Abstract
The objective of this study was to determine the potential of Bdellovibrio bacteriovorus 109J as an alternative non-chemotherapeutic treatment of infectious bovine keratoconjunctivitis (IBK). To accomplish this, various parameters of B. bacteriovorus predation of Moraxella bovis were determined in vitro. Initial passage of B. bacteriovorus using M. bovis as prey required 10 d for active cultures to develop compared with 2 d for culture on normal Escherichia coli prey; however by the 5th passage, time to active predatory morphology was reduced to 2 d. This high passage B. bacteriovorus culture [1 × 1010 plaque forming units (PFU)/mL] killed 76% of M. bovis [1 × 107 colony forming units (CFU)/mL] present in suspension broth in a 4 h assay. The minimal level of M. bovis supporting B. bacteriovorus predation was 1 × 104 CFU/mL. To assess the ability of B. bacteriovorus to kill M. bovis on an epithelial surface mimicking IBK, an in vitro assay with Madin-Darby bovine kidney (MDBK) cells inoculated with 4 × 107 CFU/mL M. bovis was used. Treatment with a B. bacteriovorus suspension (1.6 × 1011 PFU/mL) decreased adherence of M. bovis to MDBK cells by 6-fold at 12 h of treatment, as well as decreased the number of unattached M. bovis cells by 1.4-fold. This study demonstrates that B. bacteriovorus has potential as an effective biological control of M. bovis at levels likely present in IBK-infected corneal epithelia and ocular secretions.
Résumé
Cette étude visait à déterminer le potentiel de Bdellovibrio bacteriovorus 109J comme traitement alternatif non-thérapeutique de la kérato-conjonctivite infectieuse bovine (IBK). À cet effet, divers paramètres de prédation de B. bacteriovorus envers Moraxella bovis ont été déterminés in vitro. Le premier passage de B. bacteriovorus utilisant M. bovis comme proie nécessitait 10 j pour qu’une culture active se développe comparativement à 2 j pour une culture utilisant Escherichia coli comme proie; toutefois, rendu au 5e passage, le temps requis pour obtenir la morphologie de prédateur actif était réduit à 2 j. Cette culture de passage élevé de B. bacteriovorus (1 × 1010 unités formatrices de plaques (PFU)/mL) a tué 76 % des M. bovis (1 × 107 unités formatrices de colonies (CFU)/mL) présents dans un bouillon lors d’un essai d’une durée de 4 h. Le nombre minimal de M. bovis permettant la prédation par B. bacteriovorus était de 1 × 104 CFU/mL. Afin d’évaluer la capacité de B. bacteriovorus à tuer M. bovis sur une surface épithéliale imitant IBK, une épreuve in vitro avec des cellules rénales bovines Madin-Darby (MDBK) inoculées avec 4 × 107 CFU/mL M. bovis fut utilisée. Le traitement avec une suspension de B. bacteriovorus (1,6 × 1011 PFU/mL) a réduit l’adhérence de M. bovis aux cellules MDBK par un facteur de 6 après 12 h de traitement, et a également diminué le nombre de cellules de M. bovis non-attachées par un facteur de 1,4. Cette étude démontre que B. bacteriovorus a le potentiel d’être un moyen de réduction biologique efficace de M. bovis à des niveaux susceptibles d’être présents sur l’épithélium cornéen et dans les sécrétions oculaires d’animaux infectés par l’IBK.
(Traduit par Docteur Serge Messier)
Introduction
Infectious bovine keratoconjunctivitis (IBK) is a widespread, severe, contagious eye disease of bovine species that causes significant economic loss worldwide (1–3). Ocular infection of cattle with Moraxella bovis (M. bovis) is associated with the development of IBK, a significant treatment problem which may be amenable to a novel non-chemotherapeutic treatment therapy by a predatory bacterium Bdellovibrio bacteriovorus 109J (B. bacteriovorus). The clinical manifestations of IBK range from mild unilateral or bilateral conjunctivitis to central corneal ulceration and perforation (4). Bacterial culture collected from ocular secretions of calves with corneal ulcer due to M. bovis has been reported to harbor 1 × 109 to 1 × 1010 M. bovis per sample (5). Prerequisites for induction of ocular lesions by M. bovis include microbial adhesion to the corneal surface and cytotoxicity, both mediated by several virulence factors. Moraxella bovis fimbriae (Q pili) allow for bacterial adherence to the bovine conjunctival mucosa (6). Cytopathic effect of M. bovis on bovine corneal epithelial cells (BCEC), neutrophils, and erythrocytes is mediated by a cytotoxic and leukotoxic hemolysin, hydrolytic and lipolytic enzymes, proteases, and collagenases (4,7–9). However, this process can be reversible as the corneal epithelium may regenerate once ocular M. bovis infection is cleared (10). Parenteral administration of oxytetracycline (10,11), florfenicol (12,13), ceftiofur crystalline free acid (14), and tulathromycin (15) have been shown effective in the treatment of experimentally induced or naturally occurring IBK in cattle. However, the predatory bacterium B. bacteriovorus administered via ocular instillation may provide an attractive alternative to antimicrobials, especially in case of multidrug-resistant IBK infection. Its use would prevent tissue and milk residues, decrease the opportunity for development of bacterial resistance, and avoid local and systemic side effects associated with antibiotic administration.
