Abstract
Australian magpies (Gymnorhina tibicen) are notable for their vocal prowess. We investigated the syringeal and respiratory dynamics of vocalization by two 6-month-old males, whose songs had a number of adult features. There was no strong lateral syringeal dominance and unilateral phonation was most often achieved by closing the syringeal valve on the contralateral side of the syrinx. Unlike other songbirds studied, magpies sometimes used an alternative syringeal motor pattern during unilateral phonation in which both sides of the syrinx are partially adducted and open to airflow. Also, in contrast to most other songbirds, the higher fundamental frequency during two-voice syllables was usually generated on the left side of the syrinx. Amplitude modulation, a prominent feature of magpie song, was produced by linear or nonlinear interactions between different frequencies which may originate either on opposite sides of the syrinx or on the same side. Pulse tones, similar to vocal fry in human speech, were present in some calls. Unlike small songbirds, the fundamental of the modal frequency can be as low as that of the pulse tone, suggesting that large birds may have evolved pulse tones to increase acoustic diversity, rather than decrease the fundamental frequency.
Keywords: Syrinx, Birdsong, Nonlinear, Lateralization, Pulse tone
Introduction
Despite the importance of Oscine songbirds as an experimental model in which to study vocal learning and vocal communication, the syringeal and respiratory mechanisms controlling song production have been studied in only a few of the approximately 4,000 species in this diverse suborder. Even from this small sample it is apparent that different taxa have evolved different vocal motor strategies with which to produce their species-typical songs. Each of the several taxa for which there are data, uses the two sound sources in its bipartite syrinx in different ways to achieve the salient acoustic effects of its species' song (Suthers 1997, 1999; Suthers and Zollinger 2008). When a vocal mimic, the northern mockingbird (Mimus polyglottos), mimics heterospecific song, the bird uses syringeal motor patterns that are similar to those of the tutor species (Zollinger and Suthers 2004). It thus appears that each species has evolved the optimal vocal motor pattern for producing its particular song. Comparative studies of song production can provide insights into the nature of performance constraints on birdsong and their influence on the evolution of song diversity.
In this article, we present the first data on syringeal and respiratory dynamics of song production in the Australian magpie, Gymnorhina tibicen, (Artamidae) (Christidis and Boles 2008), a songbird which is noteworthy for the exceptional variety and complexity of its song (reviewed by Kaplan 2006, 2008b) and whose vocal ability earned it a Latin species name meaning `flute-player'. The Australian magpie has an adult body mass of about 340–440 g and body length of 36–44 cm, making it larger than the European and North American magpies (Pica spp), to which it is not related.
Unlike most songbirds in high latitudes, which are territorial only during the breeding season and in which females typically do not sing, Australian magpies maintain territories throughout the year and both sexes have similarly well-developed song (Brown and Veltman 1987). Some Australian magpies breed as monogamous pairs but others breed in cooperative groups maintaining permanent territories that they defend throughout the year by communal group caroling in which both sexes participate (Robinson 1956; Veltman 1989; Brown and Farabaugh 1991; Farabaugh et al. 1992). In addition to its role in territorial defense, song appears to be important in maintaining the social cohesion and group recognition that is required for this species' complex social structure (Brown and Veltman 1987; Brown et al. 1988; Kaplan 2005). Kaplan (2008b) has pointed out that attributes such as the sexually monomorphic vocal behavior of the Australian magpie, its increased use of song outside of the breeding season and the likelihood that it is an open-ended learner provide closer parallels to some important aspects of human speech than does the song of widely studied species, such as the zebra finch (Taeniopygia guttata), in which only the male sings, adults do not learn new songs, and singing is focused mainly on reproduction.
The vocal repertoire of Australian magpies includes a number of different calls, some specific to nestlings and juveniles, such as begging calls, and several categories of adult vocalizations (Kaplan 2006, 2008b). The latter include a complex set of short alarm calls of enormous variety—some 27 variations have been identified falling into 5 distinct classes (Kaplan 2008a; Kaplan et al. 2009)—and territorial vocalizations referred to as carols or warble carols. Brown and Farabaugh (1991) identified 204 carol and warble–carol syllable types belonging to 11 general classes in a population of 24 magpies. Magpies also mimic other species extensively (Kaplan 2000, 2004b).
Their adult songs are controversially referred to at times as a `subsong' because they appear to be improvised, are relatively quiet and consist of a series of short, often musical, syllables having most of their sound energy below about 2 kHz. These vocalizations are called `warbles'. Warbling bouts by individuals last typically for around 10 min but can extend to periods of hours. Bouts of 1 h are not uncommon (Kaplan 2008b). Carol syllables, though of short duration, are substantially louder and longer than warble syllables. The 1–1.5 kHz fundamental of carol syllables is accompanied by prominent overtones (Brown et al. 1988; Kaplan 2006). Warble songs are sung by one individual, but caroling may involve a pair or an entire group and thus contain syllables from two or more members of the group (Farabaugh et al. 1988). Brown et al. (1988) identified 823 syllable types of warbles from 23 birds in their study population. According to their findings, 67% of these were individually specific whereas some other syllables were shared by at least 5 other birds. Some song sharing was evident and warbling, despite its individual variability, has now been identified as having a role to play in stranger/neighbor discrimination and thus in territorial maintenance (O'Shea and Kaplan 2010).
Juvenile Australian magpies have an extended period of song development. Juvenile song begins when the young are nestlings about 3 weeks old, about a week before they fledge. Juveniles only audibly practice song when the parents and other adult magpies are at least 15–20 m away from the nest (Kaplan 2004a). Song continues to develop for at least several more months while the offspring are still in their natal territory and prior to dispersal, which typically occurs after at least 6–7 months (Brown and Veltman 1987; Kaplan 2006), and may not become fully adult-like until the age of about 15 months (Brown and Veltman 1987). Australian magpies appear to be open-ended vocal learners (Waite 1903; Brown et al. 1988; Farabaugh et al. 1988) who retain vocal plasticity in adulthood that enables them to add new syllables to their song repertoire throughout their life. The forebrain song control nuclei HVC (used as a proper name), robust nucleus of the arcopallium (RA), area X, the lateral and medial nuclei of the anterior nidopallium (LMAN and MMAN) are well developed in adult males and females and in juveniles 2–3 months after fledging (Deng et al. 2001).
The Australian magpie, like other Oscine songbirds, has a tracheobronchial syrinx in which the cranial end of each primary bronchus contains a pair of vibratory structures, the medial and lateral labia, which vibrate in response to aerodynamic forces and produce sound when adducted into the expiratory airstream of the bronchial lumen (Goller and Larsen 1997). The muscles on each side of the syrinx are innervated by the ipsilateral tracheosyringeal branch of the hypoglossal nerve so that each side of the syrinx is under independent motor control by ipsilateral motor neurons that are in turn controlled by the central song system predominantly on the same side (Nottebohm et al. 1976; Wild et al. 2000).
This project is the first to examine the syringeal mechanisms of song production in a monomorphic songbird, in which song, like speech in humans, has more generalized functions than the attraction of a mate or the defense of territory. This is important because the song system of songbirds, which includes brain nuclei and pathways underlying both the auditory-dependent learning and production of song (Brenowitz et al. 1997), is a key model system for the study of the neurobiological mechanisms by which the former makes possible speech in humans (Doupe and Kuhl 1999; Zeigler and Marler 2008).
Materials and methods
One female and two male juvenile Australian magpies were collected in the vicinity of Armidale, New South Wales, Australia (30°32′S, 151°40′E). Their sex was determined by molecular analysis of feather DNA. The birds were maintained in a large sheltered outdoor aviary (4 × 3 × 3 m) for 2 months where they were exposed on a daily basis to songs of wild magpies and other local species of birds and completely habituated to human company. Prior to the experiment, all birds were moved to the laboratory where each bird was housed individually, but in the same room, in a custom-designed cage measuring 1 × 0.6 × 0.8 m equipped with food and water ad libitum, a perch across the length of the cage and some enrichment items. An adult wild-caught magpie that was not habituated to humans, also housed in the same room as the other three, failed to produce any warbles in the laboratory and was therefore released.
