Abstract
The aim of this study was to evaluate the performance characteristics of an automated immunoassay for canine insulin-like growth factor 1 (IGF-1) measurement and to investigate the possible effects of some sources of variation, such as diurnal variations, feeding/fasting cycles, and glucocorticoid administration, in dogs. The immunoassay evaluated had an adequate analytical performance with intra- and inter-assay coefficients of variation (CVs) lower than 10%, linear regression equations with correlation coefficients of 0.9993 and 0.9988 after serial dilutions, and a limit of quantification of 7.1 ng/mL that was even lower than that reported by the manufacturer. The assay was significantly affected by hemolysis and lipemia producing a significant decrease in IGF-1 concentrations, but not by bilirubinemia.
Serum IGF-1 concentrations did not show significant diurnal changes in fed or fasted dogs and were not affected by glucocorticoid administration.
Résumé
L’objectif de la présente étude était d’évaluer les caractéristiques de performance d’une épreuve immuno-enzymatique automatisée pour la mesure du facteur de croissance 1 apparenté à l’insuline (IGF-1) et d’étudier chez les chiens les effets possibles de quelques sources de variation, telles que les variations diurnes, les cycles alimentation/jeûne, et l’administration de glucocorticoïdes. L’épreuve immuno-enzymatique évaluée avait une performance analytique adéquate avec des coefficients de variation (CV) intra- et inter-essais inférieur à 10 %, des équations de régression linéaire avec des coefficients de 0,9993 et 0,9988 après des dilutions sériées, et une limite de quantification de 7,1 ng/mL qui était inférieure à celle mentionnée par le manufacturier. L’épreuve était affectée de manière significative par de l’hémolyse dans l’échantillon et une lipémie, qui entraînaient une réduction significative des concentrations d’IGF-1, mais pas par une bilirubinémie.
Les concentrations sériques d’IGF-1 n’ont pas montré de changements significatifs associés aux variations diurnes chez des chiens nourris ou mis au jeûne et n’étaient pas affectées par l’administration de glucocorticoïdes.
(Traduit par Docteur Serge Messier)
Insulin-like growth factor 1 (IGF-1) is a small polypeptide hormone consisting of 70 amino acids that is synthesized in the liver. It is involved in the regulation of growth and metabolism and mediates many of the anabolic effects of growth hormone (GH) in different tissues, such as bones, muscles, kidneys, spleen, hearth, liver, or mammary gland (1). Serum IGF-1 measurements have garnered increased attention recently in dogs. It has been proposed as a marker of liver function with a diagnostic value nearly comparable with fasting bile acids (2). Also it has been reported to be higher in obese subjects and return to normal values after weight loss (3). Additionally, as in humans, the possible relationships between IGF-1 and neoplasia or cardiovascular disease have been studied, with a recently demonstrated a link having been found between serum IGF-1 concentrations and a worse prognosis in canine mammary cancers (4).
To facilitate studies on IFG-1 in dogs automated assays for determination of this factor need to be validated. Most of the studies published on the dog have used a multispecies radioimmunoassay (RIA) or enzyme-linked immunosorbent assay (ELISA) for IGF-1 quantification (2–4). The use of an automated solid-phase, enzyme-labelled chemiluminescent immunometric assay would have several advantages, such as easy integration into laboratory functions, high throughput, and rapid turnaround time. Secondly, knowledge about possible effects of some sources of variation, such as diurnal variations, feeding/fasting cycles, or corticoid administration on IGF-1, would allow proper interpretation of the values of this analyte. The aim of this study was to evaluate the performance characteristics of an automated immunoassay for canine IGF-1 measurement and to investigate the possible effects of some sources of variation, such as diurnal variations, feeding/fasting cycles, and glucocorticoid administration in dogs.