The genus Bdellovibrio was first described by Stolp and Starr in 1963 as bacteriolytic organisms capable of attacking a living bacterium, attaching to its surface, penetrating the cell wall, multiplying inside the host, and causing lysis of the infested cell (16) within approximately 3.5 to 4 h (17). More recent reviews describe the predatory lifestyle of B. bacteriovorus characterized by 2 differentiated cell stages (attack and growth phase) (18–21), as well as methods for its laboratory maintenance (22). Bdellovibrio bacteriovorus is a small (0.35 × 1.2 μm), obligate aerobe, motile (polar flagellum) gram-negative bacterium with obligate host-dependency on a wide range of gram-negative prey bacteria. Bdellovibrio bacteriovorus are ubiquitous and have been isolated from terrestrial and aquatic environments including soils, rice paddies, rhizosphere of plants, rivers, sewage, fish ponds, and irrigation water (16,23–25). Despite the use of Escherichia coli (E. coli) as the model prey bacterium in the majority of in vitro experiments published (17), B. bacteriovorus is selectively active against most Pseudomonas spp. and enterobacteria (16–17). Although variable between prey cells, a minimum prey density is required to sustain B. bacteriovorus life cycle. In 2 different studies, a prey concentration of approximately 1.5 × 105 E. coli per mL (26) and 3.0 × 106 Photobacterium leignathi per mL (27), was required for 50% survival of B. bacteriovorus. Optimal growth of B. bacteriovorus is seen at 30°C (range: 20°C to 45°C) and pH of 6.8 to 7.2 (range: 5.6 to 8.6) (27); values observed for the temperature of the corneal surface in horses (28) and pH of tears (29) in cattle, respectively. Bdellovibrio bacteriovorus is unlikely to cause mammalian cell toxicity because of its outer membrane characteristics and failure to grow in eukaryotic cells in vitro (23).
Reports of the use of B. bacteriovorus as a biological control or therapeutic agent are limited. Fratamico (30) demonstrated the potential use of B. bacteriovorus as biological control for pathogenic and spoilage organisms in food. Kadouri (31) demonstrated that B. bacteriovorus could attack and reduce an existing E. coli biofilm in as little as 30 min of exposure. Nakaruma (32) successfully treated Shigella flexneri-induced keratoconjunctivitis (KC) in rabbits with B. bacteriovorus. In a similar in vivo model, B. bacteriovorus effectively treated experimentally induced Pseudomonas aeruginosa KC (John J. Iandolo, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA; personal communication, November 2007).
To our knowledge, in vitro or in vivo research on the use of B. bacteriovorus on pathogenic isolates of M. bovis have not been conducted. In order to assess the potential for B. bacteriovorus for treatment of IBK, we used an in vitro IBK tissue culture model previously developed by Annuar and Wilcox (33). In vitro experiments investigating the adherence of M. bovis to cultured BCEC (8,9,33–35) and Madin-Darby bovine kidney (MDBK) cells (33), as well as associated cytopathic effects induced, have been previously published.
On the basis of preliminary experiments which confirmed that B. bacteriovorus grows on M. bovis isolates in vitro, its successful use as treatment for gram-negative bacterial KC in vivo and its reported non-toxic effect on mammalian cells, we propose that B. bacteriovorus administered by ocular instillation may represent an effective non-chemotherapeutic alternative treatment for IBK. The purpose of the study reported here was to determine the potential of B. bacteriovorus in the treatment of IBK associated with M. bovis in vitro. The first objective was to determine whether B. bacteriovorus can be trained to kill M. bovis as effectively as its normal prey E. coli. The second objective was to estimate the efficiency of low and high passage B. bacteriovorus predation on M. bovis in suspension broth culture and to establish the minimum prey concentration needed to sustain the B. bacteriovorus life cycle. The third objective was to determine the predatory effect of B. bacteriovorus on adherence of M. bovis to MDBK cells.
Materials and methods
Bacterial strains
Non-hemolytic M. bovis strain M− was kindly provided by J. Angelos (University of California at Davis, School of Veterinary Medicine, Davis, California, USA) and B. bacteriovorus strain 109J and E. coli strain 012207 by J. Iandolo (University of Oklahoma, Health Sciences Center, Oklahoma City, Oklahoma, USA).
Routine cultivation of B. bacteriovorus
Cultivation of B. bacteriovorus on E. coli was modified from that described by Ruby (21). Escherichia coli was grown overnight as lawns on 5% brain heart infusion agar with 5% sheep blood (BAP) at 37°C. Bacteria were swabbed from the surface of the BAP and used to inoculate 45 mL of dilute nutrient broth (0.16% nutrient broth, 0.02% yeast extract, and 0.1% casitone supplemented with 2 mM CaCl2 and 3 mM MgCl2) to an absorbance reading of 0.4 to 0.6 at 600 nm. The cells were immediately harvested by centrifugation (1800 × g for 5 min), the supernatant was discarded and the pellet was resuspended in 35 mL of peptone yeast extract (PYE) (1% Bacto peptone and 0.3% yeast extract supplemented with 2 mM CaCl2 and 3 mM MgCl2). The bacterial suspension was centrifuged as described, and resuspended in 5 mL of PYE to a final concentration of approximately 1 × 109 E. coli/mL. A 75 cm2 flask (Cell star tissue culture flask; Greiner Bio-One, Frickenhansen, Germany) containing 20 mL of PYE was inoculated with 5 mL of E. coli and 250 μL of an active 7 to 10 d old stock culture of B. bacteriovorus [Multiplicity of Infection (MOI) = 1; ratio 1 E. coli: 1 B. bacteriovorus], and incubated with shaking (180 rpm) at 30°C. After approximately 48 h of incubation, the solution was examined microscopically (Olympus BX41 laboratory microscope; Hitschfel Instruments, St-Louis, Missouri, USA) and considered active and ready to use when it contained motile, active attack-phase B. bacteriovorus (1 × 109 plaque forming units (PFU)/mL) with no visible E. coli or bdelloplasts (infected E. coli cells). Once active, B. bacteriovorus culture was stored at 4°C for a maximum period of 1 mo.