The experimental procedure was similar to that described in detail in Suthers (1990) and Suthers et al. (1994). Each bird was fitted with an elastic belt around its thorax which held a small self-gripping (Velcro) tab in middle of the back for later attachment of a backpack containing miniature electrical connectors for fine wires carrying physiological signals. Before surgery, the bird was anesthetized with an initial dose of ketamine HCl (50 mg/kg, im) and xylazine (5 mg/kg, im) with supplementary doses as needed. Data were obtained from the 2 males, which were about 6 months old (post hatching) at the time of the experiments. They were collected from different territories more that 5 km apart and were therefore unlikely to be genetically related. The female magpie twice rapidly removed all recording apparatus and therefore did not yield any data. The birds were released into their territories after the experiment.
Subsyringeal pressure
Fluctuations in respiratory air pressure, relative to the ambient pressure outside the bird, were monitored via a flexible 18 cm long (ID = 1.02 mm, wall thickness 0.57 mm) cannula (Dow Corning silastic tubing 602–205) inserted into an anterior thoracic air sac and connected to a miniature piezoresistive silicone diaphragm pressure transducer (Fujikura FHM 02) mounted on the backpack.
Syringeal airflow
Access to the syrinx and bronchi was gained through a mid-ventral incision between the furcula through the interclavicular membrane into the interclavicular air sac, which contains the syrinx. The rate of airflow through each side of the syrinx was monitored by a 0.13-mm diameter microbead thermistor (Thermometrics BB05JA202) which was inserted into each primary bronchus through a small hole 2 or 3 bronchial rings caudad from the sound-producing syringeal labia. The thermistors were positioned near the middle of the bronchial lumen and held in place by a micro-drop of tissue adhesive (n-butyl cyanoacrylate) applied to the external surface of the bronchus at the entry point of the flow probe. Fine, flexible wire leads from the thermistors were routed out of the air sac through the interclavicular membrane, which was closed with an airtight seal around the wire leads, and then under the skin to the backpack. Thermistor leads were connected to a feedback circuit in which the current needed to maintain the heated thermistor at a constant temperature was proportional to the rate of airflow.
Air flow and pressure signals were transmitted from the backpack along wires that exited through the top of the cage and connected to conditioning and recording electronics outside of the cage. A counter-balanced arm maintained a slight tension on the wires from the backpack that prevented the bird from becoming entangled while allowing him to move freely about the cage and sing spontaneously. Vocalizations were recorded with a condenser microphone (Audio-Technica model AT835b) positioned 0.5–1 m in front of the bird. Vocalizations, the rate of airflow through each primary bronchus and air sac pressure were recorded on separate channels of a DAT data recorder (TEAC model RD135T) having a frequency response from DC to 10 kHz (−1 dB) per channel.
Data analysis
After the experiment, taped data were reproduced at half speed and converted into “Signal” (Engineering Design) files with a real-time digitization rate of 40 kHz (Data Translation, 2821-G) per channel. Sound spectrograms and associated power spectra were computed in Igor Pro v.5 (WaveMetrics Inc., Lake Oswego, OR, USA) (1,024 points at 40,000 samples/s with a Hanning window, unless otherwise specified).
Since both inspiratory and expiratory airflow across the heated thermistor produced an upward deflection of the airflow trace, the direction of airflow was determined by the concurrent air sac pressure, which was positive during expiration and negative during inspiration. Phonation and respiration are accompanied by adduction or abduction of the medial and lateral labia at the cranial end of each primary bronchus. Qualitative changes in the aperture of the labial valve (which is analogous to the glottis in the larynx) can be inferred from changes in syringeal resistance, defined as the subsyringeal air sac pressure divided by the rate of syringeal airflow.
Two methods were used to determine the degree of song lateralization and the contribution the left and right sides of the syrinx make to the emitted vocalization. (1) Some vocalizations were accompanied by unilateral air flow. The absence of airflow through one side of the syrinx when air sac pressure is positive indicates that the labial valve on the ipsilateral side of the syrinx is closed and silent. Vocalizations must therefore be generated by airflow through the contralateral side of the syrinx. (2) Many vocalizations were accompanied by airflow through both sides of the syrinx, either or both of which could be producing sound. The fundamental frequency (f0) of magpie vocalizations was usually below 2 or 3 kHz. These low sound frequencies produce small voltage oscillations in the output signal of the ipsilateral thermistor that match the frequency and timing of the vocalization. We analyzed this near-field (i.e., within 1 wavelength of the source) `bronchial sound' by high-pass filtering the thermistor signal at 100 Hz to remove the high amplitude, low-frequency respiratory signal and then displaying the bronchial sound in spectrograms produced from fast Fourier transforms of the amplified thermistor signal (SLB and SRB in figures) (Suthers 1990; Suthers et al. 1994; Beckers et al. 2003; Zollinger et al. 2008).
When using bronchial sound to assess song lateralization in syllables with bilateral airflow, it is important to determine whether the absence of a bronchial sound signal from one bronchus indicates that side is not contributing to the vocalization or if it is because the frequency of the vocalization exceeds the frequency response of the thermistor. Thermistors behave like a low-pass filter in which the high-frequency cut-off decreases during the course of the experiment as mucus droplets and other foreign matter in the bronchial air stream gradually coat the thermistor bead, increasing its time constant and reducing its frequency response. Since it is not possible to quantify the frequency response of thermistors during an experiment and since we are only interested in the presence or absence of bronchial sound on each side of the syrinx, syllables with bilateral airflow, but unilateral evidence for bronchial sound, were not included in our assessment of song lateralization, unless there was clear spectrographic evidence from other syllables in adjacent song bouts that the fundamental frequency of the syllable in question was within the frequency response of the thermistor.
The piezoresistive pressure transducer also recorded small pressure oscillations produced in the thoracic air sac by back-propagation from the syringeal sound source during vocalization. Spectrograms of air sac sound (SAS in figures), like those of bronchial sound, were produced from fast Fourier transforms of thoracic air sac pressure, which had been high-pass filtered at 100 Hz to remove the respiratory waveform from the pressure signal. These spectrograms do not provide information regarding the side(s) of the syrinx in which the sound was produced, since they include sound from both sides of the syrinx. However, they are free from external ambient noise, including the vocalizations of other birds, and have not been filtered by resonances of the supra syringeal vocal tract. They are not a direct measure of the syringeal sound source, however, since they have been subjected to the filter properties of the subsyringeal respiratory passages including the bronchi and air sacs, as well as the silastic cannula attached to the pressure transducer. Speaker-generated pink noise was used to measure the frequency response of the pressure recording system with the 18 cm cannula. The frequency response between 300 Hz and 3 kHz varied between +4 dB and −7 dB relative to a mean attenuation of −32.7 dB and had resonance peaks at 400, 1,200, 2,000 and 2,900 Hz (Beckers et al. 2003 and unpublished data).
Results
Vocal contribution from each side of the syrinx
The songs of both birds contained complex patterns of frequency and amplitude modulation. Although similar acoustic features were sometimes repeated, the lack of acoustic stereotypy prevented a categorization of an individual's vocal repertoire into discreet syllable types. In this paper we refer to the vocalizations produced during a single expiration as a `syllable'. Temporally distinct vocalizations within a syllable are called `notes'. Song is accompanied by a marked increase in the subsyringeal (air sac) pressure due to compression of the air sacs by contraction of expiratory muscles (Hartley 1990; Goller and Suthers 1999). All the magpie vocalizations we recorded were accompanied by expiratory airflow through the syrinx. Although both bronchi are presumably exposed to a similar respiratory pressure, the ability to independently control labial adduction on each side of the syrinx allows songbirds to produce song either bilaterally or unilaterally. Successive syllables were separated from each other by a brief inspiration, often called a minibreath (Calder 1970; Hartley and Suthers 1989; Wild et al. 1998) since it is typically much shorter than inspirations when the bird is not singing (Figs. 1, 2).
