Abstract
Sleep disorders constitute major nonmotor features of Parkinson’s disease (PD) that have a substantial effect on patients’ quality of life and can be related to the progression of the neurodegenerative disease. They can also serve as preclinical markers for PD, as it is the case for rapid eye movement (REM)-associated sleep behavior disorder (RBD). Although the etiology of sleep disorders in PD remains undefined, the assessment of the components of the circadian system, including melatonin secretion, could give therapeutically valuable insight on their pathophysiopathology. Melatonin is a regulator of the sleep/wake cycle and also acts as an effective antioxidant and mitochondrial function protector. A reduction in the expression of melatonin MT1 and MT2 receptors has been documented in the substantia nigra of PD patients. The efficacy of melatonin for preventing neuronal cell death and for ameliorating PD symptoms has been demonstrated in animal models of PD employing neurotoxins. A small number of controlled trials indicate that melatonin is useful in treating disturbed sleep in PD, in particular RBD. Whether melatonin and the recently developed melatonergic agents (ramelteon, tasimelteon, agomelatine) have therapeutic potential in PD is also discussed.
Keywords: agomelatine, insomnia, light therapy, melatonin, oxidative stress, Parkinson’s disease, ramelteon, REM sleep behavior disorder, tasimelteon
Introduction
Parkinson's disease (PD) affects several million people worldwide, irrespective of gender, social, ethnic, economic, or geographic boundaries [Elbaz and Moisan, 2008]. Key symptoms, such as tremor, rigidity, bradykinesia and postural instability, develop when about three quarters of dopaminergic cells are lost in the substantia nigra pars compacta, and consequently the smooth, coordinated regulation of striatal motor circuits is hampered [Maguire-Zeiss and Federoff, 2010; Tansey et al. 2007; Zhang et al. 1999]. Other nonmotor symptoms are seen in PD, and some of them, such as hyposmia, depression, or rapid eye movement (REM)-associated sleep behavior disorder (RBD), can precede the onset of disease. Nonmotor symptoms are often misdiagnosed and untreated although their appearance is indicative of a worse prognosis and lower quality of life.
At this time, there is no treatment that will delay or stop the progression of PD, and medications currently available are mostly symptomatic. Although the increasing incidence of age-associated neurodegenerative diseases such as PD has been attributed to the augmented generation of free radicals and the associated oxidative stress, which is enhanced in certain regions of the aging brain [Gibson et al. 2010; Fahn and Cohen, 1992; Olanow, 1992], antioxidants, such as vitamin E that attenuates 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) Parkinsonism in rodents [Itoh et al. 2006], have not had significant effects on the evolution of the disease in humans [The Parkinson Study Group, 1993].
A number of studies have repeatedly documented the prevalence of sleep disturbances in PD, which in some cases was close to 90% [Comella, 2007, 2006; Garcia-Borreguero et al. 2003; Happe et al. 2002; Trenkwalder, 1998; Lees et al. 1988]. In the present manuscript attention is focused on the possible role of sleep disturbance and disruption of circadian rhythm function in the progression of PD. More particularly, the activity of melatonin, whose secretion is a key signal for sleep/wake cycle organization and has a relevant neuroprotective activity in a number of experimental models, is discussed. It has been shown that a decrease in melatonergic receptors occurs in the amygdala and the substantia nigra of PD patients [Adi et al. 2010] and that this reduction coexists with PD-associated sleep disturbances. Further, there is some clinical evidence that melatonin can be a useful add-on therapy for RBD in PD [Aurora et al. 2010].
With the introduction of new melatonergic agents such as ramelteon, tasimelteon, and agomelatine, the opportunity now exists for designing clinical trials to determine whether these agents have merit for treating sleep disorders in PD. Inasmuch as melatonin has been demonstrated to be an effective sleep regulator and multifunctional antioxidant, any possible consideration of its role in the etiology of PD could be relevant not only for providing new insights of the disease, but also for giving background to the therapeutic use of melatonergic agonists in PD.