Cultivation of B. bacteriovorus using M. bovis as prey
Moraxella bovis prey cells were prepared exactly as the E. coli inoculum. Motile and active B. bacteriovorus previously grown on E. coli were harvested as described by Rogosky (17). The E. coli cell debris was removed by centrifugation (1500 × g for 5 min). The B. bacteriovorus-rich supernatant was saved and the cells were centrifuged (8500 × g for 20 min), washed in PYE, centrifuged again (8500 × g for 20 min), and resuspended in PYE. The washed cells were filtered 3 times sequentially through 0.8 μm, then 0.45 μm, and finally 0.45 μm membranes (Acrodisc syringe filters; Pall Corporation, Ann Arbor, Michigan, USA) pre-wetted with PYE to remove residual E. coli cell debris. A 75 cm2 flask containing 15 mL of PYE was inoculated with 5 mL of M. bovis and 5 mL of triple filtered B. bacteriovorus (MOI = 0.2; ratio 1 M. bovis: 5 B. bdellovibrio), and incubated with shaking (180 rpm) at 30°C for a period of 5 to 7 d or until active. Once grown on M. bovis, subsequent subcultures were done using the same protocol, without filtration.
Preparation of M. bovis inoculum
Lawns of M. bovis were grown for 24 h at 37°C on BAP. Cells were harvested by centrifugation (13 000 × g for 15 min), washed then diluted in Hank’s balanced salt solution containing phenol red and supplemented with 25 μg/mL of glucose (HBSS + G) to an absorbance reading of 0.14 at 600 nm, for a final bacterial concentration of 4 × 107 M. bovis/mL.
Preparation of B. bacteriovorus inoculum
Ten milliliters of active and motile B. bacteriovorus previously grown on M. bovis was centrifuged (1500 × g for 5 min) to remove cell debris. The B. bacteriovorus-rich supernatant was saved and the cells were centrifuged (8500 × g for 20 min) and resuspended in PYE. The washed B. bacteriovorus cells were filtered twice (0.8 μm, 0.45 μm) to remove residual M. bovis cell debris. This suspension was cultured on BAP and was shown to be free of M. bovis. However, this filtration process also decreased the number of B. bacteriovorus present by 30% or greater. Therefore, the number of active B. bacteriovorus in the filtered bacterial suspension was estimated under light microscopy (100×).
Enumeration of B. bacteriovorus using plaque assays
A modification of the double–layer plaque assay technique used for counting bacteriophage described by Varon and Shilo (36) was used for enumeration of B. bacteriovorus. A 200 μL sample of B. bacteriovorus dilutions 10−5, 10−6, 10−7, 10−8, and 10−9 and 200 μL of the E. coli suspension (as prepared in routine cultivation of B. bacteriovorus) were mixed in 3 mL of liquefied overlay agar (PYE medium containing 0.6% agar) kept on a hot plate at 45°C. The mixtures were inverted 6 or 7 times to allow proper mixing and immediately spread over the surface of PYE medium containing 1.5% agar in 92 × 16 mm in Petri dishes. Plates were incubated upright at 30°C for 3 to 7 d until clear circular plaques appeared in the lawn of prey cells.
Efficiency of killing assay
Bdellovibrio bacteriovorus and M. bovis inocula were prepared as described except that prey cells were resuspended in HBSS to a final M. bovis concentration of approximately 1 × 108/mL. The number of live B. bacteriovorus present in the inoculum was determined via plaque assay technique. Five hundred microliters of the original M. bovis inoculum was serially diluted in 4.5 mL of HBSS (10−1 to 10−4). In group A (control group), 50 μL of PYE was added to triplicate tubes of 250 μL of each M. bovis dilutions (1 × 104/mL to 1 × 108/mL). The same method was used for group B (treatment group), except that PYE was replaced by 50 μL of B. bacteriovorus inoculum (1 × 1010 PFU/mL), resulting in a final 1:1 predator to prey ratio. Serial dilution tubes were incubated at 35°C for 4 h with shaking (180 rpm). To determine the M. bovis colony forming units (CFU)/mL at time 0 and 4 h, each M. bovis dilution from the 3 replicates of group A and B were further serially diluted, and serial dilution 10−1, 10−2, 10−3, and 10−4 were plated on BAP and incubated at 37°C for 24 h. The percent of M. bovis killed by B. Bdellovibrio predation was calculated using the formula described previously by Rogosky (17):
where: A0 and A4 are the mean CFU/mL M. bovis in the absence of B. bacteriovorus (group A) at 0 and 4 h, respectively, and B0 and B4 are the mean CFU/mL M. bovis in the presence of B. bacteriovorus (group B) at 0 and 4 h.