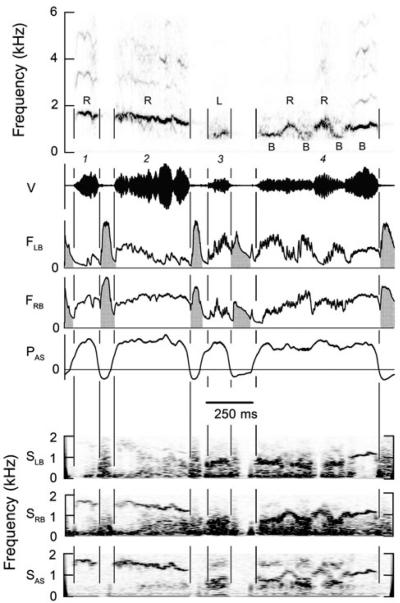
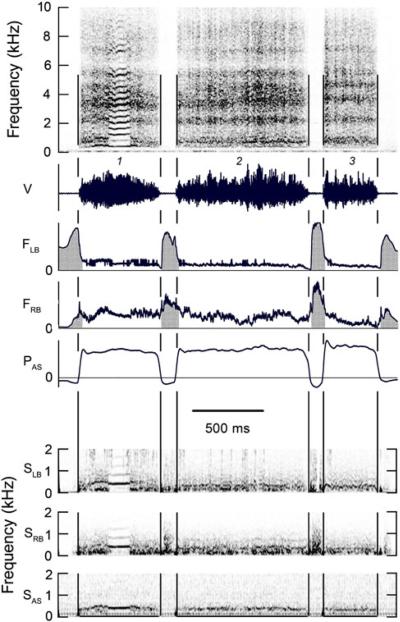
Fig. 1.
Four syllables, separated by minibreaths, illustrating sequential switching of phonation between the left and right sides of the syrinx. Left side of syrinx is closed during Wrst half of syllable 1 and even when this side is open during the latter part of the syllable there is no detectable sound from the left side of the syrinx. Despite the presence of bilateral airflow during syllables 2-4 analysis of the bronchial sound indicates that syllable 2 is generated on the right side and periodic sound in syllable 3 is generated on the left side while the right side produces wide band noise. Syllable 4 contains two sinusoidal frequency sweeps preceded and followed by a relatively CF (constant frequency) component. Bronchial sound indicates that the two `CF' elements and the low-frequency trough at the end of each sinusoidal peak contain contributions from both sides of the syrinx, but the sounds generated on the two sides of the syrinx are not identical. The two sinusoidal peaks of syllable 4 are produced only on the right side of the syrinx, despite bilateral airflow. (Magpie 1, 01-922). R, L, B on spectrogram indicate vocalization originates on right, left or both sides of syrinx, respectively. V time waveform of vocalization with syllables numbered; FLB and FRB rate of airflow through left and right sides of syrinx. Horizontal line zero airflow. Both inspiratory and expiratory flow cool heated thermistor and cause an upward deflection of trace. Inspiratory flow is accompanied by negative air sac pressure and is indicated with grey shading. PAS subsyringeal pressure in thoracic air sac. Horizontal line indicates external ambient pressure. SLB and SRB, spectrogram of signal from thermistor in the left or right bronchus, respectively. Frequency range is limited by response time of the thermistor. SAS spectrogram of sound in thoracic air sac recorded by piezoresistive pressure transducer. Low-frequency fluctuations in bronchial airflow and air sac pressure associated with the respiratory cycle have been attenuated by a 100 Hz high-pass digital Wlter. Time bar applies to entire figure
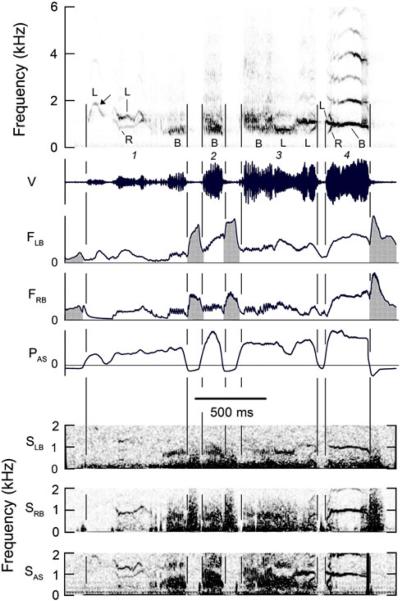
Fig. 2.
A sequence of syllables in which different production techniques increase the acoustic diversity of the song. First note of syllable 1 (arrow) arises on the left side of the syrinx while right side is closed [indicated by zero airflow (FRB) despite positive air sac pressure (PAS)]. This note is followed by a two-voice component in which the high-frequency element comes from the left syrinx and the low-frequency from the right syrinx. The last part of syllable 1, all of syllable 2 and the initial note of syllable 3 exhibit rapid AM associated with oscillating rates of airflow through both sides of the syrinx. Air sac pressure lacks prominent oscillations, suggesting the rapid AM is controlled by the syrinx. Bronchial sound indicates that the last two-thirds of syllable 3 is produced on the left side of the syrinx. The beginning of syllable 4 contains three short independent, simultaneous frequency components: a steep upsweep from the right side of the syrinx, a gradual down-sweep from the left side and a steep down-sweep of unknown origin, possibly biphonation that mirrors the upsweep. The remaining CF portion of syllable 4 is produced bilaterally. Noise or chaos during last 40 ms originates mostly in the right side of the syrinx. See Fig. 1 legend for abbreviations. (Magpie 2, 99–100)
The f0 of vocalizations ranged between about 200 Hz and 2 kHz. Some syllables, or parts thereof, were sung on the left side of the syrinx (e.g., Fig. 1, syllable 3), others on the right side (e.g., Fig. 1, syllables 1 and 2) and in some cases both sides simultaneously produced the same sound (e.g., Fig. 1, syllable 4, last note). Phonation was also often switched from one side of the syrinx to the other during the course of a note or syllable (e.g., Fig. 1, syllable 4 and Fig. 2, syllables 3 and 4). Bilateral phonation requires airflow through both sides of the syrinx.
Unilateral phonation was usually accomplished, as in other songbirds, by closing the labial valve on one side to silence it while allowing air to flow through the partially adducted labia on the contralateral side. However, the magpies also used a different alternative syringeal mechanism, rarely observed in other songbirds studied, in which the silent side of the syrinx was only partially adducted (reduced rate of airflow without a reduction in air sac pressure indicates increased syringeal resistance) during unilateral phonation, so that expiratory air continued to flow through the silent, as well as the phonating side of the syrinx. This is the case during syllable 2 in Fig. 1 and where the spectrograms of bronchial sound (SLB and SRB in Fig. 1) show that this syllable is present only in the right bronchus.
During some bilaterally produced vocalizations the left and right side of the syrinx simultaneously generated different, harmonically unrelated frequencies, referred to as `two-voice' elements (Figs. 2 and 3). Analysis of two-voice syllables, in which the frequency of the left and right components was clear, showed that, with one exception, the higher frequency was produced on the left side (left f0 1.30 ± 0.48 kHz; right g0 1.03 ± 0.45 kHz; n = SD; 3 syll Magpie #1 + 8 syll Magpie #2). The mean difference between sides (left-right) was 300 ± 260 Hz. Despite this difference, there was extensive overlap in the frequency range of each side of the syrinx during two-voice elements (left f0, 0.5–2.0 kHz; right g0, 0.2–1.6 kHz; n = 11). This lateralization of frequency ranges is in the opposite direction from most other songbirds studied, in which the right side of the syrinx produces the highest fundamental.
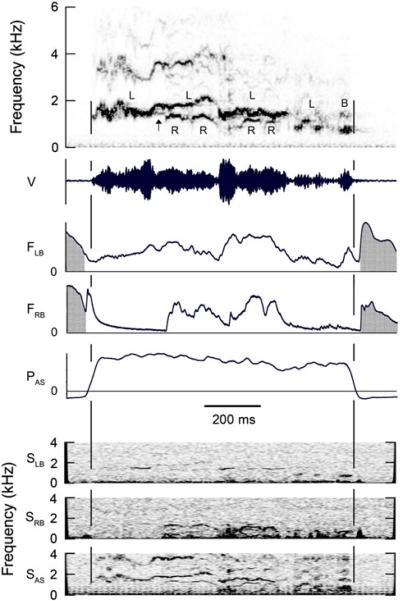
Fig. 3.