Basic sleep physiology
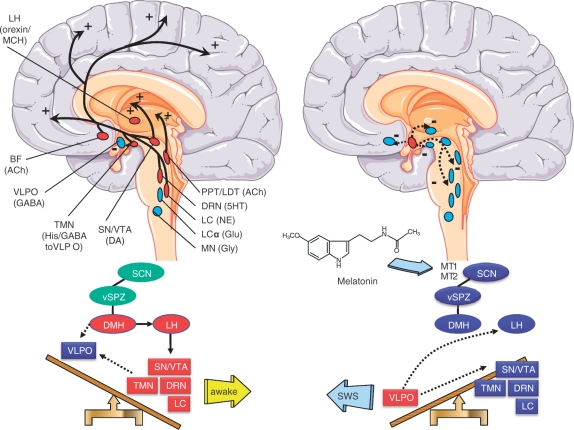
The sleep-switch model (Figure 1), originally proposed by Saper and colleagues, describes flip–flop mutual inhibitions among sleep-associated activities in the ventrolateral preoptic nucleus and wakefulness associated activities in the tuberomammillary nucleus, dorsal raphe nucleus and locus coeruleus (LC) [Saper et al. 2005]. The components of the ascending reticular activating system also include dopamine (DA)-containing neurons in the substantia nigra pars compacta (SN) and the ventral tegmental area (A10 area), the basal forebrain (cholinergic neurons) and the pedunculopontine and laterodorsal tegmenti (PPT/LDT) (cholinergic neurons). Orexin–melanocyte concentrating hormone neurons in the lateral hypothalamus provide stimulatory input to the wakefulness-promoting areas (Figure 1).
Figure 1.
Upper part: Schematic representation (modified from Saper et al. [2005]) of key components of the ‘ascending reticular arousal system’ mediating wakefulness (left) and the inhibitory control on these components during slow-wave sleep (SWS) by GABAergic neurons of the ventrolateral preoptic nucleus (VLPO), located at the bottom of the anterior hypothalamus (right). During wakefulness the histaminergic neurons in the ventral tuberomammillary nucleus (TMN) at the bottom of the posterior hypothalamus provide a strong inhibitory influence to the VLPO. The components of the ascending reticular activating system further include the dorsal raphe nuclei (DRN, 5HT neurons), the locus coeruleus (LC, noradrenergic neurons), the pedunculopontine and laterodorsal tegmenti [PPT/LDT, acetylcholine (Ach)-containing neurons], DA-containing neurons in the substantia nigra (SN) and the ventral tegmental area (VTA), and the basal forebrain (BF, cholinergic neurons). Orexin–melanocyte concentrating hormone (MCH) neurons on the lateral hypothalamus (LH) provide stimulatory input to the wakefulness-promoting areas. The LC-α and magnocellular nuclei (MN), participating in REM-induced atonia (see Figure 2) are also depicted. Red labels denote activation, while blue labels denote inhibition. In PD several of these monoaminergic areas promoting wakefulness degenerate (see the text) which is a feasible explanation for the increased somnolence found. Lower part: the SCN, projecting through the hypothalamic ventral subparaventricular zone (sPVZ), promotes wakefulness principally by augmenting the activity of orexinergic/MCH-containing neurons in LH. Melatonin inhibits via MT1 and MT2 receptors, SCN activity and promotes sleep. In PD a decrease of MT1 and MT2 receptors are found in SN and amygdala [Adi et al. 2010], which could explain the impoverishing of sleep.
The daily sleep/wake cycle is influenced by two separate processes: (1) the endogenous biological clock that drives the circadian rhythm of sleep/wake cycle (Process C, for ‘circadian’) and (2) a homeostatic component (Process S, for ‘sleep’) that influences sleep propensity, a state which is determined by the immediate history of sleep and wakefulness and additionally by the duration of previous sleep episodes [Borbely, 1982]. These two processes, which interact continuously, determine the consolidated bout of sleep at night and the consolidated bout of wakefulness during daytime.