Madin-Darby bovine kidney cell culture
Immortalized MDBK cells (50th to 60th passage levels) were kindly provided by R. Fulton (Oklahoma State University Center for Veterinary Health Sciences, Stillwater, Oklahoma). Tissue culture growth media consisted of Dulbecco’s modification of Eagle’s medium with glucose (4.5 g/L) and L-glutamine (DMEM), supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) of penicillin-streptomycin (10 000 IU/mL, 10 000 μg/mL). For the adherence assay, antibiotic-free media was used. Cells were incubated at 37°C, 5% CO2 and 90% to 100% humidity and cell culture media was changed 3 times weekly until cells reached 90% confluency. Cells formed monolayers within 3 to 4 d of incubation, and additional passages of the cells were done using conventional procedures at a split ratio of 1:14.
Cell monolayers on coverslips
Madin-Darby bovine kidney cells were grown as monolayers on coverslips for adherence experiments as described by Moore and Rutter (35). Prior to cell seeding, 13 mm round coverslips (Thermanox cell culture coverslips; Nalge Nunc International, Rochester, New York, USA) were placed at the bottom of each well of four 24-well tissue culture plates coated with type 1 collagen (Greiner Bio-One, Frichenhansen, Germany). The cell culture was maintained coated side up. Ninety percent confluent MDBK monolayers were released from their original tissue culture flask with trypsin-EDTA (0.05%, 0.53 mM EDTA). The detached cells were centrifuged (200 × g for 5 min) and then resuspended in fresh tissue culture growth media. The suspension was adjusted to 2 × 105 viable cells per mL. Four 24-well plates were seeded with 1 × 105 cells per coverslip. Each coverslip was incubated in their respective well with 0.5 mL of cell suspension at 37°C, 5% CO2, and 90% to 100% humidity. The cells were used after 36 to 48 h when the monolayers were 75% to 80% confluent as estimated by light microscopy (Olympus CKX-41 inverted microscope; Hitschfel Instruments, St. Louis, Missouri, USA).
Attachment assay
Attachment of M. bovis to MDBK cells was done as described by Annuar and Wilcox (33), with slight modifications. Bdellovibrio bacteriovorus and M. bovis inocula, as well as MDBK monolayers grown on coverslips, were prepared as described. The media type and amount per well, incubation temperature and time, and the concentration of the bacterial suspension used were determined by preliminary experiments (data not shown) to give optimum MDBK cells viability, preserve M. bovis viability and prevent overgrowth, provide optimum M. bovis attachment to MDBK cells, and mimic what would occur at the level of the ocular surface in case of naturally occurring IBK.
To begin the assay, tissue culture growth media was removed from each well of 24-well plate group 1 (control plate) and group 3 (B. bacteriovorus plate), and replaced by 400 μL of HBSS + G. The same process was repeated for group 2 (M. bovis plate) and group 4 (M. bovis + B. bacteriovorus plate) except that each well received 400 μL of M. bovis inoculum. All 4 plates were incubated at 37°C for 45 min. Following incubation, coverslips were transferred to 4 new corresponding 24-well tissue culture plates using 25 gauge needles and sterile tissue forceps with non-serrated tips leaving the majority of the non-adherent M. bovis inocula in the original wells. The coverslips were not washed to remove residual bacteria inocula because this process also resulted in detachment of variable numbers of MDBK cells. At time zero, 250 μL of HBSS + G and 50 μL of PYE was added to each well of plate 1 and plate 2, and 250 μL of HBSS + G and 50 μL of B. bacteriovorus inoculum was added to each well of plate 3 and plate 4. To determine the CFUs per well at 0, 6, and 12 h, media from 6 randomly selected wells from each treatment group was removed, serially diluted (up to 10−3), and plated on BAP, which were incubated at 37°C for 24 h. The MDBK coverslips from corresponding wells were fixed in methanol and acetone (1:1 ratio) for 2 min and stained with Wright-Giemsa stain with phosphate buffered saline added (3:1 ratio) for 10 min. To quantify the mean number of adherent bacteria per MDBK cell, attached M. bovis were counted on 40 MDBK cells per coverslip. The number of live B. bacteriovorus present in the inoculum in the combined media from 2 randomly selected wells in plate 3 (B. bacteriovorus group) and plate 4 (M. bovis + B. bacteriovorus group) at time 0 and at 12 h, was determined via plaque assay technique.
Results
Efficiency of B. bacteriovorus predation following serial passages on M. bovis
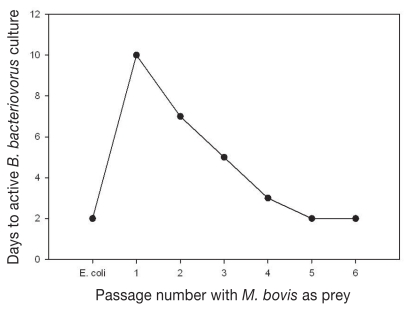
As shown in Figure 1, initial passage of B. bacteriovorus using M. bovis as prey required 10 d for active cultures to develop compared with 2 d for culture on normal E. coli prey; however, by the 5th passage of B. bacteriovorus on M. bovis, time to active predatory morphology was reduced to 2 d.
Figure 1.