This two-voice syllable begins with sound from left side of syrinx while right side is closed. About 200 ms after start of syllable the right side of syrinx begins to produce notes with an f0 lower than that from the left side. Simultaneous sounds from the two sides are not harmonically related and show independent FM. Arrow indicates a brief biphonation on the left side while the right side is closed. Amplitude of the lower frequency component is too low to be visible in bronchus. Entire syllable is produced during one expiration. See Fig. 1 legend for abbreviations. (Magpie 2, 01–601)
Many begging calls consisted of harmonic stacks with a fundamental between about 0.4 and 2.0 kHz. Some calls contained prominent frequency modulation (FM) (e.g., Fig. 4, syllable `3') but others were relatively constant in frequency.
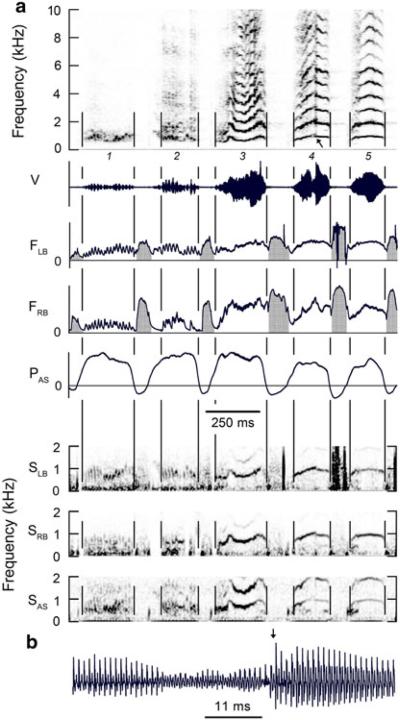
Fig. 4.
a Syllables 1 and 2 exhibit rapid AM that is associated with oscillating airflow rates on each side of the syrinx. Absence of oscillation in air sac pressure suggests AM is produced by varying aperture of the syringeal valve rather than using respiratory muscles to modulate subsyringeal pressure. A frequency jump (slanted arrow) occurs in the middle of syllable 4. This jump is present in bronchial sound on both sides of the syrinx. The apparent overlap of the frequency immediately before and after the jump is probably an artifact of the spectrograms limited time resolution. b Expanded waveform of jump (vertical arrow). See Fig. 1 legend for abbreviations. (Magpie 1, 02–216)
Nonlinear phenomena
The calls of both magpies often contained one or more of four types of nonlinear phenomena, i.e., deterministic chaos, subharmonics, biphonation and frequency jumps, which are associated with nonlinear systems and occur in the vocalizations of many vertebrates, including humans (Fee et al. 1998; Wilden et al. 1998; Fitch et al. 2002). In songbirds, each coupled pair of vibrating labia is a nonlinear oscillator with intrinsic properties that can cause it to go into different states of complex oscillation.
We analyzed about 500 vocalizations from each bird. In both birds, nonlinear phenomena were prominent features of begging calls, but were much less common in `song syllables', i.e., syllables with most of their energy in a f0 below about 3 kHz (e.g., Figs. 1, 2, 3).
Deterministic chaos
Many begging calls of both birds were composed of broadband aperiodic sound. The abrupt onset of the call and the occasional presence of periodic `windows' are consistent with our assumption that this broad-band sound is deterministic chaos. These chaotic vocalizations typically contained prominent formants, i.e., frequency bands where acoustic energy is concentrated. The first of the three chaotic begging calls shown in Fig. 5 also contain a harmonic window with a f0 at about 400 Hz. Despite the low rate of airflow through the left side of the syrinx, the harmonic window occurs in both bronchi at the same time, indicating that the unknown variable controlling its occurrence is expressed simultaneously on both sides of the syrinx. It is interesting that only the f0 appears in the thoracic air sac, despite the fact that the pressure transducer has a higher frequency response than the bronchial thermistors.
Fig. 5.
Three begging calls that appear to consist of deterministic chaos with six formants. Chaos in syllable 1 is interrupted by a harmonic window. Bronchial sound indicates that both sides of the syrinx are contributing to the chaos and the harmonic window. The ca 400 Hz f0 of the harmonic window is continuously present in the cranial thoracic air sac during the full duration of each syllable, but is not detectable outside the window in either the bronchial sound or vocalization. See Fig. 1 legend for abbreviations. (Magpie 2, 01–175)
Subharmonics
Some magpie vocalizations contain subharmonics. This nonlinear phenomenon appears in spectrograms of the vocalization as integer fractional values of f0, which are evenly spaced below f0 and between its higher harmonics. The bilaterally produced vocalization in Fig. 6 consists of a harmonic stack with an f0 of 660 Hz. A subharmonic at 0.5 f0 is present during the first half of the vocalization. Spectrographs of bronchial sound indicate that this subharmonic arises mainly or entirely on the right side of the syrinx. Sometimes a subharmonic appears in the bronchial sound but is not present in the emitted vocalization. The sequence of four calls in Fig. 7 have an f0 at about 600 Hz that is being generated on both sides of the syrinx. Aperiodic noise is present below the f0 in the left bronchus of all four calls and in the right bronchus of the first two calls. A clear subharmonic at 0.5 f0 is present in the right bronchus during the third and fourth calls, but is filtered out of the vocalization.
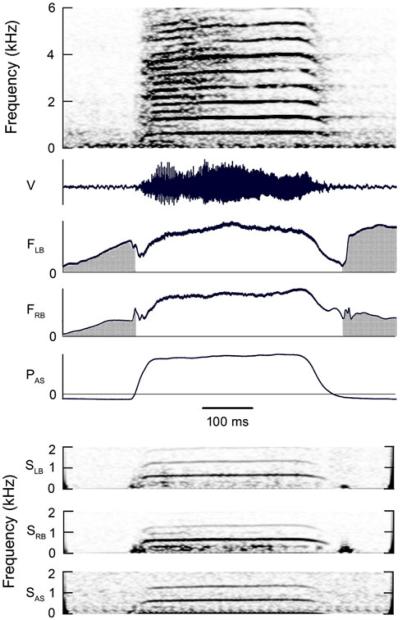
Fig. 6.
Syllable composed of harmonic stack having a f0 that rises gradually from about 640 Hz at beginning of syllable to 660 Hz later in syllable. A subharmonic at 0.5 f0, which appears to originate on the right side of the syrinx, is present during Wrst half of the syllable. This 320 Hz subharmonic modulates the 640 Hz f0 to produce the rapid AM near beginning of syllable. Unlike, the subharmonic in Fig. 7, this sub-harmonic was not Wltered out of the emitted vocalization. Higher harmonics, not shown, extend up to about 9 kHz. Some echoes, due to sound reflected from objects near the bird, are visible after the end of the vocalization. See Fig. 1 legend for abbreviations. (Magpie 2, 01–266)
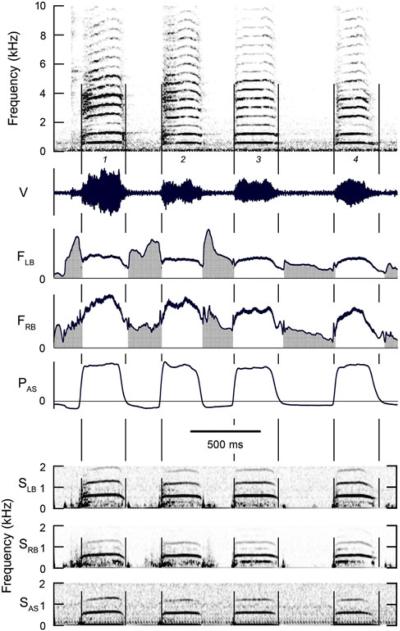
Fig. 7.
Four syllables consisting of harmonic stacks with a f0 ca 600 Hz. Bronchial sound indicates these harmonics are generated bilaterally. There is some broad-band noise below the f0 in both bronchi that is perhaps due to turbulent airflow. In the right bronchus this `noise' resolves itself into a subharmonic at 0.5 f0 in syllables 3 and 4, but this subharmonic is Wltered out of the emitted vocalization. See Fig. 1 legend for abbreviations. (Magpie 2, 01–261)
Biphonation
Cases in which two or more harmonically unrelated frequencies were produced on the same side of the syrinx were rare. Two-voice syllables differ from biphonation in that, in the former, each pair of oscillators, i.e., the medial and lateral labia on each side of the syrinx, generates a single fundamental frequency that is different from that produced on the contralateral side of the syrinx. In order to distinguish between biphonation and two voices, one must know whether the two sounds in question arise on the same side of the syrinx or on opposites sides (Zollinger et al. 2008). Biphonation occurs briefly in the left side of the syrinx in Fig. 3 (arrow) while the right side is closed.