A ‘forced desynchrony paradigm’ (in which the subject’s circadian rhythms are experimentally desynchronized) has been used to study the contribution of each of these components. Observations of subjects whose circadian rhythms had been experimentally desynchronized have supported the inference that REM sleep is driven by the circadian component whereas slow-wave sleep (SWS) (or non-REM sleep) is driven by the homeostatic component [Scheer et al. 2007].
The circadian system is also known as a wake-promoting system because it determines the strength of wakefulness [Borbely, 1982]. The circadian rhythm in the secretion of the pineal hormone melatonin has been shown to be responsible for the sleep rhythm in both normal and blind subjects (i.e. in the absence of the synchronizing effect of light). It is possible that melatonin feedbacks at the suprachiasmatic nucleus (SCN) to inhibit the circadian signal responsible for promoting wakefulness [Wehr et al. 2001; Dijk et al. 1997; Sack et al. 1992] (Figure 1).
Since the SCN interacts with both sleep regulatory mechanisms, Process S and Process C, it was suggested that a functional disruption of the SCN is involved in disorders of sleep and wakefulness [Saper et al. 2005]. The role of the SCN in sleep regulation was first studied in the squirrel monkey. In this primate species lesions made in the SCN caused either loss of sleep or prolonged sleep [Edgar et al. 1993]. It is suggested that circadian signals emanating from the SCN promote wakefulness during the day and may facilitate sleep during the subjective night. It is interesting to note that the neural pathways from the SCN that promote wakefulness are complemented by those that are involved in the promotion of sleep (Figure 1) [Saper et al. 2005].
Pathophysiology of sleep disorders in Parkinson’s disease
Several studies have revealed that Lewy’s body pathology, a characteristic feature of cellular PD lesions, is pronounced not only in SN but also in other central nervous system (CNS) regions such as the lower brainstem and several nuclei of the autonomic nervous system [Beach et al. 2009; Braak et al. 2007, 2003]. According to Braak and colleagues, the first stage of PD involves deposition of α-synuclein in the anterior olfactory nucleus, the olfactory bulb, and the dorsal motor nucleus of the vagus [Braak et al. 2007, 2003]. In addition, neurons of the unmyelinated lamina-1 spinal cord and peripheral autonomic ganglia are also affected. Stage 2 is characterized by neurodegenerative processes comprising brainstem cholinergic, serotoninergic, and noradrenergic regions, such as the PPT/LDT, LC and the and reticular-activating system, the dopaminergic neurons of the SN being affected only at stage 3. At more advanced stages (4–6) cortical neurons become affected [Beach et al. 2009; Braak et al. 2007, 2003].
Despite some debates on the preclinical processes [Wu et al. 2011], there can be no doubt about a remarkably extended period of neurological changes prior to the appearance of classic PD symptoms. In several cases, these changes have been assumed to date back to the perinatal period [Wu et al. 2011].
This particular pattern of neurodegeneration explains why sleep disorders have such a high prevalence rate in PD and why they deserve consideration for clinical prediction of the disease [Postuma et al. 2010]. Indeed, neurological nonmotor and psychiatric manifestations of PD often precede the traditionally recognized motor manifestations [Postuma et al. 2010; Savica et al. 2010]. Case–control and cohort studies suggest that depression and anxiety disorders are also one of the earliest manifestations of PD [Postuma et al. 2010; Weisskopf et al. 2003; Shiba et al. 2000]. Since sleep disturbances in PD patients are associated with cognitive decline and psychiatric symptoms [Comella, 2007, 2006], it has been suggested that attention be focused on the development of targeted interventions for early correction of nonmotor disorders [Naismith et al. 2010].
Sleep and chronobiological disorders in PD
Most patients with PD experience sleep-related symptoms, such as difficulty in initiating and maintaining sleep, excessive daytime somnolence (EDS), and parasomnias such as RBD [Ceravolo et al. 2010]. When occurring in the absence of medication these PD sleep disorders are classified as primary and are regarded as intrinsic to PD, while those appearing subsequent to medications are classified as secondary [Friedman and Chou, 2004].