Canine serum samples used for the validation study were obtained from 3 apparently healthy dogs of different sex, breeds, and ages that were presented at the Veterinary Teaching Hospital of Murcia University for routine check-ups. Dogs showed no remarkable findings on physical and clinical examination and in routine hematology and biochemistry analysis. In addition, samples were obtained from 3 dogs with chronic hepatitis, the diagnosis of hepatopathy was made as described by Neumann et al (2).
Eight young intact beagle dogs [4 females and 4 males; age range: 3.2 to 5.1 y; bodyweight (BW) range: 12.6 to 18.9 kg; body condition scores (BCS): 3 to 4], were included to investigate diurnal variations and influence of food intake on IGF-1. Fourteen young intact beagle dogs (8 males and 6 females; age range: 3.2 to 5.5 y; BW range: 10.1 to 17.9 kg; BCS: 3 to 4), were used to investigate possible effects of glucocorticoids on serum IGF-1. None of these dogs had ever received exogenous glucocorticoids. A single breed (beagle) within a narrow age range was used to avoid heterogeneity. All animals had a negative serological titer for Leishmania infantum and Erlichia canis. Animal care and procedures were in accordance with the guidelines of the University of Murcia, Spain. The assessment of the nutritional condition was based on a 5-scale BCS: 1 — thin; 2 — lean; 3 — optimal; 4 — obese; 5 — gross (5).
An automated solid-phase, enzyme-labelled chemiluminescent immunometric assay (Immulite 1000 IGF-1 assay; Diagnostic Products, Los Angeles, California, USA) was used. It was configured and calibrated on an instrument in our laboratory according to manufacturer’s instructions. The assay has test units containing one bed coated with monoclonal murine anti-IGF-1, alkaline phosphatase (bovine calf intestine) conjugated to polyclonal rabbit anti-IGF-1 in buffer, chemiluminescent substrate (phosphate ester of adamantyl dioxetane in an AMP buffer with enhancer).
Cortisol was analyzed with using an automated chemiluminescent immunoassay (Immulite System; Siemens Health Diagnostics, Deerfield, Illinois, USA). Glucose was analyzed on an automated clinical chemistry analyser (Olympus AU2700; Olympus Diagnostica GmbH, Hamburg, Germany) as per the manufacturer’s instructions.
For analytical validation of the method, the precision; accuracy; lower limit of quantification (LLOQ); and effects of hemolysis, lipemia, and bilirubinemia were investigated.
Two serum pools with different IGF-1 activities were prepared, one from the population of healthy dogs (mean IGF-1 close to 315.20 ng/mL), and the other from dogs with chronic hepatopathies (mean IGF-1 close to 38.02 ng/mL) and were used for precision studies. Intra- and inter-assay coefficients of variation (CV) were calculated after analysis of these 2 serum pools 5 times in a single assay or on 5 consecutive days, respectively.
Accuracy of the assay was evaluated indirectly by linearity under dilution. This procedure is an alternative used in situations, as in this study, when no reference method is available and no commercially available certified reference materials exist (6).
To evaluate effect of hemolysis, lipemia, and bilirubinemia, 2 canine serum samples were mixed with different concentrations of hemoglobin, lipid, or bilirubin solution following previously described procedures (7) and each preparation was run in duplicate.
To investigate diurnal variation and possible effect of food intake on serum IGF-1, a previously described protocol (8) with some modifications was used. Briefly: 8 adult beagle dogs were divided into 2 groups of 4 dogs (2 males and 2 females in each). One group, defined as group Fed+, was fed at 09:00 as on previous days. The other group, defined as group Fed−, was fasted 24 h. Blood samples were taken prior to feeding at 08:00 and at intervals of 2, 4, 8, 10, 12, 16, and 20 h.
