed human skin in vivo
Expression of CLA on peripheral Vγ9Vδ2 T cells suggested the existence of a specialized skin homing Vγ9Vδ2 T cell subset possibly recruited to skin under conditions of skin perturbation. To assess in vivo dynamics of cutaneous Vγ9Vδ2 T cell homing we performed skin suction blisters in healthy volunteers (Figure 3A). The skin suction blister model allows for the ex vivo analysis of cells present within skin under conditions of tissue homeostasis and pathology (26, 27). We compared Vγ9Vδ2 T cells present in skin blisters induced on normal skin (“non-perturbed” condition) with those induced on “perturbed” skin previously injected with physiological saline. Within the CD3+ T cell population, perturbed skin contained significantly higher percentages of Vγ9Vδ2 T cells than non-perturbed skin (1.2% (±0.16%) vs. 0.7% (±0.08%), p<0.05) (Figure 3B). Absolute numbers of Vγ9Vδ2 T cells (4.6*102 (±1.06*102) vs. 1.02*103 (±1.5*102) per ml blister fluid, p<0.05) and of non-Vγ9Vδ2 T cells (7.3*104 (±2.1*104) vs. 1.04*105 (±2.7*104) per ml blister fluid, p<0.05) both increased in perturbed skin (Figure 3, C and D). However, the relative increase of Vγ9Vδ2 T cells between non-perturbed and perturbed skin of the same individual was significantly higher (125.9% (±29.1%)) compared to the increase of non-Vγ9Vδ2 T cells (44.5% (±11.1%), p<0.05)) (Figure 3E). Vγ9Vδ2 T cells isolated from perturbed skin contained significantly more CLA+Vγ9Vδ2 T cells than circulating Vγ9Vδ2 T cells from the same individuals (perturbed: 63.8% (±7.1%), circulating: 28.8% (±5.0%), p<0.01) (Figure 3F). This data indicates migration of CLA+Vγ9Vδ2 T cells to skin and highlights the existence of a specialized circulating immune cell subset with the potential for immediate skin recruitment.
Figure 3. CLA+ Vγ9Vδ2 T cells are recruited to human skin.
Suction blisters were performed on normal skin (“non-perturbed”) and skin that was injected 24 hours before with sterile saline (“perturbed”) (n=6, matched) of healthy volunteers (A). Blister fluid was harvested 16-20 hours after blister induction and assessed for absolute and relative Vγ9Vδ2 T cells and non-Vγ9Vδ2 CD3+ cells. Perturbed skin contained significantly higher percentages of Vγ9Vδ2 T cells than non-perturbed skin (p<0.05) (B). Absolute numbers of Vγ9Vδ2 T cells and non-Vγ9Vδ2 CD3+ cells were both significantly higher in perturbed compared to non-perturbed skin (both p<0.05) (C+D) but Vγ9Vδ2 T cells increased to a significantly greater extent than non-Vγ9Vδ2 CD3+ cells (p<0.05) (E). Vγ9Vδ2 T cells in perturbed skin were significantly enriched for CLA+Vγ9Vδ2 T cells as compared to circulating Vγ9Vδ2 T cells of the same individuals (p<0.01) (F+G).