The most common sleep-related manifestation of PD is sleep fragmentation, with a prevalence of 39% [Tandberg et al. 1999], concomitant with PD motor disorders [Larsen, 2003] or nocturia [Lees et al. 1988]. Nocturnal motor activity, including off-state dystonia, cramps, tremor or severe akinesia, and rigidity tend to produce painful postures or problems in turning in bed [Chaudhuri et al. 2006; Dhawan et al. 2006b].
Overall, difficulties in falling asleep or in maintaining restorative sleep are found in PD. Disorders of sleep initiation and maintenance are characterized by either a reduction in the stages 3/4 of non-REM sleep or by a decrease in REM sleep [Adler, 2005; Adler and Thorpy, 2005]. They may represent a direct effect of disease on sleep architecture or could be an indirect effect mediated by either the cardinal parkinsonian symptoms or by the medication employed [Suzuki et al. 2007; Kaynak et al. 2005; Garcia-Borreguero et al. 2003; Young et al. 2002].
Restless legs syndrome is also prevalent in PD patients and has been considered the consequence of local iron deficiency in the SN, although evidence is this respect is not compelling [Nomura et al. 2006; Ondo et al. 2002]. About 20% of PD patients exhibit either of obstructive or central type sleep apnea [Arnulf et al. 2002].
EDS [Comella, 2006; Garcia-Borreguero et al. 2003], ultimately affecting health-related quality of life [Weintraub et al. 2004; Scaravilli et al. 2003], affects up to 50% of PD patients [Monderer and Thorpy, 2009; Comella, 2006; Hobson et al. 2002]. Factors that contribute to EDS in PD include PD motor disability, impact of PD and other medications on alertness, presence of depression and dementia, and concurrent medical illnesses [Simuni, 2004]. The severity of EDS does not correlate with the degree of nocturnal sleep impairment in PD [Arnulf et al. 2002] and increases with the progression of the disease. Although it has been suggested that intake of both dopaminergic agonists and levodopa are contributing factors in the development of EDS [Mehta et al. 2008], it could also be due to the underlying pathology of PD [Erro et al. 2010; Arnulf et al. 2002]. In a study performed in one of our laboratories, day-to-day sleep quality self-evaluations by sleep log and actigraphy, PD sleep scale (PDSS), daytime somnolence and sleep log daily wellbeing could differentiate PD patients from healthy controls, but only daily wellbeing segregated moderately/severely affected PD patients from the less affected patients [Pérez Lloret et al. 2009]. Nocturnal activity, as assessed by actigraphy, increased only in PD patients taking higher doses of levodopa, independently of their disease’s severity. Multivariate regression analysis showed that PD severity and depression were the only predictors of reduced sleep quality [Pérez Lloret et al. 2009].
Neuropsychiatric symptoms, such as vivid dreams, nightmares and nocturnal hallucinations, or RBD, are commonly observed in PD [Chaudhuri et al. 2006, 2002; Dhawan et al. 2006a, 2006b; Grandas and Iranzo, 2004]. RBD in PD may occur as a prodromal feature, predating motor symptoms by several years [Postuma et al. 2010]. Its prevalence, which is thought to be 60%, has been identified in longitudinal studies and is suspected to be a predictor for dementia in longitudinal studies [Marion et al. 2008; Vendette et al. 2007]. RBD is characterized by a loss of skeletal muscle atonia with prominent motor activity and dreaming [Boeve et al. 2007a; Olson et al. 2000] (Figure 2).