Frequency jumps
We define a frequency jump as an abrupt step-like, up or down change in f0 with <5 ms silent interval separating the f0 before and after the jump. Frequency jumps were not common in magpie vocalizations. A downward frequency jump is present in the middle of syllable `4' in Fig. 4.
Amplitude modulation
Both magpies often included prominent rapid amplitude modulation (AM) in their vocalizations, including song syllables. Many of these were produced by linear (additive) or nonlinear (multiplicative) acoustic interactions between different, simultaneously generated frequency components that often originated on opposite sides of the syrinx.
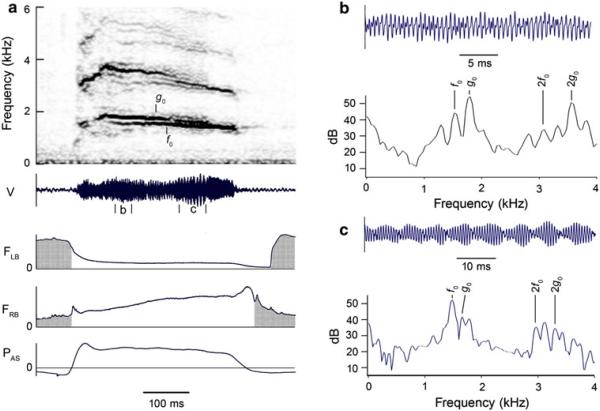
Inspection of the bronchial sound (not shown) of the two-voice syllable in Fig. 8 shows an f0 on the left side that is lower than the fundamental (g0) on the right side. The two-voice portion of the syllable is accompanied by strong amplitude modulation that is not due to changes in syringeal resistance or in the subsyringeal pressure, since there is no concurrent cyclical oscillation in either the expiratory pressure or the rate of syringeal airflow. This AM is likely due to the linear interaction of f0 and g0, which differ by about 237 Hz, during time interval b. As the syllable progresses, g0 bifurcates, resulting in biphonation on the right side just before the beginning of time interval c. Most of the sound energy is in the lower frequency of the biphonation and this frequency sums with f0 to produce AM at about 127 Hz during time interval c.
Fig. 8.
a Rapid amplitude modulation due to linear acoustic interaction of slightly different fundamental frequencies on the left (f0 = 1,538 Hz) and right (g0 = 1,775 Hz) sides of the syrinx during a two-voice syllable. The modulation rate decreases gradually during the course of the syllable as the difference between the f0 and g0 decreases. b Modulation frequency during segment b is about 237 Hz (period = 4.2 ms), which is equal to the difference between the f0 and g0 from the left and right sides of the syrinx, respectively, during this portion of the syllable. c Modulation frequency during segment c is 127 Hz (period = 7.9 ms), which is equal to the difference between the left (f0 = 1,463 Hz) and right (g0 = 1,590 Hz; the lower frequency component of the biphonation) at this time in the syllable. See Fig. 1 legend for abbreviations. (Magpie 2, 01–562)
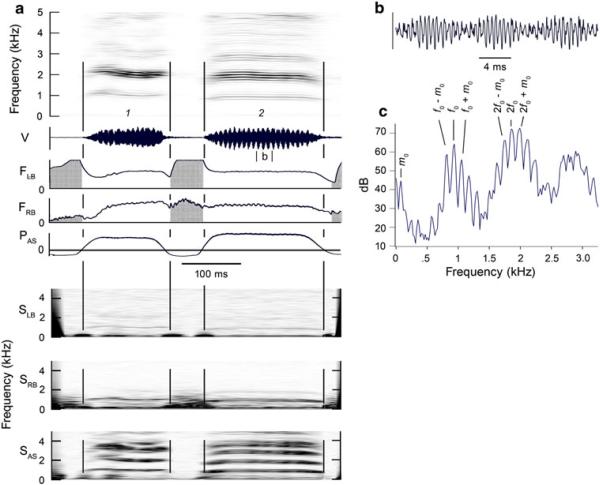
The rapid AM in the vocalization shown in Fig. 9 appears to be the result of nonlinear interaction between a 917 Hz fundamental (the carrier frequency) produced in the right side of the syrinx and a 113 Hz modulating frequency of unknown origin. This generates sum and difference tones equal to f0 ± m0 which appear in the spectrogram as side bands around the carrier frequency.
Fig. 9.
a and b Example of two vocalizations exhibiting strong AM. Bilateral airflow and the presence of the same f0 in both bronchi and in the thoracic air sac, indicate that both sides of the syrinx are producing the same f0, which in the middle of the second syllable, is about 917 Hz. c This f0 is accompanied by a modulating frequency m0 at about 110 Hz, which generates side bands at f0 ± m0 and 2f0 ± m0. Additional side bands are also present. Note the prominent formants produced by the vocal tract resonance Wlter. The airflow signal for the left bronchus is clipped during peak inspiratory flow rates which exceeded the dynamic range of the amplifier. 2048 point FFT. See Fig. 1 legend for abbreviations. (Magpie 2, 01–569)
Pulse tone vocalizations
Humans are able to change the quality, or register, of their voice by altering the mode of vocal fold vibration (Hollien 1974; Titze 1994). Normal speech is produced in the modal register. Other vibratory modes include a high-pitched falsetto register and a low-pitched pulse tone or vocal fry register. Evidence for a pulse tone register in songbirds was reported by Jensen et al. (2007).
The begging calls of both magpies often exhibited an acoustic waveform consisting of repetitive very brief high amplitude sound pulses (Fig. 10, arrow) which are produced by rapidly closing and opening the labial valve on the right side of the syrinx. Each biphasic sound pulse is followed by a lower amplitude acoustic pressure wave, which is presumably produced by a resonant damped oscillation of the labia. The result is a broad-band vocalization consisting of a harmonic stack with an f0 equal to the pulse rate (Fig. 10). Complex patterns of subharmonics are often present. Usually this vocalization is generated on the right side of the syrinx, but sometimes trains of pulses are produced on both sides of the syrinx and the phase relationship between the two sides can vary. The time constant of the heated thermistor bead measuring syringeal airflow limits its frequency response so that the signal from the thermistor does not return to zero flow at the reversal points between inspiration and expiration or during the postulated brief closure associated with each sound pulse.
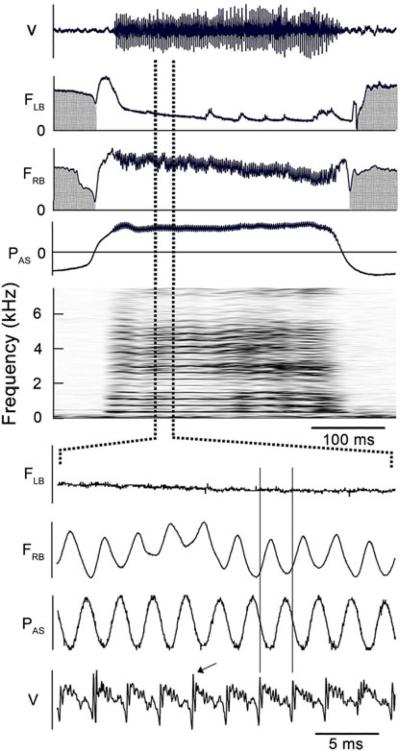
Fig. 10.