Time to active B. bacteriovorus predatory morphology for passages 1 through 6 on M. bovis with comparison with normal prey E. coli.
Efficacy of B. bacteriovorus predation of M. bovis in broth cultures
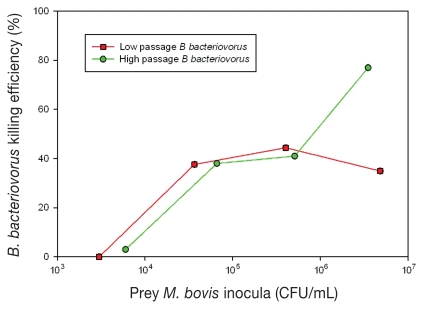
The level of M. bovis prey required for B. bacteriovorus to demonstrate predation was assessed in suspension broth cultures for M. bovis prey levels of 1 × 103 to 1 × 107 CFU/mL with a B. bacteriovorus inoculum of 1 × 1010 PFU/mL. As shown in Figure 2, B. bacteriovorus passaged on M. bovis 3 times showed killing of ~40% for M. bovis prey levels greater than 4 × 104 CFU/mL. However after 6 passages, killing efficiency increased to ~76% only for M. bovis prey levels greater than 9 × 106 CFU/mL.
Figure 2.
Efficacy of B. bacteriovorus predation of M. bovis in broth cultures. Initial inocula levels of M. bovis (CFU/mL) are plotted on the x-axis versus the B. bacteriovorus killing efficacy (%) at 4 h of exposure on the y-axis. Low and high passages of B. bacteriovorus on M. bovis correspond with 2 to 3 and 6 passages, respectively.
Efficacy of B. bacteriovorus predation of M. bovis in an in vitro model of IBK
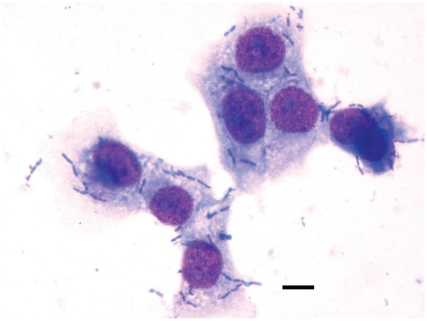
Moraxella bovis attaches specifically to epithelial cells of bovine origin (8,33–35), and MDBK cells have been used by others as an in vitro model of M. bovis attachment for IBK (33). Using this model M. bovis attached to MDBK cells appeared as darkly stained rods or coccobacillary shapes, characteristically in pairs, adhered to the surface of the larger polygonal MDBK cells (Figure 3). At the time of inoculation in the presence of 1.6 × 1011 PFU/mL of B. bacteriovorus, the mean number of bacteria attached per MDBK cell was ~4 M. bovis per MDBK cell (Table I). The number of M. bovis attached per MDBK cell remained relatively constant during the 12 h incubation period with no statistical difference between the 0, 6, and 12 h time points for the controls lacking B. bacteriovorus. When compared with the presence of B. bacteriovorus, there was 1.9-fold decrease of M. bovis attached to MDBK cells after 6 h and 6-fold decrease after 12 h, which were statistically significant at P < 0.05 and P < 0.001, respectively.
Figure 3.
Light microscopic appearance of M. bovis adhering to Madin-Darby bovine kidney (MDBK) cells. Moraxella bovis attached to MDBK cells appeared as darkly stained rods or coccobacillary shapes, characteristically in pairs, adhered to the surface of the larger polygonal MDBK cells. Wright-Giemsa stain. Magnification: 1000×. Bar = 4.5 μm.
Table I.
Bdellovibrio bacteriovorus clearance of Moraxella bovis attached to Madin-Darby bovine kidney (MDBK) cells
| Attachment (M. bovis per MDBK)e |
Unattached M. bovis (103 CFU/mL)
|
|||
|---|---|---|---|---|
| Exposure time (h) | No B. bacteriovorus | With B. bacteriovorus | No B. bacteriovorus | With B. bacteriovorus |
| 0 | 4.13 ± 2.62 | 4.62 ± 1.31 | 80 ± 152c | 998 ± 571c |
| 6 | 3.41 ± 1.27a | 1.77 ± 0.45a | 277 ± 65 | 213 ± 87 |
| 12 | 5.35 ± 1.96b | 0.88 ± 0.11b | 1.520 ± 860d | 691 ± 72d |
Statistically significant at P < 0.05.
Statistically significant at P < 0.001.
Statistically significant at P < 0.005.
Statistically significant at P < 0.005.
Results represent mean ± 1 standard deviation from 6 coverslips.
In addition to M. bovis attached to MDBK cells, 0.8 × 105 to 1 × 106 CFU/mL M. bovis were present in the culture media at the beginning of the incubation period (Table I). In the absence of B. bacteriovorus, the M. bovis level increased to 1.5 × 106 CFU/mL at 12 h, whereas in the presence of B. bacteriovorus the supernatant M. bovis declined at 6 h and were near the pre-inoculation value of 0.7 × 106 CFU/mL at 12 h. These values were significantly different (P < 0.005) from that for the supernatant M. bovis CFU/mL in the absence of B. bacteriovorus.
The supernatant B. bacteriovorus level increased from 8 × 109 PFU/mL at 0 h to 3 × 1010 PFU/mL and 5 × 1010 PFU/mL at 12 h in the absence and presence of M. bovis, respectively. The number of active B. bacteriovorus in the original filtered bacterial suspension ranged from 15 to 20 per 100 × field under light microscopy.