Vγ9Vδ2 T cells are increased in skin of psoriasis patients
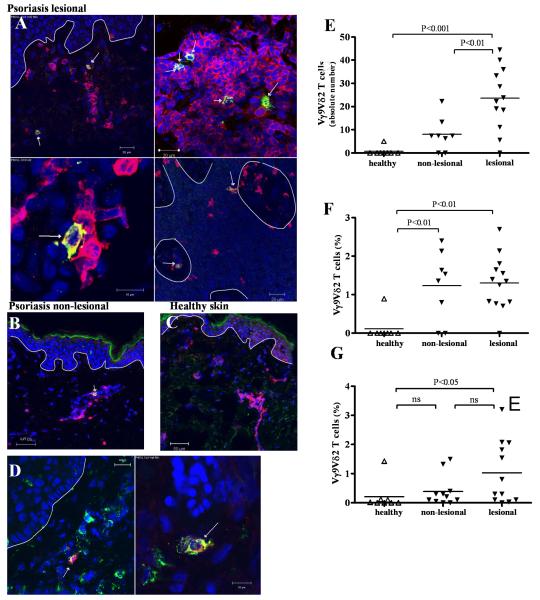
Skin homing properties of peripheral Vγ9Vδ2 T cells suggested that the selective reduction of Vγ9Vδ2 T cells in the blood of psoriasis patients might be due to their redistribution into the skin. Therefore, we examined the presence of Vγ9Vδ2 T cells in psoriatic skin lesions. Vδ2+ T cells co-expressing CD3 were preferentially located in psoriatic dermis (Figure 4A). Few Vδ2+ T cells were identified in clinically normal appearing psoriatic skin (“non-lesional psoriatic skin”) (Figure 4B) whereas Vδ2+ T cells were rarely found in healthy skin (Figure 4C). Immunohistochemical staining for Vγ9+ cells revealed positive cells around blood vessels in the dermis and scattered in the epidermis (Supplementary Figure 2E). Vγ9+ T cells were also detected in non-lesional psoriatic skin (Supplementary Figure 2F) whilst being rare in normal skin (Supplementary Figure 2G). Vδ2 and CLA were commonly co-expressed in psoriatic skin confirming the infiltration of a skin-homing Vγ9Vδ2 T cell subset (Figure 4D).
Figure 4. Vγ9Vδ2 T cells are increased in skin of psoriasis patients.
Frozen skin sections were stained with anti-CD3 mAb (red) and anti-Vδ2 mAb (green), identifying Vδ2 T cells as yellow. Nuclei were stained with To-Pro-3 (blue) and dermo-epidermal barrier is indicated with a white line. Vδ2 T cells were present in lesional psoriatic dermis as well as scattered in the epidermis (A). Vδ2 T cells were found in non-lesional psoriatic skin (B) while in healthy skin, Vδ2 T cells were rare (C). Co-staining of anti-CLA (green) and anti-Vδ2 (red) revealed CLA expression on skin Vγ9Vδ2 (D). Psoriatic skin harbored significantly higher absolute numbers of Vδ2 T cells than non-lesional (p<0.01) or healthy skin (p<0.001) as calculated in 1 cm of skin section (E). Relative quantification of Vγ9Vδ2 T cells in skin sections revealed that a higher percentage of T cells in non-lesional (p<0.01) as well as lesional (p<0.01) psoriatic skin express Vδ2 than in normal skin (F). We established T cell lines from lesional (n=12), edge (n=11) and healthy (n=8) skin by culturing skin pieces in the presence of IL-2 (60 IU/ml) and IL-15 (12 ng/ml) for 2-3 weeks and harvesting cells that migrated out of the tissue. Skin T cell lines were analyzed for γδ T cell subsets after gating on CD3+ cells. In lesional skin there were more Vγ9Vδ2 T cells (1.03% (±0.31%)) than in edge (0.39% (±0.16%), ns) or healthy skin (0.21% (±0.18 %), p<0.05) (G).
Absolute numbers of Vγ9Vδ2 T cells were significantly higher in psoriatic lesions (23.62 (±4.95)/cm) than in non-lesional psoriatic skin (8.00 (±2.55)/cm, p<0.01) and healthy skin (0.63 (±0.63)/cm, p<0.001) (Figure 4E). Quantifying Vγ9Vδ2 T cells in skin sections as percentage of total CD3+ cells we found a significantly higher percentage of Vγ9Vδ2 T cells in lesional (1.31% (±0.19%), n=13) as well as non-lesional (1.23% (±0.32%), n=8) psoriatic skin compared to healthy skin (0.12 (+0.12%), n=8, both p<0.01) (Figure 4F). Higher proportions of Vγ9Vδ2 T cells already strategically positioned in non-lesional psoriatic skin indicate a potential role in development of early lesions.
Flow cytometry analysis of T cell lines from lesional (n=12), non-lesional (n=11) and healthy (n=8) dermis revealed significantly higher percentages of Vγ9Vδ2 T cells in lesional psoriatic skin compared to healthy skin (1.03% (±0.31%) vs. 0.21% (±0.18%), p<0.05) whilst the percentage of Vγ9Vδ2 T cells in non-lesional psoriatic skin was also slightly increased (0.39% (±0.16%), ns) (Figure 4G).