The pulse tone of this begging call is generated by the opening and closing of the labial valve in the right side of the syrinx. Labial adduction increases syringeal resistance causing the rate of syringeal airflow to decrease and the driving expiratory pressure to increase, so that air flow and pressure are 180° out of phase. The airflow trace does not reach zero due to the time constant of the thermistor. A biphasic sound pulse (arrow) occurs during each cycle. The period of the 370 Hz fundamental is equal to the interval between sound pulses. No correction has been made in the Wgure for the delay in the sound recording relative to that of airflow and pressure (see text). The interval between pulses appears to contain damped resonant oscillations. The absence of any oscillation in FLB indicates the left side of the syrinx is not producing sound. See Fig. 1 legend for abbreviations. (Magpie 1, 02–391)
The expiration that produced the call shown in Fig. 10 begins with both sides of the syrinx opening and reducing syringeal resistance to allow a high rate of airflow bilaterally. About 15 or 20 ms after the start of expiration the reduced airflow through the left side, despite a relatively constant subsyringeal pressure, indicates that the left labial valve partially closes, but the sustained low rate of airflow through the left side does not appear to generate sound. The expanded traces in the lower portion of Fig. 10 show that subsyringeal pressure is 180° out of phase with the rate of airflow through the right side of the syrinx. The acoustic waveform, V, in Fig. 10 is delayed relative to the airflow and pressure traces by the time required for the sound to travel from the bird to the microphone which was 0.5–1 m from the bird. This delay was probably about 2 ms, but since the exact distance is not known it is not possible to determine the precise time during the pressure and airflow cycles when the pulse is generated.
Discussion
The experiments we report in this article provide a first look at the peripheral vocal mechanisms which underlie the exceptional vocal versatility of the Australian magpie. Already in young males during the late stages of song development, our data show impressive flexibility in the use of independent sound sources in the left and right sides of the syrinx and in the bird's ability to achieve precise motor coordination between sides in order to produce very rapid amplitude modulation or to operate its vocal organ in a pulse tone register. Although the repertoires of older magpies include more stereotyped syllables than were evident in the males we studied, the vocal motor plasticity of these males emphasizes the varied motor mechanisms, which contribute to the acoustic diversity of magpie vocalizations.
Comparison with adult song
Magpies fledge when they are about 1 month old. Juvenile song begins well before fledging. Juvenile song shortly after fledging is highly variable with a “squeaky and jumbled” quality. The time spent singing continues to increase, resulting in more vocalizations and song of increasing complexity (Brown and Veltman 1987). Much of this vocal development occurs during the first 4–6 months post-fledging (Kaplan 2004a) although fully adult-like renditions of song elements may not appear until about 15 months of age (Brown and Veltman 1987). Even as adults, magpies continue to combine song elements in new ways.
The song we recorded from our 6-month-old birds was generally adult-like in its frequency range and sound level. Many of the syllables were similar to adult song but lacked the stereotypy that may be present in adult song. It is interesting that many song syllables of our birds did not fit easily into either of the two major types of adult vocalization: warble song and caroling. Both warble song and caroling have fundamental frequencies below about 2 kHz, but adult warble song is low-intensity musical song consisting of a series of rapidly produced syllables with a duration that is usually less than 200 ms. Carols on the other hand are about 400–650 ms long with a high-intensity and multiple overtones that may extend to about 8 kHz (Brown et al. 1988). Many of the syllables from our birds, that were similar to warble song in lacking the strong overtones, were longer than typical adult warble syllables, suggesting that syllable length in adult warble song emerges relatively late in song development, perhaps by segmentation of complex, long-duration juvenile vocalizations. Some of the diversity in vocal motor gestures, which we observed in our young birds, likely reflects an increased vocal plasticity compared to adults. As song becomes more stereotyped some motor correlates of juvenile song, such as airflow through the silent side of the syrinx, may no longer be used by adults. Such changes may reflect maturation of song control nuclei, such as HVC and RA, which, although near adult-like in size in juvenile female magpies, may be smaller in males of similar age (Deng et al. 2001).
Song lateralization
Both of our magpies demonstrated bilaterally independent control of both the timing of phonation and the frequency composition of sounds generated on each side of their syrinx. The majority of syllables from each bird included contributions from both sides of the syrinx. Sometimes both sides produced the same f0. At other times the frequency from each side was different, resulting in a two-voice vocalization. Unilaterally generated syllables were more commonly produced on the right side than on the left, but neither side strongly dominated song production. Patterns of song lateralization in our young magpies were more similar to those in the brown thrasher (Toxostoma rufum) and grey catbird (Dumatella carolinensis) (both family Mimidae) (Suthers et al. 1994, 1996) than to the motor patterns of other species that have been studied.
It is interesting that our magpies sometimes sang syllables unilaterally while maintaining bilateral airflow through the syrinx. This motor pattern is rare in the other songbirds we have studied, which nearly always silence the contralateral side of their syrinx during unilateral phonation by fully adducting the labia to prevent airflow through that side (Suthers and Zollinger 2008). A presumed advantage of this unilateral closure is that it avoids `wasting' a portion of the expiratory vital capacity that would otherwise be available for phonation (Hartley and Suthers 1989; Suthers and Goller 1997). Although magpies also often closed the non-phonating side of their syrinx, it was not uncommon for them to leave it open. In these cases the labia are not fully abducted out of the expiratory air stream. The presence of a relatively low rate of airflow through the silent side—compared to the phonating side, which is exposed to a similar subsyringeal pressure—indicates that the labia on the silent side maintain a relatively high syringeal resistance by remaining partially adducted into the expiratory airflow (e.g., Fig. 1, FLB). Since the two sides of the syrinx are in parallel, this high resistance is necessary to maintain adequate airflow for phonation through the opposite side of the syrinx. Under these conditions it is not clear how the bird keeps the labia on the silent side from vibrating and producing sound. Presumably motor commands to the syringeal muscles on the silent side prevent that side of the syrinx from assuming a phonatory configuration (Goller and Suthers 1996).
A syringeal motor pattern allowing bilateral airflow during unilateral sound production may be advantageous for some kinds of vocalizations by large birds if their expiratory volume exceeds that needed to produce the syllable. Otherwise the unexpelled air would have to either be forcibly exhaled between syllables by increased activity of the expiratory muscles, increasing the energetic cost of song production, or the silent interval between syllables would have to be increased in order to accommodate a longer exhalation, thereby decreasing song tempo.
The left and right sides of the magpie syrinx are specialized for partially different ranges of f0, as they are in most other songbirds studied. However, the Australian magpie's lateralization of high frequencies to the left side of its syrinx is the opposite of that found in most other songbirds studied, in which high fundamental frequencies are produced on the right side of the syrinx. In other species of songbirds for which data are available, the two sides of the syrinx differ in the range of fundamental frequencies they can produce (Suthers 1997, 1999). Although there is considerable overlap in mid-range frequencies, the left and right sides of the syrinx are specialized to produce frequencies at the low and high ends, respectively, of the vocal range. This lateral specialization for different frequency ranges presumably increases the range of frequencies that the bird can sing.
Pulse register vocalizations
In humans, the pulse, or vocal fry, register is characterized by low fundamental frequency, below about 100 Hz, with a crackly voice quality. The rapid closing/opening of the glottis produces short open pulses followed by a much longer period, which may occupy up to 90% of the cycle, when the glottis is closed. The vibrating medial margin of the vocal fold is flaccid due to reduced tension of the vocalis muscle. Tension in the lateral portion of the vocal fold causes it to shorten and thicken, moving the margin of the vocal folds medially (Whitehead et al. 1984; Blomgren and Chen 1998; Seikel et al. 2005). Pulse phonation has also been recorded from other mammals including felids and primates (e.g., Riede and Zuberbühler 2003).
A pulse tone register was first described in songbirds by Jensen et al. (2007) who used an angioscope to obtain high-speed films of syringeal dynamics in anesthetized hooded crows when air was forced through the syrinx in an expiratory direction. Angioscopic images of the labia during forced airflow in anesthetized crows showed the labia opened during the acoustic pulse for about half (1.5 ms) of each cycle. Jensen et al. also identified pulse register-like acoustic features in some of the spontaneous vocalizations of unanesthetized hooded crows (Corvus corone cornix), starlings (Sturnus vulgaris) and zebra finches. The presence of expiratory airflow during the interval between sound pulses in the natural vocalizations of these birds, and of our magpies, suggests that closure of the syringeal valve may be very brief. If so, the labial dynamics of the natural vocalizations of these songbirds differ in this respect from those of anesthetized crows and from the laryngeal dynamics of human speech, in which the labial or laryngeal valve is closed during most of each cycle. Unfortunately, the waveform of the flow signal may not accurately follow the fine temporal dynamics of the labial valve within each cycle, making it impossible to determine the temporal pattern of the gating mechanism without additional data.