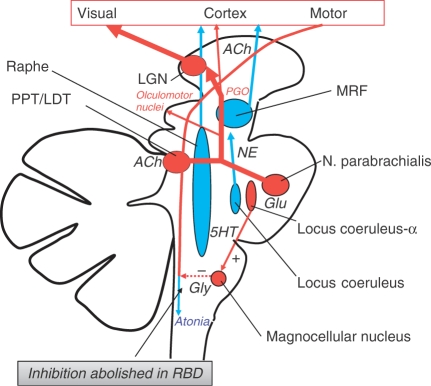
Figure 2.
A schematic drawing of nuclei and neural structures involved in the regulation of REM sleep. Pontogeniculooccipital (PGO) activity is typical of REM sleep and can be recorded in animals with the pons isolated from the rest of the brain. The PGO system can be activated by drugs or lesions that lead to a suppression of 5HT secretion in the Raphe nuclei. PGO generators use acetylcholine (Ach) as a transmitter. During REM, the increasing loss of activity in the locus coeruleus leads to disinhibition of locus coeruleus-α which in turn activates the magnocellular nucleus via glutamatergic (Glu) projections. Glycine (Gly)-mediated inhibition of motor neurons in spinal cord is activated and atonia ensues. The Locus coeruleus-α begins to be active, a few minutes before the actual onset of REM. In PD, loss of REM sleep atonia and/or increased locomotor drive have been suggested as likely mechanisms for the clinical expression of REM-associated sleep behavior disorder (RBD) [Boeve et al. 2007b]. LGN: lateral geniculate nucleus. PPT/LDT: pedunculopontine and laterodorsal tegmenti.
Numerous cases of RBD have been found in clinically diagnosed PD patients [Boeve et al. 2007a; Schenck et al. 1996]. In several studies RBD has been demonstrated to be more frequent in the male than female gender, but the reasons for this male predominance are not yet known [Ceravolo et al. 2010]. Loss of REM sleep atonia and/or increased locomotor drive have been suggested as likely mechanisms for the clinical expression of human RBD [Boeve et al. 2007b] (Figure 2). There is a tendency for the dream content to involve an aggressive, attacking, or chasing theme. Nightmare behaviors such as screaming, kicking, punching, and injuring the bed partner are quite common [Jahan et al. 2009].
Nocturnal disturbance and sleep arousals as measured by actigraphy are specific to RBD seen in PD [Naismith et al. 2010]. A loss of orexin neurons and of cells secreting melanin-concentrating hormone in the hypothalamus of PD patients is said to be responsible for nocturnal insomnia, RBD and hallucinations [Thannickal et al. 2008, 2007]. However, changes in orexin do not necessarily underpin associated RBD sleep disturbances [Compta et al. 2009]. The investigation of the components of the circadian system (that mediates the onset and timing of REM sleep), including the pattern and timing of melatonin secretion, combined with clinicopathological assessments, could be useful for defining the chronobiological correlates of RBD in PD.
Imaging studies have been useful in identifying the role of dopaminergic alterations in the pathogenesis of PD sleep disorders [Mehta et al. 2008]. In a study conducted on 10 PD patients, polysomnographic (PSG) parameters were compared with dopaminergic functions in the striatum and upper brainstem using [18F] fluoro-DOPA (F-DOPA) positron emission tomography. Decreased F-DOPA uptake was found to correlate with increased REM sleep duration in early PD [Hilker et al. 2003]. Treatment of PD with dopaminergic drugs has been shown to promote sleep when given in low doses, but when administered in higher doses it prolonged sleep latency and caused fragmentation of sleep [van Hilten et al. 1994]. Selegiline, when given to PD patients, caused alerting effects and caused difficulty in falling asleep [Lavie et al. 1980]. PD patients when given pergolide as an add-on drug increased nocturnal activity and worsened sleep fragmentation as compared with placebo [Comella, 2006].
Thus, both dopaminergic drugs as well as nondopaminergic drugs commonly used for treatment of PD may affect sleep and degrade the quality of life in these patients. Hence, there is a necessity to introduce clinically effective nontoxic sleep-promoting drugs for treatment of sleep disorders seen in PD. In this context the neurohormone melatonin and particularly its agonists ramelteon, tasimelteon, and agomelatine offer promising features for the treatment of sleep disorders associated with PD.