Correlation between severity of psoriatic disease and percentage of circulating Vγ9Vδ2 T cells
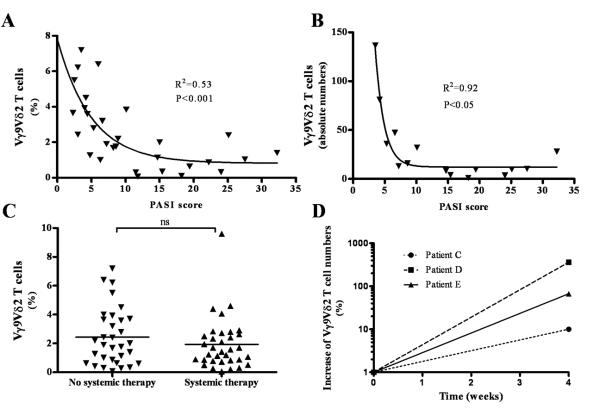
To evaluate the clinical relevance of Vγ9Vδ2 T cells we analyzed circulating Vγ9Vδ2 T cells in various disease severity states as reflected by the Psoriasis Area and Severity (PASI) index. We found a significant correlation between severe clinical disease (higher PASI) and lower percentages of circulating Vγ9Vδ2 T cells (R2=0.53, p<0.001, n=30) (Figure 5A). Absolute Vγ9Vδ2 T cell numbers were obtained in a subpopulation of patients (n=16) and also negatively correlated with disease severity (R2=0.92, p=0.05, n=16) (Figure 5B). In addition there was no correlation between age or gender with levels of peripheral Vγ9Vδ2 T cells (data not shown). None of the other immune cell subsets investigated (total CD3+, CD4+, CD8+, Vδ1+, CD56+ and CD161+ NK like T cells, CD56+CD16+ NK cells, CD56bright NK cells) showed a significant positive or negative correlation with disease severity as indicated by the PASI index (data not shown).
Figure 5. Clinical correlation between psoriasis severity and circulating Vγ9Vδ2 T cells.
Lower percentages of circulating Vγ9Vδ2 T cells significantly correlated with severe clinical disease as reflected in a higher PASI score (p<0.001, R2=0.53, n=30) (A). Absolute Vγ9Vδ2 T cell numbers were obtained in a subpopulation of patients (n=16) and also correlated with disease severity (p<0.05, R2=0.92) (B). Comparing percentage of peripheral Vγ9Vδ2 T cells of patients without systemic treatment to treated patients with the same PASI score we did not observe any significant difference (2.44% (+0.35%) vs. 1.93 (+0.31%), ns) (C). We analysed 3 patients before and during the course of their treatment at week 0 and week 4. In all of these patients PASI had dropped by at least 40% after 4 weeks of systemic treatment (Patient C: 4 weeks - 40%; Patient D: 4 weeks - 48%, Patient E: 45%) In all three patients, the absolute numbers of peripheral Vγ9Vδ2 T cell increased with successful treatment (Patient C: 4 weeks – 10%; Patient D: 4 weeks - 360%; Patient E: 4 weeks - 67%) (D).
We selected 2 patients with a PASI >20 for detailed calculation of Vγ9Vδ2 T cell numbers. We took advantage of a method developed for quantification of T cell numbers in skin by the group of Kupper and Clark (20). Based on this method, calculations in patient A (with 50% of the body surface involved) showed a total of 7.7*108 Vγ9Vδ2 T cells present in inflamed skin. This patient had approximately 14 times more Vγ9Vδ2 T cells in inflamed psoriatic skin than in peripheral blood (0.53*108 Vγ9Vδ2 T cells) (Supplementary Figure 3A) Similar numbers were obtained for a further patient B with a PASI score >20 (Supplementary Figure 3B). These data are in line with our hypothesis that Vγ9Vδ2 T cells re-distribute from blood to skin during skin inflammation.