Jensen et al. (2007) suggest that the pulse tone register may enable small birds to produce lower f0s than would otherwise be possible considering their small size. This may not be a factor in large birds such as the Australian magpie, however, which can produce an f0 in the modal register that is as low as that produced in the pulse tone register. The presence of pulse tones in some of the begging calls of our magpies may provide a means of increasing the acoustic complexity of the vocal repertoire or generating a vocalization with distinctive acoustic properties in order to attract parental attention. It is not known if pulse tones are present in vocalizations of older adults.
Rapid amplitude modulation
A striking feature of many magpie vocalizations is the presence of prominent amplitude modulation at high modulation rates exceeding 100 Hz. Some of this AM is a beat signal produced by the linear addition of relatively high-intensity sounds emanating from each side of the syrinx at slightly different frequencies. Other AM appears to result from the nonlinear modulation of a carrier frequency by a much lower modulating frequency. The source of the low modulating frequency is unclear. Jensen et al. (2007) found that songbirds can produce low-frequency sounds by changing their syringeal dynamics on one or both sides of the syrinx from a modal to a pulse-tone register. In magpie vocalization in Fig. 9 it is possible that the modulating frequency, m0, is generated on one side of the syrinx and the carrier frequency, f0, is generated on the opposite side but spectrogram of the bronchial sound indicate both sides are producing the same f0 at about 900 Hz. An alternative to either mechanical or acoustic source-source coupling between sides of the syrinx is source-filter coupling in which the labia are coupled to the vocal tract's resonance filter (Nowicki and Capranica 1986; Laje and Mindlin 2005; Laje et al. 2008; Zollinger et al. 2008). The northern cardinal (Cardinalis cardinalis) can tune its primary vocal tract resonance to frequencies below 2 kHz by expanding its oropharyngeal-esophageal cavity (Fletcher et al. 2006; Riede et al. 2006) so it is reasonable to assume that the much larger magpie can achieve a vocal tract resonance around 900 Hz. Further information and analysis is needed to fully understand the mechanisms responsible for these vocalizations, but their complexity underlines the important contribution a duplex syrinx with two independent sound sources makes to the acoustic diversity of these songbirds.
Presence of nonlinear phenomena
The past decade has witnessed an increased awareness of and interest in nonlinear acoustic phenomena such as sub-harmonics, chaos, biphonation and frequency jumps as normal, potentially information bearing, components of vocal communication (e.g., Wilden et al. 1998; Fitch et al. 2002). They arise from the intrinsic nonlinear dynamics of coupled oscillators, which in birds are the syringeal labia or membranes. Nonlinear phenomena are present in the vocalizations of many vertebrates from frogs to primates, including humans. It is not clear to what extent they are “unintended” by-products of sound production or whether they have in some cases evolved as an “inexpensive” way to increase vocal diversity or complexity that does not require elaborate neuromotor control mechanisms. Fee et al. (1998) described the nonlinear dynamics in the excised zebra finch larynx and nonlinear phenomena have been found in the natural vocalizations of non-passerines such as parrots (Fletcher 2000) and doves (Beckers and ten Cate 2006). Identification of some kinds of nonlinear phenomena in songbirds is complicated by the presence of an independent sound source on each side of the syrinx since a frequency jump or apparent subharmonic might be produced by generating different frequencies on each side of the syrinx rather than by a bifurcation in the oscillatory dynamics of a single sound source. Zollinger et al. (2008) distinguished between these two alternatives by monitoring airflow and bronchial sound on each side of the syrinx in a vocal mimic, the northern mockingbird. They found that 8.5% of 1,000 syllables from each of four mockingbirds contained one or more of these nonlinear phenomena; the most common being chaos and biphonation. Although their presence increases acoustic complexity, it remains to be determined what role, if any, nonlinear phenomena have in vocal communication by mockingbirds.
In juvenile Australian magpies the most striking examples of nonlinear phenomena are the chaotic begging calls (Fig. 5). These are the only vocalizations we recorded that usually consist of almost continuous broad-band, high-intensity chaos. We speculate that the distinctive acoustic properties of these calls may be advantageous in attracting the parents' attention. The broad frequency spectrum of the calls is also well suited to convey their formant structure. Since formant frequencies are determined by the vocal tract resonances, which in turn depend on the detailed shape and dimensions of the vocal tract, they are potentially well designed to convey acoustic cues for individual recognition (Williams et al. 1989; Suthers 1994; Fitch and Kelley 2000). Such cues might be used by a parent magpie to recognize its own young when juvenile song is still highly variable.
Acknowledgments
We thank Drs. Gabriel Beckers, Kenneth K. Jensen and Gabriel Mindlin for their constructive comments on this manuscript and Amy Coy for her assistance in data analysis and preparation of the Wgures. All experimental procedures were approved by the University of New England Animal Ethics Committee. Supported by a grant to R.A.S from the US National Institutes of Health.
Abbreviations
- AM
Amplitude modulation
- CF
Constant frequency
- FLB
Rate of airflow through left bronchus
- FRB
Rate of airflow through right bronchus
- FM
Frequency modulation
- f0
Fundamental frequency
- LMAN
Lateral nucleus of the anterior nidopallium
- MMAN
Medial nucleus of the anterior nidopallium
- m0
Second fundamental frequency or modulating frequency
- PAS
Pressure in the thoracic air sac
- RA
Robust nucleus of the arcopallium
- SAS
Sound in thoracic air sac
- SLB
Sound in left bronchus
- SRB
Sound in right bronchus
- V
Time domain microphone recording of vocalizations
References
- Beckers GJL, ten Cate C. Nonlinear phenomena and song evolution in Streptopelia doves. Acta Zool Sin. 2006;52(Suppl):482–485. [Google Scholar]
- Beckers GJL, Suthers RA, ten Cate C. Pure-tone birdsong by resonance Wltering of harmonic overtones. Proc Nat Acad Sci USA. 2003;100:7372–7376. doi: 10.1073/pnas.1232227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren M, Chen Y. Acoustic, aerodynamic, physiologic and perceptual properties of modal and vocal fry registers. J Acoust Soc Am. 1998;103:2649–2658. doi: 10.1121/1.422785. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- Brown ED, Farabaugh SM. Song sharing in a group-living song-bird, the Australian magpie, Gymnorhina tibicen. Part III. Sex specificity and individual specificity of vocal parts in communal chorus and duet songs. Behav. 1991;118:244–274. [Google Scholar]
- Brown ED, Veltman CJ. Ethogram of the Australian magpie (Gymnorhina tibicen) in comparison to other Cracticidae and Corvus species. Ethology. 1987;76:309–333. [Google Scholar]
- Brown ED, Farabaugh SM, Veltman CJ. Song sharing in a group-living songbird, the Australian magpie, Gymnorhina tibicen Part I. Vocal sharing within and among social groups. Behaviour. 1988;104:1–28. [Google Scholar]
- Calder WA. Respiration during song in the canary (Serinus canaria) Comp Biochem Physiol. 1970;32:251–258. doi: 10.1016/0010-406x(70)90938-2. [DOI] [PubMed] [Google Scholar]
- Christidis L, Boles WE. Systematics and taxonomy of Australian birds. CSIRO Publishing; Canberra: 2008. [Google Scholar]
- Deng C, Kaplan G, Rogers LJ. Similarity of the song nuclei of male and female Australian magpies (Gymnorhina tibicen) Behav Brain Res. 2001;123:89–102. doi: 10.1016/s0166-4328(01)00200-5. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Ann Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Brown ED, Veltman CJ. Song sharing in a group-living songbird, the Australian magpie, Gymnorhina tibicen Part II. Vocal sharing between territorial neighbors, within and between geographic regions, and between sexes. Behaviour. 1988;104:105–125. [Google Scholar]