In the last decade many studies of molecular clock mechanisms involved in regulating the circadian physiology and behavior in mammals have been carried out. These have focused on central circadian pacemaker, the SCN of the anterior hypothalamus, as well as on a number of peripheral tissues and cells [Dibner et al. 2010; Liu et al. 2007]. Several circadian genes, known as ‘clock genes’, have been identified. Among these Per1 and Bmal1 are regarded as the best markers of the molecular clock. Disruptions of Bmal1 and Per1 expression in mice brought about altered circadian behavior and dysregulation of circadian patterns in cell function [Kondratov et al. 2006; Cermakian et al. 2001]. The circadian clock genes Per1 and Bmal1, which are expressed in many peripheral cells, are also located in leukocytes of healthy humans [Fukuya et al. 2007], and studies of these genes have been undertaken in total leukocytes of PD patients [Cai et al. 2010b]. During the dark phase expression of Bmal1 but not of Per1 was much reduced in PD patients, suggesting an alteration of the peripheral molecular clockwork in Parkinsonism. The decrease in expression of Bmal1 in PD patients correlated with the United Parkinson’s Disease Rating Scale score and Pittsburgh Sleep Quality Index score [Cai et al. 2010b].
Alteration of clock gene expression could be the basis for many of the circadian rhythm disturbances that are commonly seen in PD. However, it must noted that the discussion of circadian aspects of PD in a clinically meaningful context is an exceedingly complex problem due to the numerous confounding factors, such as pain, bladder dysfunction, sedation from medications, depression, anxiety, daytime naps, etc. Therefore, caution is needed in interpreting clinical data.
Parkinsonian symptoms themselves undergo circadian fluctuations [Willis, 2008]. Patients with PD often experience worsening of symptoms in the afternoon and the evening. Patients with PD also experience time-dependent responsiveness to dopaminergic stimulation [Bruguerolle and Simon, 2002]. In PD patients Fertl and coworkers reported that levodopa-treated patients but not the ‘de novo’ patients showed a phase advance of the melatonin circadian rhythm [Fertl et al. 1993, 1991]. Bordet and colleagues confirmed those results and showed no changes in locomotor activity, cortisol nor temperature rhythms in ‘de novo’ PD patients [Bordet et al. 2003]. In this study a decrease in amplitude of circulating melatonin rhythm was also observed.
Corresponding data were obtained in a recent study performed in one of our laboratories in which untreated (‘de novo’), levodopa-treated without motor fluctuations (stable) and levodopa-treated fluctuating idiopathic PD patients were examined in their sleep/wake rhythmicity [Pérez Lloret et al. 2010]: treated patients of stable and fluctuating groups showed significantly earlier waking and ending of the immobility time. Indeed, sleep/wake rhythm alterations in PD patients are expressed along a continuum, varying as a function of levodopa-treatment intensity.
A primary approach to the treatment of sleep disorders in PD concerns to sleep hygiene, since so far there are no controlled data on any specific hypnotic agent in PD. Since previous sleep hygiene measures are often not sufficient, it is recommended that hypnotic agents must be prescribed with caution with close monitoring for potential side effects, specifically confusion and daytime sleepiness [Ceravolo et al. 2010]. This relative lack of safe pharmacological tools to treat PD sleep disorders has focused interest on recently developed pharmacological agents such as ramelteon, agomelatine, and tasimelteon that are melatonin receptor agonists which, compared with melatonin itself, have a longer half life and greater affinity for melatonin receptors. Consequently, they are thought to hold promise for treating a variety of sleep disorders.
One important point when dealing with the effect of melatonin analogs is to understand that they are not regular hypnotic drugs resembling benzodiazepines and their derivatives. Melatonin-like compounds exert a promoting effect on sleep by amplifying day/night differences in alertness and sleep quality and displaying a modest sleep inducing effect, quite mild as compared with the efficacy of benzodiazepines.