Discussion
Efficacy of low and high passages B. bacteriovorus predation of M. bovis in suspension broth cultures
It is difficult to replicate the in vivo conditions of IBK in an in vitro model for assessing the efficiency of predation of M. bovis by B. bacteriovorus. The 4 h time period was selected to provide sufficient time for B. bacteriovorus to complete its life cycle and the incubation temperature of 35°C was selected (instead of the 30°C optimum for B. bacteriovorus) for optimal M. bovis growth and viability. Bdellovibrio bacteriovorus requires adequate levels of prey bacteria, typically > 1 × 106 CFU/mL (26), in order to maintain its predatory lifestyle. The efficiency of predation followed a predicable trend in that at low prey levels (< 104 CFU/mL) there were insufficient prey to either trigger or sustain B. bacteriovorus predation, but at higher prey levels (> 105 CFU/mL and > 107 CFU/mL) efficiency of predation increased from ~40% to ~76%, respectively. The range of this amount is less efficient compared with the reported B. bacteriovorus predation efficiency of other gram-negative bacteria such as E. coli, but comparable to various strains of Enterobacter spp, Erwinia spp, and Salmonella spp. Rogosky et al (17) reported that B. bacteriovorus does have prey preference, killing some prey more efficiently than others. Pantoea agglomerans, E. coli, and S. marcescens were reported to be preferred prey for B. bacteriovorus with efficiency of predation of ~90% compared with E. aerogenens, E. carotova subsp. carotova, and S. enterica with efficiency of predation of ~60%. It has been observed here and by others that B. bacteriovorus can be trained to kill less preferred prey more efficiently by continuous passage on that prey. For this study, B. bacteriovorus was initially passaged on M. bovis 3 times with modest shortening of the passage time (from 10 d to 5 d after 3rd passage) to fully active B. bacteriovorus cultures. Improved efficiency of predation at high prey concentration (> 107 CFU/mL) was attained when B. bacteriovorus number of passages on M. bovis increased to 5 times, with corresponding passage time of 2 d to fully active B. bacteriovorus culture (Figure 1).
Efficacy of B. bacteriovorus predation of M. bovis in an in vitro model of IBK
Our initial experiments with the in vitro IBK model used a β-hemolytic strain of M. bovis, but this strain produced significant cytotoxicity resulting in MDBK cell detachment from coverslips such that the mean number of M. bovis attached per MDBK cell could not be consistently determined. Therefore, a non β-hemolytic strain of M. bovis was used in this study. No difference in efficiency of predation by B. bacteriovorus of hemolytic versus non-hemolytic strains of M. bovis was observed.
A paramount question for the use of B. bacteriovorus as a non-chemotherapeutic alternative therapy for IBK is whether B. bacteriovorus can efficiently decrease the number of M. bovis adhered to corneal epithelial cells in infected bovine eyes. To study this in vitro, a co-culture model of M. bovis with MDBK cells was used, with the media selected to support both MDBK and M. bovis viability while attempting to mimic the ocular environment. To reproduce the physical ocular environment, round coverslips with the approximate dimension of the bovine cornea were used and to mimic the ocular tear film, the media column above the coverslip was kept to the minimum that supported MDBK cell viability for a 12 h exposure period. Selected media was also tailored to simulate tears. It contained minimum levels of nutrients in an aqueous salts composition to reduce M. bovis growth rate to one simulating the slower growth rate in vivo. This was accomplished in that the doubling time for M. bovis in the in vitro IBK model was 4.3 h (calculated from data for Table I). During the 12 h experimental period, M. bovis in the unexposed control IBK model underwent approximately 3 doublings, but M. bovis attachment to MDBK cells only increased 1.3-fold. However, exposure to B. bacteriovorus decreased the number of adherent M. bovis on MDBK cells in vitro by 6-fold (Table I).
Bdellovibrio bacteriovorus appears to be an effective predator of bacterial prey fixed on surfaces as demonstrated by its efficacy against bacteria in surface biofilms (31). Exposure to B. bdellovibrio increased the levels of planktonic M. bovis by 12-fold at the beginning of the experiment, suggesting that the predatory bacteria is efficient in preventing initial attachment of M. bovis on MDBK cells in vitro. Although B. bacteriovorus was not as effective in clearing M. bovis from the aqueous media above the coverslip, it did reduce the CFU/mL by 1.4-fold as compared to a 19-fold increase in the controls without B. bacteriovorus (Table I). The apparent bactericidal effect of M. bovis attached to bovine epithelial cells may be crucial for reducing M. bovis ulceration of the cornea of infected cattle because pathogenesis of these lesions appears to be the result of direct contact of M. bovis with corneal epithelial cells (37). In contrast, although M. bovis attached to corneal epithelia extend into the corneal stroma where they may be protected from innate and acquired immunity, M. bovis in the tear film will not only be exposed to B. bacteriovorus predation but also to innate immune response of infiltrating neutrophils; tear antimicrobial proteins and enzymes, such as lysozyme (29), the latter reported to increase up to 33-fold in cattle with inflamed corneas (38); and to acquired immunity through secreted IgA and leaked serum IgG (29,39), and therefore, bacteriostatic activity of B. bacteriovorus against M. bovis in the tear film may be adequate to resolve infection. Due to its poor specificity against gram-negative bacteria, the true protective effect of tear lysozyme against the invasion of the ocular surfaces by M. bovis is unknown (29).