Next, we sought to investigate whether treatment would affect peripheral Vγ9Vδ2 T cells. We found that Vγ9Vδ2 T cells percentage (2.44% (±0.35%) vs. 1.93 (±0.31%), ns) (Figure 5C) and absolute number (data not shown) were similar in peripheral blood of untreated patients and patients on systemic therapy (Figure 5C), having the same median PASI score (5.95 (1.2-32.2) vs. 5.35 (0.6-22.4)). However, when we followed three patients before and 4 weeks after successful therapy (PASI score reduced by more than >40% from baseline), we found that alleviation of psoriasis was accompanied by an increase of absolute T cell numbers (Figure 5D) and percentage (data not shown) in peripheral blood. Possibly due to residual disease, Vγ9Vδ2 T cell levels did not consistently reach levels seen in healthy controls (data not shown).
Taken together, psoriasis severity correlated with decreased numbers of circulating Vγ9Vδ2 T cells and was reversed after successful treatment with psoriasis-targeted therapy, suggesting a clinically significant role of Vγ9Vδ2 T cells in psoriasis.
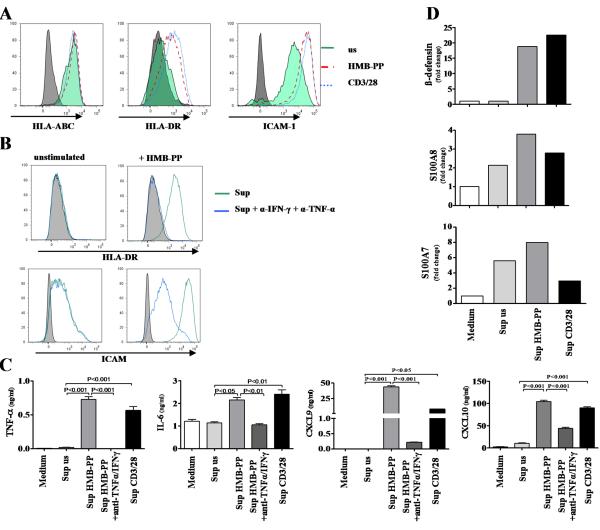
CLA+Vγ9Vδ2 T cells produce psoriasis–relevant pro-inflammatory cytokines, chemokines and growth factors
To further define the role of Vγ9Vδ2 T cells in psoriasis, we investigated a range of pro-inflammatory mediators with potential relevance to psoriasis pathogenesis. CLA+Vγ9Vδ2 T cells produced high levels of TNF-α and IFN-γ upon TCR-specific (HMB-PP) and unspecific stimulation while constitutive production in resting cells was low to undetectable (representative experiment, n=8) (Figure 6A). Vγ9Vδ2 T cells produced IL-17A and IL-17A producing Vγ9Vδ2 T cells were enriched in the CLA+ population (CLA+: 0.4% (±0.07) vs. CLA−: 0.15% (±0.05%), p=0.0313) (Figure 6B). CLA+Vγ9Vδ2 T cells did not produce IL-22 (data not shown).
Figure 6. CLA+Vγ9Vδ2 T cells produce psoriasis-relevant inflammatory mediators.
CLA+Vγ9Vδ2 T cells from healthy controls were sorted and stimulated with HMB-PP, CD3/28 beads or PMA/Ionomycin before analyzing supernatant by multiplex bead assay. Activated CLA+Vγ9Vδ2 T cells up-regulated their IFN-γ and TNF-α production (representative experiment, n=8) (A). IL-17A production was assessed in circulating Vγ9Vδ2 T cells by flow cytometry after gating on the Vγ9 subset. A small percentage of Vγ9+ T cells produced IL-17A which was enriched in the CLA+Vγ9+ subset (B). CLA+Vγ9Vδ2 T cells produced the chemokine IL-8 as assessed by multiplex bead assay (C). The CC-chemokines CCL3, CCL4 and CCL5 were produced already constitutively. The production of CCL3 and CCL4 was increased upon activation, whereas CCL5 production could not be further up-regulated (all one representative experiment, n=11) (C) IGF-1 production was assessed by flow cytometry. Unstimulated cells cultured with medium alone showed a subtle constitutive production of IGF-1 (light grey). PMA/Ionomycin (red) and HMB-PP (dark grey) led to up-regulation of IGF-1 production in CLA+Vγ9Vδ2 T cells (D). Relative IGF-1 expression as assessed by quantitative PCR showed an up-regulation of IGF-1 expression upon activation also with IFN-α (n=4) (D).