Melatonin: physiological role
Melatonin, which occurs ubiquitously in nature, is a remarkable molecule with diverse physiological functions [Hardeland et al. 2011; Reiter et al. 2010; Pandi-Perumal et al. 2006b]. In mammals circulating melatonin is synthesized by the pineal gland with a nocturnal maximum of about 200 pg/ml and a daytime nadir of less than 10 pg/ml [Arendt and Skene, 2005]. Although synthesis also occurs in various other regions of the body such as the gastrointestinal tract, skin, lymphocytes, platelets, bone marrow cells, retina, or thymus [Hardeland et al. 2011; Reiter et al. 2010; Pandi-Perumal et al. 2006b], in all of these tissues melatonin has either an autocrine or a paracrine role.
Once formed melatonin is not stored within the pineal gland but diffuses into the capillary blood and cerebrospinal fluid (CSF) [Tricoire et al. 2003]. In a recent study conducted in humans, CSF melatonin levels were found to be higher in the third ventricle compared with the lateral one, thus indicating that melatonin enters the CSF through the pineal recess, even during daytime [Leston et al. 2010]
In all mammals pineal melatonin biosynthesis is synchronized to light/dark cycle by the SCN, which receives its input from the retinohypothalamic tract. Special photoreceptive retinal ganglion cells containing melanopsin as a photopigment are involved in the projection from retina [Berson et al. 2002; Brainard et al. 2001]. Fibers from the SCN pass through a circuitous route involving the paraventricular nucleus of the hypothalamus, medial forebrain bundle, reticular formation, lateral horn cells of the spinal cord, superior cervical ganglion, and then proceed to innervate pineal gland as postganglionic sympathetic fibers. NE released from these fibers regulate melatonin biosynthesis mainly through a β-adrenergic receptors–adenylyl cyclase–cyclic AMP mechanism [Klein et al. 1971].
In the circulation melatonin is partially bound to albumin [Cardinali et al. 1972], and can also bind to hemoglobin [Gilad and Zisapel, 1995]. Circulating melatonin is metabolized mainly in the liver where it is first hydroxylated by cytochrome P450 mono-oxygenases (isoenzymes CYP1B1, CYP1A2, CYP1A1) and thereafter conjugated with sulphate to be excreted as 6-sulfatoxymelatonin. Melatonin can be metabolized nonenzymatically in all cells of the body. It is converted into 3-hydroxymelatonin when it scavenges two hydroxyl radicals [Tan et al. 1998]. In the brain a substantial amount of melatonin can be metabolized to kynuramine derivatives [Hirata et al. 1974], especially under brain inflammatory conditions [Hardeland et al. 2009; Silva et al. 2005]. These metabolites of melatonin which are formed in the brain, namely, N1-acetyl-N2-formyl-5-methoxy kynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), also share the antioxidant and anti-inflammatory properties of melatonin [Hardeland et al. 2009; Tan et al. 2001].
Melatonin exerts its physiological actions through G-protein MT1 and MT2 melatonin receptors expressed both singly and together in various cells and tissues of the body [Dubocovich et al. 2010]. Functional melatonin receptors have been localized in different areas of the brain such as the SCN [Liu et al. 1997], cerebellum [Al Ghoul et al. 1998], hippocampus [Savaskan et al. 2002] and central dopaminergic pathways, including the SN, caudate putamen, ventral tegmental areas, and nucleus accumbens [Uz et al. 2005]. MT1 and MT2 receptors, which have been found in the human amygdala and SN of normal subjects, have shown decreased expression in patients with PD [Adi et al. 2010].
Melatonin exerts some of its actions by binding to calmodulin [Benitez-King, 2006; Benitez-King and Anton-Tay, 1993], as well as to nuclear receptors of retinoic acid receptor family RZR β, RORα-1, and RORα-2 [Wiesenberg et al. 1998]. Melatonin’s antioxidant actions comprise receptor-dependent and receptor-independent effects and, in particular, modulation of mitochondrial metabolism [Hardeland et al. 2011].