To study effects of glucocorticoids on serum IGF-1, 14 adult beagles were assigned to control (n = 4) and test groups (n = 10). Dogs in the control group (2 males and 2 females) were injected SC 0.9% NaCl (0.1 mL/kg). Dogs in the test group were divided into 2 subgroups: group 1 (n = 5, 3 males and 2 females) and group 2 (n = 5, 3 males and 2 females). Methylprednisolone (Urbason 40 mg; Aventis Pharma S.A., Alcorcon, Madrid, Spain) was injected once SC 1 mg/kg BW and 5 mg/kg BW in groups 1 and 2, respectively. The experiment was started at 08:00 after 12 h fasting in all groups. Blood samples were collected prior to treatment at 08:00 and at intervals of 2, 4, 8, 12, and 20 h. This protocol was chosen on the basis of data from previous studies (9,10).
Blood samples were collected by venipuncture in the cephalic vein into tubes containing clotting accelerator (TapVal; Aquisel, Barcelona, Spain). Samples were centrifuged at 2000 × g for 10 min at room temperature to obtain serum, which was then stored in plastic vials at −20°C for less than 6 mo (11). Serum samples used for repetitive analysis were frozen in aliquots and only vials needed for each run were used, to avoid possible changes due to repetitive thawing and freezing.
Arithmetic means, medians, intra-assay and inter-assay CVs were calculated using routine descriptive statistical procedures and software (GraphPad Prism, GraphPad Software; San Diego, California, USA). Linearity under dilution was accomplished by using ordinary linear regression analysis comparing the measured levels of IGF-1 with the expected levels. Interferograms were prepared to show the differences in IGF-1 concentration when hemoglobin, triglycerides, or bilirubin were added. The influence of hemoglobin and triglycerides was investigated using a 1-way analysis of variance (ANOVA) and Dunnetts’ post test. Repeated measures ANOVA with Newman-Keuls multiple comparison post test was used to analyze the possible effects of feeding/fasting cycles and glucocorticoids on IGF-1 levels in dogs. A two-way ANOVA with Bonferoni post test was used to compare the biochemical parameters between fed and fasted animals, and between different groups of dogs in glucocorticoid experiment. The significance level used in each case was P < 0.05.
Intra-assay CVs were 7.4% and 4.1%, while inter-assays CVs of the same serum pools were 9.2% and 8.6%. In the present study, CV values from the assay (IMMULITE IGF-1) gave slightly higher intra- and inter-assay CVs than those reported by the manufacturers. Nevertheless, the assay appears to be suitable for detecting canine IGF-1, as CV values did not exceed 15%, the limit of the objective analytic performance standard for precision (12).
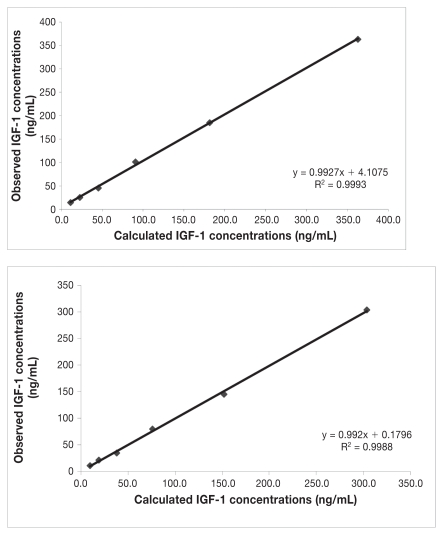
Dilution of 2 serum samples resulted in linear regression equations with correlation coefficients of 0.9993 and 0.9988 (Figure 1). The LLOQ was 7.1 ng/mL with the precision within 15% verified by repeated analysis (13), and it was lower than that reported by manufacturers (20 ng/mL). This fact could help for detecting the low values of IGF-1 that can be expected in liver diseases.
Figure 1.
Linearity under dilution of 2 canine serum samples with different insulin-like growth factor (IGF-1) concentrations measured with an assay (IMMULITE 1000).