Activated CLA+Vγ9Vδ2 T cells produced the psoriasis-relevant chemokine IL-8 (Figure 6C). In addition they produced the pro-inflammatory chemokines CCL3 (MIP-1α) and CCL4 (MIP-1β) which were markedly up-regulated upon activation (Figure 6C). CCL5 (RANTES) was constitutively produced at high levels (Figure 6C).
Psoriasis is an immune mediated disease associated with hyperproliferation of keratinocytes and angiogenesis; we therefore assessed whether CLA+Vγ9Vδ2 T cells produced growth factors such as VEGF, KGF, FGF-2, EGF and IGF-1. CLA+Vγ9Vδ2 T cells up-regulated IGF-1 production when stimulated with HMB-PP or PMA/Ionomycin (Figure 6D). This was confirmed at RNA level where the psoriasis-relevant cytokine IFN-α was identified as a strong inducer of IGF-1 mRNA (n=3) (Figure 6D). CLA+Vγ9Vδ2 T cells produced low amounts of VEGF (<100pg/ml) and FGF-2 (<15pg/ml) upon activation while KGF and EGF production was absent (data not shown). These data establish CLA+Vγ9Vδ2 T cells as potent pro-inflammatory cells and potential contributors to psoriasis immunopathogenesis.
CLA+Vγ9Vδ2 T cells activate keratinocytes
We next investigated the effects of CLA+Vγ9Vδ2 T cells on skin epithelial cells. Incubation of primary human keratinocytes with CLA+Vγ9Vδ2 T cell supernatant up-regulated the activation markers ICAM-1, HLA-DR and HLA-ABC on keratinocytes (Figure 7A). Keratinocyte activation with CLA+Vγ9Vδ2 T cell supernatant was partly blocked by adding anti-IFN-γ and TNF-α antibodies to the culture (Figure 7B). CLA+Vγ9Vδ2 T cell supernatant induced keratinocytes to produce the psoriasis-relevant mediators TNF-α, IL-6, CXCL9 and CXCL10 in an IFN-γ and TNF-α dependent manner (Figure 7C). A major defense mechanism of keratinocytes against invading pathogens is their production of anti-microbial peptides (28) which are also up-regulated in psoriasis (29). CLA+Vγ9Vδ2 T cell supernatant induced production of β-defensin 2, S100A7 and S100A8 but not LL37 in keratinocytes (n=6) (Figure 7D and data not shown).
Figure 7. CLA+Vγ9Vδ2 T cells activate keratinocytes.
Keratinocytes were cultured for 48 hours with the supernatant of stimulated and resting CLA+Vγ9Vδ2 T cells. Supernatant of Vγ9Vδ2 T cells activated with HMB-PP (red) or CD3/28 beads (blue) strongly up-regulated the activation markers HLA-DR and ICAM-1 on keratinocytes, while HLA-ABC levels were comparable to the supernatant of unstimulated cells (green) (A). Up-regulation of HLA-DR and ICAM was inhibited when adding anti-TNF-α and α-IFN-γ antibodies to the culture (green: supernatant of HMB-PP activated CLA+Vγ9Vδ2 T cells only, blue: supernatant of HMB-PP activated CLA+Vγ9Vδ2 T cells + α-IFN-γ and α-TNF-α antibodies) (B). Keratinocytes exposed to supernatant of HMB-PP activated CLA+Vγ9Vδ2 T cells produced high amounts of the CXC chemokine CXCL9 and CXCL10 in a largely TNF-α and IFN-γ dependent fashion (C). Activated CLA+Vγ9Vδ2 T cell supernatant induced production of β-defensin, S100A7 and S100A8 but not LL37 in keratinocytes at RNA level (representative experiment, n=6) (D).
These data demonstrate a pro-inflammatory cross talk between CLA+Vγ9Vδ2 T cells and keratinocytes.
Discussion
Murine skin has an abundant population of epidermal γδ T cells which serve an important role as early immune sentinels. γδ T cells are exceedingly rare in human skin and it has remained controversial if and to what extent γδ T cells play a role in human skin homeostasis or pathology. This study revisits the role of γδ T cells in skin of healthy individuals and in the chronic inflammatory skin disease psoriasis.