In summary, the present study confirms that B. bacteriovorus can be trained to kill M. bovis as effectively as its normal prey E. coli. The efficiency of low passage B. bacteriovorus predation on M. bovis in suspension broth culture was ~40% at prey levels > 4 × 104 CFU/mL though, at high passage on M. bovis and prey levels > 9 × 106 CFU/mL, the efficiency was increased to ~76%. The minimum M. bovis concentration to sustain B. bacteriovorus life cycle was established to be < 104 CFU/mL. In the in vitro model of IBK, exposure to B. bacteriovorus not only significantly decreased the number of adherent M. bovis on MDBK cells, but also had a bacteriostatic effect on planktonic M. bovis. Therefore, we conclude that B. bacteriovorus can act as an effective M. bovis predator at levels present in IBK infected corneal epithelia and ocular secretions. Future studies are needed to evaluate the cytoprotective effects of B. bacteriovorus on M. bovis infected MDBK cells and to characterize the effect of high lysozyme concentration on B. bacteriovorus in vitro.
Acknowledgments
This research was supported by grant 5T20RR015564, Center of Biomedical Research Excellence (COBRE) from the National Center for Research Resources to the University of Oklahoma Health Sciences Center, and the Oklahoma State University Research Advisory Committee. The authors thank Huda Mussa and Asitha Vasudevan Pillai for technical assistance.
References
- 1.Baptista PJHP. Infectious bovine keratoconjunctivitis: A review. Br Vet J. 1979;135:225–242. doi: 10.1016/s0007-1935(17)32882-8. [DOI] [PubMed] [Google Scholar]
- 2.George LW. Antibiotic treatment of infectious bovine keratoconjunctivitis. Cornell Vet. 1990;80:229–235. [PubMed] [Google Scholar]
- 3.Thrift FA, Overfield JR. Impact of pinkeye (infectious bovine keratoconjunctivitis) on weaning and postweaning performance of Hereford calves. J Ani Sci. 1974;38:1179–1184. doi: 10.2527/jas1974.3861179x. [DOI] [PubMed] [Google Scholar]
- 4.Brown MH, Brightman AH, Fenwick BW, et al. Infectious bovine keratoconjunctivitis: A review. J Vet Intern Med. 1998;12:259–266. doi: 10.1111/j.1939-1676.1998.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith JA, George LW. Treatment of acute ocular Moraxella bovis infections in calves with a parenterally administered long-acting oxytetracycline formulation. Am J Vet Res. 1985;46:804–807. [PubMed] [Google Scholar]
- 6.Ruehl WW, Marrs C, Beard MK, et al. Q pili enhance the attachment of Moraxella bovis to bovine corneas in vitro. Mol microbiol. 1993;7:285–288. doi: 10.1111/j.1365-2958.1993.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 7.Clinkenbeard KD, Thiessen AE. Mechanism of action of Moraxella bovis hemolysin. Infect Immun. 1991;59:1148–1152. doi: 10.1128/iai.59.3.1148-1152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kangonyera GM, George LW, Munn R. Cytopathic effects of Moraxella bovis on cultured bovine neutrophils and corneal epithelial cells. Am J Vet Res. 1989;50:10–17. [PubMed] [Google Scholar]
- 9.Marrion RM, Riley LK. Detection of cell detachment activity induced by Moraxella bovis. Am J Vet Res. 2000;61:1145–1149. doi: 10.2460/ajvr.2000.61.1145. [DOI] [PubMed] [Google Scholar]
- 10.George LW, Mihalyi J, Edmondson A, et al. Topically applied furazolidone or parenterally administered oxytetracycline for the treatment of infectious bovine keratoconjunctivitis. J Am Vet Med Assoc. 1988;192:1415–1422. [PubMed] [Google Scholar]
- 11.Eastman TG, George LW, Hird DW, et al. Combined parenteral and oral administration oxytetracycline for control of infectious bovine keratoconjunctivitis. J Am Vet Med Assoc. 1998;121:560–563. [PubMed] [Google Scholar]
- 12.Angelos JA, Dueger EL, George LW, et al. Efficacy of florfenicol for treatment of naturally occurring infectious bovine keratoconjunctivitis. J Am Vet Med Assoc. 2000;216:62–64. doi: 10.2460/javma.2000.216.62. [DOI] [PubMed] [Google Scholar]
- 13.Dueger EL, Angelos JA, Cosgrove S, et al. Efficacy of florfenicol in the treatment of experimentally induced infectious bovine keratoconjunctivitis. Am J Vet Res. 1999;60:960–964. [PubMed] [Google Scholar]
- 14.Dueger EL, George LW, Angelos JA, et al. Efficacy of a long- acting formulation of ceftiofur crystalline-free acid for the treatment of naturally occurring infectious bovine keratoconjunctivitis. Am J Vet Res. 2004;65:1185–1188. doi: 10.2460/ajvr.2004.65.1185. [DOI] [PubMed] [Google Scholar]
- 15.Lane VM, George LW, Cleaver DM. Efficacy of tulathromycin for treatment of cattle with acute ocular Moraxella bovis infections. J Am Vet Med Assoc. 2006;229:557–561. doi: 10.2460/javma.229.4.557. [DOI] [PubMed] [Google Scholar]