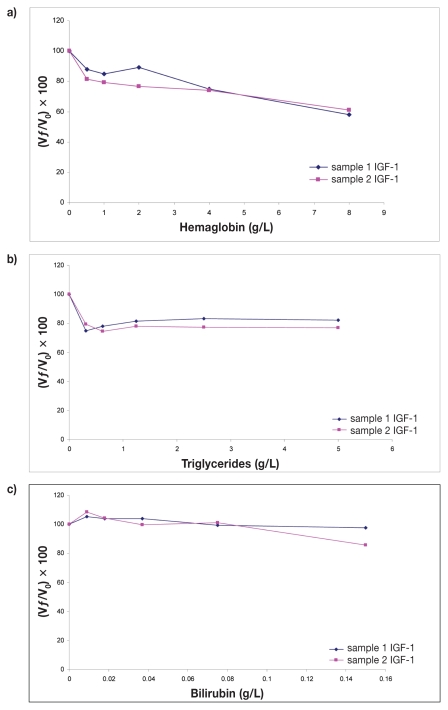
Interferograms showing effects of hemolysis, lipemia, and bilirubinemia on determining IGF-1 concentrations are presented in Figure 2. On the graphs, X-axes show increasing concentrations of hemoglobin, triglycerides, or bilirubin while Y-axes show percentage change in IGF-1 [(Vƒ/Vo) × 100]. A one-way ANOVA showed significant decrease in IGF-1 concentrations in presence of hemolysis (P = 0.0015) and lipemia (P = 0.0043), but not bilirubinemia (P = 0.1). Dunnetts’ post test showed a statistically significant decrease in IGF-1 concentrations when hemoglobin concentrations were 1, 2, 4, and 8 g/L (P < 0.05, P < 0.05, P < 0.01, P < 0.005, respectively), and when any amount of triglycerides was added to the samples (P < 0.01, in all cases). These results would partly agree with ones reported by manufacturers, as they indicate the influence of low grade hemolysis on IGF-1 levels and that the presence of conjugated and unconjugated bilirubin in concentrations up to 200 mg/L had no effect on the results. But it is contradictory in the case of lipemia, as the manufacturers have reported that the presence of triglycerides, up to 3000 mg/dL, had no effect on the IGF-1 levels.
Figure 2.
Interferograms corresponding to the effect of hemoglobin (a), triglycerides (b), and bilirubin (c) concentrations on insulin-like growth factor (IGF-1) determination by assay (IMMULITE 1000). Y-axes show percentage of change in IGF-1 [(Vƒ/V0) × 100] for a given concentration of hemoglobin, triglycerides, or bilirubin, respectively. Vƒ, final value; V0, original value.
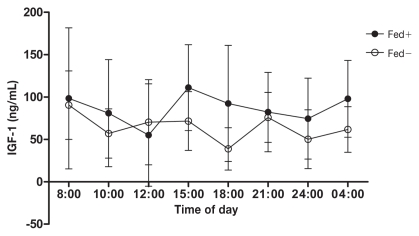
Figure 3 shows serum IGF-1 levels over a 20 h period in fed and fasted dogs. No consistent statistically significant diurnal patterns in total IGF-1 levels were observed in both groups. These data are in accordance with results published in human medicine, in which most studies agree that circulating IGF-1 levels remain relatively stable during the day and are unaffected by meal intake, as well as the type of meal (14–16). Conversely, in other studies on humans, there is evidence of a nocturnal decline in IGF-1 levels from midnight to 04:00 h, following which the levels return to baseline (16,17). This can be explained by shifts in plasma volumes (18).
Figure 3.
Mean ± standard deviation variation of serum insulin-like growth factor (IGF-1) concentrations. Four beagle dogs were fed at 09:00 h (Fed+) and 4 beagle dogs were fasted (Fed−) all day long.