In a first instance we had performed an extensive flow cytometry based screen of unconventional T cell populations including NKT cells and γδ T cells in healthy subjects and psoriasis patients. While we were unable to find significant differences in most of the cell populations investigated, there was a reproducible and significant reduction of Vγ9Vδ2 cells in the blood of psoriasis patents compared to healthy controls.
Here, we identify and characterize a novel subset of skin homing, pro-inflammatory Vγ9Vδ2 T cells which migrates to perturbed human skin in vivo. We suggest that Vγ9Vδ2 T cells are clinically relevant in psoriasis since reduction of circulating Vγ9Vδ2 T cell numbers correlated with increased psoriasis severity and was restored by successful psoriasis-targeted therapy.
Few studies have investigated Vγ9Vδ2 T cell in skin homeostasis and pathology and, even though several reports show that Vγ9Vδ2 T cells are present in skin, their role and function has remained ill explored. Using spectrotyping techniques Holtmeier et al. described the presence ofVγ9Vδ2 T cells in healthy human skin (12). Other studies using immunohistology techniques detected Vγ9Vδ2 T cells in a variety of infectious, inflammatory and malignant cutaneous diseases (8-9, 32-35). Interestingly, the majority of primary cutaneous γδ T cell lymphomas display a Vδ2 gene usage (30) supporting the concept of a specialized subset of skin homing Vγ9Vδ2 T cells.
We show that a reduction of circulating Vγ9Vδ2 T cells in psoriasis patients compared to healthy controls is specific for Vγ9Vδ2 T cells as other circulating lymphocyte subsets, such as conventional T cells, Vδ1 T cells, NKT cells or NK cells, did not show significant differences.
More detailed analysis revealed the existence of a Vγ9Vδ2 T cell subset expressing the E-selectin ligand and skin homing marker CLA. This subset was selectively decreased in psoriasis compared to the CLA− subset indicating the existence of a skin homing Vγ9Vδ2 T cell subset with the potential to be recruited into psoriatic skin. This was further supported by the reduction of peripheral Vγ9Vδ2 T cells expressing skin homing chemokine receptors in psoriasis.
While the majority of CLA+ conventional αβ T cells co-expresses the homeostatic skin homing chemokine receptors CCR4 and CCR10 (20), only a minority of Vγ9Vδ2 T cells expressed these receptors indicating differences in skin homing behavior between conventional αβ T cells and Vγ9Vδ2 T cells. Predominant expression of the inflammatory skin chemokine receptor CCR6 on Vγ9Vδ2 T cells supported the possibility of a coordinated and selective recruitment of circulating Vγ9Vδ2 T cells into skin upon skin perturbation as opposed to homeostatic migration into the skin.
We studied the skin homing capability of Vγ9Vδ2 T cells in vivo using a skin suction blister model. This model has been widely used to study skin immune cells under homeostasis and in the context of skin perturbation (26, 27, 31). Immune cells present in blister fluid have been shown to represent cells, phenotypically and numerically, found in situ by histology (32). Vγ9Vδ2 T cells were significantly increased following skin perturbation against a background of Vγ9Vδ2 T cells present in unperturbed skin. Vγ9Vδ2 T cells in unperturbed skin could represent a rare skin resident population (14). Vγ9Vδ2 T cells in perturbed skin increased to a significantly higher extent than total T cells and expressed significantly more CLA than circulating Vγ9Vδ2 T cells. Taken together these results support the existence of a specialized circulating skin homing Vγ9Vδ2 T cell subset in vivo.
In order to place Vγ9Vδ2 T cells in the context of pathology we investigated psoriasis, one of the most common inflammatory skin conditions. Psoriasis has served a model of inflammatory disease in which to study the involvement of immune cell subsets.
While the presence of cells expressing the pan-γδ TCR has been previously described in psoriasis (33) our results show for the first time the infiltration of Vγ9Vδ2 T cells in psoriasis skin. Calculating absolute Vγ9Vδ2 T cell numbers in psoriatic skin, we found substantial amounts of Vγ9Vδ2 T cells in psoriatic lesions. The number of Vγ9Vδ2 T cells in the skin of patients with severe psoriasis was an order of magnitude higher than the number of circulating Vγ9Vδ2 T cells; e.g. patient A had 14 fold higher numbers of Vγ9Vδ2 T cells in inflamed skin compared to that in the peripheral circulation (770 million Vγ9Vδ2 T cells in skin versus 53 million circulating Vγ9Vδ2 T cells).