- Farabaugh SM, Brown ED, Hughes JM. Cooperative territorial defence in the Australian magpie, Gynmorhina tibicen (Passeriformes, Cractididae), a group-living songbird. Ethology. 1992;92:283–292. [Google Scholar]
- Fee MS, Shraiman B, Pesaran B, Mitra PP. The role of nonlinear dynamics of the syrinx in the vocalizations of a songbird. Nature. 1998;395:67–71. doi: 10.1038/25725. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Kelley JP. Perception of vocal tract resonances by whooping cranes Grus americana. Ethology. 2000;106:559–574. [Google Scholar]
- Fitch WT, Neubauer J, Herzel H. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim Behav. 2002;63:407–418. [Google Scholar]
- Fletcher NH. A class of chaotic bird calls? J Acoust Soc Am. 2000;108:821–826. doi: 10.1121/1.429615. [DOI] [PubMed] [Google Scholar]
- Fletcher NH, Riede T, Suthers RA. Model for vocalization by a bird with distensible vocal cavity and open beak. J Acoust Soc Am. 2006;119:1005–1011. doi: 10.1121/1.2159434. [DOI] [PubMed] [Google Scholar]
- Goller F, Larsen ON. A new mechanism of sound generation in songbirds. Proc Nat Acad Sci USA. 1997;94:14787–14791. doi: 10.1073/pnas.94.26.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in gating air-flow and sound production in singing brown thrashers. J Neurophysiol. 1996;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Bilaterally symmetrical respiratory activity during lateralized birdsong. J Neurobiol. 1999;41:513–523. doi: 10.1002/(sici)1097-4695(199912)41:4<513::aid-neu7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hartley RS. Expiratory muscle activity during song production in the canary. Respir Physiol. 1990;81:177–187. doi: 10.1016/0034-5687(90)90044-y. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Suthers RA. Airflow and pressure during canary song: evidence for mini-breaths. J Comp Physiol A. 1989;165:15–26. [Google Scholar]
- Hollien H. On vocal registers. J Phon. 1974;2:125–143. [Google Scholar]
- Jensen KK, Cooper BG, Larsen ON, Goller F. Songbirds use pulse tone register in two voices to generate low-frequency sound. Proc R Soc B Biol Sci. 2007;274:2703–2710. doi: 10.1098/rspb.2007.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. Song structure and function of mimicry in the Australian magpie (Gymnorhina tibicen) compared to the lyrebird (Menura ssp.) Int J Comp Psychol. 2000;12:219–241. [Google Scholar]
- Kaplan G. Australian magpie: biology and behaviour of an unusual songbird. Univ New South Wales Press/CSIRO; Sydney: 2004a. [Google Scholar]
- Kaplan G. Magpie mimicry. In: BekoV M, Goodall J, editors. Encyclopedia of animal behaviour. Greenwood Publishing; Westport, CT: 2004b. pp. 772–774. [Google Scholar]
- Kaplan G. School of Veterinary Sciences. University of Queensland; St Lucia, Brisbane: 2005. The vocal behaviour of the Australian magpies (Gymnorhina tibicen): a study of vocal development, song learning communication and mimicry in the Australian magpie. [Google Scholar]
- Kaplan G. Australian magpie (Gymnorhina tibicen). Voice. In: Higgins P, Peter JM, Cowling SJ, editors. The handbook of Australian, New Zealand and Antarctic birds. vol 7. Oxford University Press; Melbourne: 2006. pp. 605–608.pp. 613–616. Pt A. [Google Scholar]
- Kaplan G. Alarm calls and referentiality in Australian magpies: between midbrain and forebrain, can a case be made for complex cognition? Brain Res Bull. 2008a;76:253–263. doi: 10.1016/j.brainresbull.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Kaplan G. The Australian magpie (Gymnorhina tibicen): an alternative model for the study of songbird neurobiology. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge, UK: 2008b. pp. 50–57. [Google Scholar]
- Kaplan G, Johnson G, KoboroV A, Rogers LJ. Alarm calls of the Australian magpie (Gymnorhina tibicen): I. Predators elicit complex vocal responses and mobbing behaviour. Open Ornithol J. 2009;2:7–16. [Google Scholar]
- Laje R, Mindlin GB. Modeling source-source and source-Wlter acoustic interaction in birdsong. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72:036218–036211. doi: 10.1103/PhysRevE.72.036218. [DOI] [PubMed] [Google Scholar]
- Laje R, Sciamarella D, Zanella J, Mindlin GB. Bilateral source acoustic interaction in a syrinx model of an oscine bird. Phys Rev E Stat Nonlinear Soft Matter Phys. 2008;77:011912. doi: 10.1103/PhysRevE.77.011912. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes T, Leonard CM. Central control of song in the canary, Serinus canarius. Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Capranica RR. Bilateral syringeal coupling during phonation of a songbird. J Neurosci. 1986;6:3595–3610. doi: 10.1523/JNEUROSCI.06-12-03595.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea K, Kaplan G. Warbles and vigilance: neighbour/stranger discrimination by territorial Australian magpies (Gymnorhina tibicen) J Avian Biol. 2010 submitted. [Google Scholar]
- Riede T, Zuberbühler K. Pulse register phonation in Diana monkey calls. J Acoust Soc Am. 2003;113:2919–2926. doi: 10.1121/1.1567278. [DOI] [PubMed] [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins W. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc Nat Acad Sci USA. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. The annual reproductory cycle of the magpie, Gymnorhina dorsalis, Campbell, in south-western Australia. Emu. 1956;56:232–336. [Google Scholar]
- Seikel JA, King DW, Drumright DG. Anatomy and physiology for speech language and hearing. Thomson Delmar Learning; Clifton Park, NY: 2005. [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA. Variable asymmetry and resonance in the avian vocal tract: a structural basis for individually distinct vocalizations. J Comp Physiol A. 1994;175:457–466. doi: 10.1007/BF00199253. [DOI] [PubMed] [Google Scholar]
- Suthers RA. Peripheral control and lateralization of birdsong. J Neurobiol. 1997;33:632–652. [PubMed] [Google Scholar]
- Suthers RA. The motor basis of vocal performance in songbirds. In: Hauser M, Konishi M, editors. The design of animal communication. MIT Press; Cambridge, MA: 1999. pp. 37–62. [Google Scholar]
- Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. In: Nolan V Jr, Ketterson E, Thompson CF, editors. Curr Ornithol. vol 14. Plenum Press; New York: 1997. pp. 235–288. [Google Scholar]
- Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge: 2008. pp. 78–98. [Google Scholar]
- Suthers RA, Goller F, Hartley RS. Motor dynamics of song production by mimic thrushes. J Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Goller F, Hartley RS. Motor stereotypy and diversity in songs of mimic thrushes. J Neurobiol. 1996;30:231–245. doi: 10.1002/(SICI)1097-4695(199606)30:2<231::AID-NEU5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of voice production. Prentice Hall; Englewood Cliffs, NJ: 1994. [Google Scholar]
- Veltman CJ. Flock, pair and group-living lifestyles without cooperative breeding by Australian magpies, Gymnorhina tibicen. Ibis. 1989;131:6016–6018. [Google Scholar]
- Waite ER. Sympathetic song in birds. Nature. 1903;68 [Google Scholar]
- Whitehead RL, Metz DE, Whitehead BH. Vibratory patterns of the vocal folds during pulse register phonation. J Acoust Soc Am. 1984;75:1293–1297. doi: 10.1121/1.390737. [DOI] [PubMed] [Google Scholar]
- Wild JM, Goller F, Suthers RA. Inspiratory muscle activity durig birdsong. J Neurobiol. 1998;36:441–453. doi: 10.1002/(sici)1097-4695(19980905)36:3<441::aid-neu11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Neural pathways for bilateral vocal control in songbirds. J Comp Neurol. 2000;423:413–426. doi: 10.1002/1096-9861(20000731)423:3<413::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wilden I, Herzel H, Peters G, Tembrock G. Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics. 1998;8:1–30. [Google Scholar]
- Williams H, Cynx J, Nottebohm F. Timbre control in zebra finch (Taeniopygia guttata) song syllables. J Comp Psychol. 1989;103:366–380. doi: 10.1037/0735-7036.103.4.366. [DOI] [PubMed] [Google Scholar]
- Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- Zollinger SA, Suthers RA. Motor mechanisms of a vocal mimic: implications for birdsong production. Proc R Soc Lond B. 2004;271:483–491. doi: 10.1098/rspb.2003.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger SA, Riede T, Suthers RA. Two-voice complexity from a single side of the syrinx in northern mockingbird Mimus polyglottos vocalizations. J Exp Biol. 2008;211:1978–1991. doi: 10.1242/jeb.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]