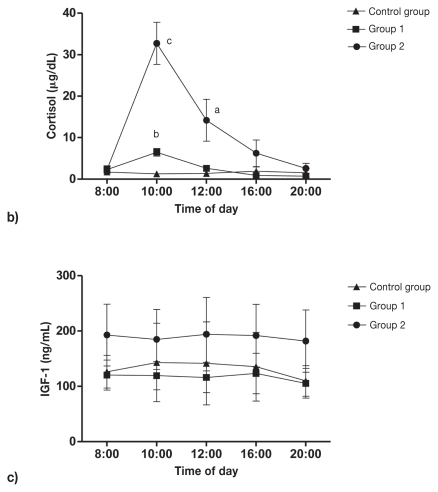
Responses of cortisol and total IGF-1 to methylprednisolone, in comparison with placebo injection, are shown in Figure 4. Circulating cortisol levels increased 180.5% in group 1 (P = 0.0008) and 1318.9% in group 2 (P = 0.0003) 2 h after methylprednisolone injection. Four hours after methylprednisolone injection cortisol levels in group 1 returned to normal values (P = 0.5), while in group 2 cortisol levels were still significantly increased 36.4% (P = 0.0089) and returned to normal values 12 h after corticoid injection. Methylprednisolone 1 mg/kg BW and 5 mg/kg BW subcutaneously did not cause alterations in circulating IGF-1 levels, and no significant difference was found between the control group and groups that received glucocorticoids. Similar results were reported by Wolthers et al (19,20) as no changes in circulating IGF-1 levels in children with asthma treated with low doses of prednisolone (2.5 mg) or inhaled glucocorticoids were detected. In addition, normal IGF-1 levels have been reported in humans with Cushing’s syndrome (21). Conversely, Miell et al (22) reported that treatment of men with dexamethasone (2.5 mg) for 3 d significantly stimulated total IGF-1 levels.
Figure 4.
Mean ± standard deviation plasma cortisol (a) and insulin-like growth factor (IGF-1) (b) response to subcutaneous (SC) bolus of methylprednisolone: 1 mg/kg BW (group 1; n = 5) and 5 mg/kg BW (group 2; n = 5). Four dogs in the control group were injected SC 0.9% NaCl (0.1 mL/kg). a Statistically significant (P < 0.01). b Statistically significant (P < 0.001). c Statistically significant (P < 0.0005).
In conclusion, the automated method that was tested in the present study exhibited analytical characteristics validating its use in the laboratory, including adequate precision and linearity under dilution. Since it is robust, safe, and totally automated, this method will facilitate widespread studies and applications of IGF-1 measurements in dogs. Furthermore, we have demonstrated, that canine IGF-1 was significantly influenced by hemolysis and lipemia, so hemolytic or lipemic samples should, ideally, be discarded or interpreted with caution. However it was not affected by meal, fasting, or methylprednisolone administration, so in practice no strict standardization of the sampling condition (time of the day, fasting versus non-fasting, no previous corticoid treatment) is required for collection of blood for IGF-1 measurements.