Perhaps our most striking finding was the fact that psoriasis disease severity significantly correlated with lower relative and absolute numbers of Vγ9Vδ2 T cells in the circulation and that successful anti-psoriatic therapy leads to increase of peripheral Vγ9Vδ2 T cells. This establishes Vγ9Vδ2 T cells as potential biomarkers for psoriasis and supports their clinical significance. The mechanism by which Vγ9Vδ2 T cells increase with successful therapy remains to be determined. Possible scenarios involve re-entry of skin resident Vγ9Vδ2 T cells into the circulation, reduced recruitment of peripheral Vγ9Vδ2 T cells to resolving psoriatic lesions or decrease of CLA expression on peripheral Vγ9Vδ2 T cells.
We suggest that circulating CLA+Vγ9Vδ2 T cells are early immune sentinels recruited to perturbed skin in the context of trauma or infection. In this scenario, Vγ9Vδ2 T cells might be protective/beneficial in conditions of skin homeostasis and help in maintenance of skin integrity and in anti-microbial responses (34, 35) On the other hand, Vγ9Vδ2 T cells might be pathogenic in an inflammatory environment contributing to psoriasis pathogenesis as potentially early players in the development of a psoriatic plaque (Supplementary Figure 4).
Plasmacytoid DCs (pDCs) triggering the development of psoriatic lesions through their IFN-α production (36). It is thus conceivable that Vγ9Vδ2 T cells are activated by IFN-α in psoriatic skin as IFN-α potently induces IFN-γ production in Vγ9Vδ2 T cells (37). In addition we show that IFN-α induces production of the psoriasis relevant growth factor IGF-1 (38, 39) in Vγ9Vδ2 T cells. Furthermore, Vγ9Vδ2 T cells produce the psoriasis-relevant cytokines IFN-γ, TNF-α and IL-17A, in line with previously reported IL-17A production of blood derived Vγ9Vδ2 T cells (40). Vγ9Vδ2 T cells also release large amounts of pro-inflammatory chemokines such as IL-8, CCL3, CCL4 and CCL5 which have been shown to be of importance for recruitment of key immune effector cells to skin (41, 42) and efficiently activate keratinocytes. Thus, Vγ9Vδ2 T cells might play an important role in psoriasis disease pathogenesis.
There are questions remaining. While it is the nature of translational human immunology studies that most of the data are observational, it will be interesting to study the role of Vγ9Vδ2 T cells in the context of targeted interventions using biologics. Also, more insights into the functional nature of these cells might be obtained in clinically relevant model systems such as animal xenotransplantation models.
Taken together, we propose that CLA+Vγ9Vδ2 T cells represent an innate immediate response tissue surveillance cell, constituting a first wave of T cells that enter skin upon tissue perturbation, such as trauma, adding to the intricate immune surveillance network that operates in the skin (43). Our data points towards three major roles for skin homing Vγ9Vδ2 T cells: (1) the rapid release of pro-inflammatory cytokines influencing resident immune and epithelial cells; (2) the recruitment of immune cells from the circulation; and (3) the release of growth factors resulting in tissue remodeling.
Our data puts the emphasis on a previously overlooked T cell subset in skin immunology. The discovery of a potential role of Vγ9Vδ2 T cells in psoriasis pathogenesis provides the basis for the discovery of novel biomarkers and potential therapeutic targets in chronic inflammatory skin diseases.
Supplementary Material
Acknowledgements
We thank Karen Robertson, Naomi Hare, Gemma Ash and Darren Geoghegan from the skin therapy research unit, Mrs. Jenny Geh and Mr. Ciaran Healy from plastic surgery as well as Dr. Rose Mak, Angela Clifford and Sharon Jones for help with collecting clinical samples. In addition we thank Isabella Tosi for excellent management and coordination of tissue bank as well as Jennifer Mollon for advice on statistical analysis. This work would not have been possible without the generous help of healthy volunteers and of patients from Guy’s and St. Thomas’ Hospital, London.
We acknowledge support by the following grant funding bodies: National Institute of Health RO1AR040065, Wellcome Trust Programme GR078173MA, Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust, Medical Research Council UK Programme G0601387, and Dunhill Medical Trust.