- 16.Stolp H, Starr MP. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek J Microbiol Serol. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- 17.Rogosky Am, Moak PL, Emmert EAB. Differential predation by Bdellovibrio bacteriovorus 109J. Curr Microbiol. 2006;52:81–85. doi: 10.1007/s00284-005-0038-6. [DOI] [PubMed] [Google Scholar]
- 18.Sockett RE. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol. 2009;63:523–539. doi: 10.1146/annurev.micro.091208.073346. [DOI] [PubMed] [Google Scholar]
- 19.Strauch E, Schwudke D, Linscheid M. Predatory mechanisms of Bdellovibrio and like organisms. Future Microbiol. 2007;2:63–73. doi: 10.2217/17460913.2.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Lambert C, Morehouse KA, Chang C-Y, et al. Bdellovibrio: Growth and development during the predatory cycle. Curr Opin Microbiol. 2006;9:639–644. doi: 10.1016/j.mib.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Ruby EG. The genus Bdellovibrio. In: Balows A, Truper HG, Dworking M, Harder W, Schleifer KH, editors. The Prokaryotes. 2nd ed. NewYork: Springer-Verlag; 1991. pp. 3400–3415. [Google Scholar]
- 22.Lambert C, Sockett RE. Laboratory maintenance of Bdellovibrio. Curr protoc Microbiol. 2008;chapter 7(Unit 7B.2):7B.2.1–7B.2.13. doi: 10.1002/9780471729259.mc07b02s9. [DOI] [PubMed] [Google Scholar]
- 23.Sockett Re. Nature Reviews. Bdellovibrio as therapeutic agents: A predatory renaissance? Microbiol. 2004;2:669–675. doi: 10.1038/nrmicro959. [DOI] [PubMed] [Google Scholar]
- 24.Fry JC, Staples DG. Distribution of Bdellovibrio bacteriovorus in sewage works, river water, and sediments. Appl Environ Microbiol. 1976;31:469–474. doi: 10.1128/aem.31.4.469-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolp H. The Bdellovibrios: Bacterial parasites of bacteria. Annu Rev Phytopathol. 1973;11:53–76. [Google Scholar]
- 26.Hespell RB, Thomashow MF, Rittenberg SC. Changes in cell composition and viability of Bdellovibrio during starvation. Arch Microbiol. 1974;97:313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- 27.Varon M, Zeigler BP. Bacterial predator-prey interaction at low prey density. Appl Environ Microbiol. 1978;36:11–17. doi: 10.1128/aem.36.1.11-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann SM, Kainer RA, Severin GA. Reaction of normal equine eyes to radio-frequency current-induced hyperthermia. Am J Vet Res. 1982;43:1938–1944. [PubMed] [Google Scholar]
- 29.Gionfriddo JR, Melgarejo T, Morrison EA, et al. Comparison of tear proteins of llamas and cattle. Am J Vet Res. 2000;61:1289–1293. doi: 10.2460/ajvr.2000.61.1289. [DOI] [PubMed] [Google Scholar]
- 30.Fratamico PM, Whiting RC. Ability of Bdellovibrio bacteriovorus 109J to lyse gram-negative food-borne pathogenic and spoilage bacteria. J Food Prot. 1995;58:160–164. doi: 10.4315/0362-028X-58.2.160. [DOI] [PubMed] [Google Scholar]
- 31.Kadouri D, O’Toole GA. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol. 2005;71:4044–4051. doi: 10.1128/AEM.71.7.4044-4051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M. Alteration of Shigella pathogenicity by other bacteria. Am J Clin Nutr. 1972;25:1441–1451. doi: 10.1093/ajcn/25.12.1441. [DOI] [PubMed] [Google Scholar]
- 33.Annuar BO, Wilcox GE. Adherence of Moraxella bovis to cell cultures of bovine origin. Res Vet Sci. 1985;39:241–246. [PubMed] [Google Scholar]
- 34.Jackman SH, Rosenbush RF. In vitro adherence of Moraxella bovis to intact corneal epithelium. Curr Eye Res. 1984;3:1107–1112. doi: 10.3109/02713688409000809. [DOI] [PubMed] [Google Scholar]
- 35.Moore LJ, Rutter JM. Attachment of Moraxella bovis to calf corneal cells and inhibition by antiserum. Aust Vet J. 1989;66:39–42. doi: 10.1111/j.1751-0813.1989.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 36.Varon M, Shilo M. Interaction of Bdellovibrio bacteriovorus and host bacteria. J Bacteriol. 1968:744–753. doi: 10.1128/jb.99.1.136-141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodgers DG. Conjunctival lesions caused by Moraxella bovis in gnotobiotic calves. Vet Pathol. 1987;24:554–559. doi: 10.1177/030098588702400614. [DOI] [PubMed] [Google Scholar]
- 38.Prieur DJ. Tissue specific deficiency of lysozyme in ruminants. Comp Biochem Physiol. 1986;85B:349–353. doi: 10.1016/0305-0491(86)90011-8. [DOI] [PubMed] [Google Scholar]
- 39.Eichenbaum JD, Lavach JD, Severin GA, et al. Immunology of the ocular surface. Comp Cont Ed Pract Vet. 1987;9:1101–1109. [Google Scholar]