References
- 1.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 2.Neumann S, Welling H, Thuere S. Insulin-like growth factor I concentration in dogs with inflammatory and neoplastic liver diseases. J Vet Med Assoc. 2007;54:612–617. doi: 10.1111/j.1439-0442.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard G, Nguyen P, Gayet C, Leriche I, Siliart B, Paragon BM. Rapid weight loss with high-protein low-energy diet allows the recovery of ideal body composition and insulin sensitivity in obese dogs. J Nutr. 2004;134:2148S–2150S. doi: 10.1093/jn/134.8.2148S. [DOI] [PubMed] [Google Scholar]
- 4.Queiroga FL, Perez-Alenza D, Silvan G, Peña L, Lopes CS, Illera JC. Serum and intratumoural GH and IGF-1 concentrations: Prognostic factors in the outcome of canine mammary cancer. Res Vet Sci. 2010;89:396–403. doi: 10.1016/j.rvsc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 5.McGreevy PD, Thompson PC, Pride C, Fawcett A, Grassi T, Jones B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Record. 2005;156:695–702. doi: 10.1136/vr.156.22.695. [DOI] [PubMed] [Google Scholar]
- 6.Feldman BF, Zinkl JG, Jain NC. Schalm’s Veterinary Hematology. 5th ed. Ames, Iowa: Blackwell Publ; 2006. pp. 20–28. [Google Scholar]
- 7.Martínez-Subiela S, Ceron JJ. Effects of hemolysis, lipemia, hyperbilirubinemia, and anticoagulants in canine C-reactive protein, serum amyloid A, and ceruloplasmin assays. Can Vet J. 2005;46:625–629. [PMC free article] [PubMed] [Google Scholar]
- 8.Ishioka K, Hatai H, Komabayashi K, et al. Diurnal variations of serum leptin in dogs: Effects of fasting and re-feeding. Vet J. 2005;169:85–90. doi: 10.1016/j.tvjl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Subiela S, Ginel PJ, Ceron JJ. Effects of different glucocorticoid treatments on serum acute phase proteins in dogs. Vet Rec. 2004;154:814–817. doi: 10.1136/vr.154.26.814. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz Z, Ilcol YO, Golcu E. Serum leptin levels in response to methylprednisolone injection in healthy dogs. Res Vet Sci. 2007;82:187–194. doi: 10.1016/j.rvsc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Nakachi K, Imai K, et al. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor β, and superoxide dismutase activity for the JACC study. J Epidemiol. 2005;15:S67–S73. doi: 10.2188/jea.15.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine; 2001. [Last accessed 2011]. Available from www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. [Google Scholar]
- 13.Tholen DW, Kroll M, Astles JR. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; approved guideline NCCLS document EP6-A. NCCLS; Pennsylvania, USA: 2003. [Last accessed 2011]. Available from http://www.clsi.org/source/orders/free/ep6-a.pdf. [Google Scholar]
- 14.Belobrajdic DP, Priebe IK, Forbes B, et al. Assesing the potential usefulness of IGF-related peptides and adiponectin for predicting disease risk. Growth Horm IGF Res. 2008;18:198–204. doi: 10.1016/j.ghir.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am J Clin Nutr. 2005;82:350–354. doi: 10.1093/ajcn.82.2.350. [DOI] [PubMed] [Google Scholar]
- 16.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Gu X, Pan H, et al. Reference ranges for serum IGF-1 and IGFBP-3 levels in chinese children during childhood and adolescence. Endocr J. 2010;57:221–228. doi: 10.1507/endocrj.k09e-200. [DOI] [PubMed] [Google Scholar]
- 18.Frystyk J, Freda P, Clemmons DR. The current status of IGF-I assay — A 2009 update. Growth Horm IGF Res. 2010;20:8–18. doi: 10.1016/j.ghir.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolthers OD, Juul A, Hansen M, Muller J, Pedersen S. The insulin-like growth factor axis and collagen turnover during prednisolone treatment. Arch Dis Child. 1994;71:409–413. doi: 10.1136/adc.71.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolthers OD, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion, and measures of the insulin-like growth factor axis and collagen turnover in children treated with inhaled glucocorticosteroids. Pediatr Res. 1997;41:44–50. doi: 10.1203/00006450-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gourmelen M, Girard F, Binoux M. Serum somatomedin/insulin-like growth factor (IGF) and IGF carrier levels in patients with Cushings syndrome or receiving glucocorticoid therapy. J Clin Endocrinol Metab. 1982;54:885–892. doi: 10.1210/jcem-54-5-885. [DOI] [PubMed] [Google Scholar]
- 22.Miell JP, Taylor AM, Jones J, et al. The effects of dexamethasone treatment on immunoreactive and bioactive insulin-like growth factors (IGFs) and IGF-binding proteins in normal male volunteers. J Endocrinol. 1993;136:525–533. doi: 10.1677/joe.0.1360525. [DOI] [PubMed] [Google Scholar]