References
- 1.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 3.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 5.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 6.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 7.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 9.Bachelez H, Flageul B, Degos L, Boumsell L, Bensussan A. TCR gamma delta bearing T lymphocytes infiltrating human primary cutaneous melanomas. J Invest Dermatol. 1992;98:369–374. doi: 10.1111/1523-1747.ep12499808. [DOI] [PubMed] [Google Scholar]
- 10.Elbe A, Foster CA, Stingl G. T-cell receptor alpha beta and gamma delta T cells in rat and human skin--are they equivalent. Semin Immunol. 1996;8:341–349. doi: 10.1006/smim.1996.0045. [DOI] [PubMed] [Google Scholar]
- 11.Alaibac M, Morris J, Chu AC. Gamma delta T-cells in human cutaneous immunology. Int J Clin Lab Res. 1997;27:158–164. doi: 10.1007/BF02912452. [DOI] [PubMed] [Google Scholar]
- 12.Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR-delta repertoire in normal human skin is restricted and distinct from the TCR-delta repertoire in the peripheral blood. J Invest Dermatol. 2001;116:275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 13.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 15.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR, Nair RP. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 17.Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 20.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 21.Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis. J Invest Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 22.Robak E, Niewiadomska H, Robak T, Bartkowiak J, Blonski JZ, Wozniacka A, Pomorski L, Sysa-Jedrezejowska A. Lymphocyctes Tgammadelta in clinically normal skin and peripheral blood of patients with systemic lupus erythematosus and their correlation with disease activity. Mediators Inflamm. 2001;10:179–189. doi: 10.1080/09629350124724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- 24.Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2010;59:1109–1120. doi: 10.1007/s00262-010-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitzalis C, Kingsley GH, Covelli M, Meliconi R, Markey A, Panayi GS. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991;21:369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- 27.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MH, Akbar AN. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol. 2007;25:616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripodo C, Iannitto E, Florena AM, Pucillo CE, Piccaluga PP, Franco V, Pileri SA. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6:707–717. doi: 10.1038/nrclinonc.2009.169. [DOI] [PubMed] [Google Scholar]
- 31.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed JR, Vukmanovic-Stejic M, Fletcher JM, Soares MV, Cook JE, Orteu CH, Jackson SE, Birch KE, Foster GR, Salmon M, Beverley PC, Rustin MH, Akbar AN. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433–1443. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ, Cho HJ, Park HR. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol. 2007;34:903–911. doi: 10.1111/j.1600-0560.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 34.Alaibac M, Harms G, Zwingenberger K, Morris J, Yu R, Chu AC. Gamma delta T lymphocytes in oriental cutaneous leishmaniasis: occurrence and variable delta gene expression. Br J Dermatol. 1993;128:388–392. doi: 10.1111/j.1365-2133.1993.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 35.Flageul B, Bachelez H, Boumsell L, Degos L, Bensussan A. Infiltrating lymphocytes in benign and malignant naevomelanocytic lesions. Nouv Rev Fr Hematol. 1990;32:9–11. [PubMed] [Google Scholar]
- 36.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 38.Wraight CJ, White PJ, McKean SC, Fogarty RD, Venables DJ, Liepe IJ, Edmondson SR, Werther GA. Reversal of epidermal hyperproliferation in psoriasis by insulin-like growth factor I receptor antisense oligonucleotides. Nat Biotechnol. 2000;18:521–526. doi: 10.1038/75382. [DOI] [PubMed] [Google Scholar]
- 39.Pirgon O, Atabek ME, Sert A. Psoriasis following growth hormone therapy in a child. Ann Pharmacother. 2007;41:157–160. doi: 10.1345/aph.1H324. [DOI] [PubMed] [Google Scholar]
- 40.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine Requirements for the Differentiation and Expansion of IL-17A- and IL-22-Producing Human V{gamma}2V{delta}2 T Cells. J Immunol. 2010 doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunstfeld R, Lechleitner S, Wolff K, Petzelbauer P. MCP-1 and MIP-1alpha are most efficient in recruiting T cells into the skin in vivo. J Invest Dermatol. 1998;111:1040–1044. doi: 10.1046/j.1523-1747.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 42.Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–559. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 43.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.