Melatonin is involved in the control of various physiological functions of the body such as seasonal control of reproductive processes [Srinivasan et al. 2009b; Reiter, 1980], sleep regulation [Wurtman and Zhdanova, 1995], immune mechanisms [Srinivasan et al. 2005; Guerrero and Reiter, 2002] and regulation of circadian [Deacon and Arendt, 1995; Armstrong, 1989] and sleep/wake rhythms [Arendt and Skene, 2005; Rajaratnam et al. 2004]. In addition to the above-mentioned physiological actions melatonin in pharmacological doses inhibits tumor growth and may have a potential therapeutic value in treating breast cancer, prostate cancer, melanoma, and cancer of gastrointestinal tract [Srinivasan et al. 2008; Blask et al. 2005]. Melatonin also exerts antinociceptive and antiallodynic actions [Srinivasan et al. 2010]. As the prototype of the chronobiotic class of drugs [Cardinali et al. 2006; Arendt and Skene, 2005; Pévet et al. 2002; Dawson and Armstrong, 1996] melatonin restores the phase and amplitude of circadian rhythmicity by interaction with MT1 and MT2 receptors expressed in the SCN.
Conflicting results have been published on the circulating levels of melatonin in PD patients. In one study in which the levels of melatonin were measured twice daily at morning hours (10:00 and 12:00) the circulating melatonin levels from healthy subjects were lower than those of PD patients [Catala et al. 1997]. No information on nocturnal melatonin levels was provided in this study. Phase advances of the melatonin circadian rhythm were reported in PD patients [Bordet et al. 2003; Fertl et al. 1993, 1991] and a decrease in the amplitude of circulating melatonin rhythm was also observed [Bordet et al. 2003].
Owing to the lower rates of cancer mortality/incidence in patients with PD, speculations about risk or preventative factors common to both diseases, including lifestyle factors (such as smoking) and genetic susceptibility have been entertained [Rod et al. 2010]. Relevant to the subject of the present review is that preliminary epidemiological evidence suggests that longer years of working night shifts is associated with reduced melatonin levels and reduced risk of PD among whereas longer hours of sleep appear to increase their risk [Schernhammer et al. 2006]. While lower melatonin concentrations may predict a higher cancer risk [Stevens et al. 2011], there is also some evidence that they may be associated with a lower risk of PD.
Melatonin’s neuroprotective role in animal models of Parkinson’s disease
Animal models employed for studying the efficacy of various therapeutic agents in PD commonly produce behavioral deficits by suppressing brain dopaminergic function [Terzioglu and Galter, 2008; Schober, 2004]. This is achieved by injecting 6-hydroxydopamine (6-OHDA) into the nigrostriatal pathway of the rat or by the systemic or intracerebral administration of neurotoxins such as MPTP, rotenone, or maneb–paraquat. The loss of dopamine neurons occurring in these animal models causes severe sensory and motor impairment which in turn gives rise to tremor, rigidity, and akinesia similar to those seen in PD patients [Terzioglu and Galter, 2008; Schober, 2004]. A major concern on these neurotoxin models is the obvious difference between models comprising attenuation of disease symptoms and models of the disease process itself, e.g. the α-synuclein knock out model [Ubhi et al. 2010; Rockenstein et al. 2007]. There is no information on melatonin efficacy to modify PD phenotype in genetic models of PD.
In a study using the MPTP model to produce PD symptoms, melatonin administration was found to counteract MPTP-induced lipid peroxidation in the striatum, hippocampal, and midbrain regions [Acuña-Castroviejo et al. 1997]. Using the same MPTP model, melatonin’s ability to prevent neuronal cell death in the nigrostriatal pathway was demonstrated [Antolin et al. 2002].
MPTP elicits its neurotoxic effects by increasing the amount NO radicals derived from inducible nitric oxide synthase (iNOS) [Terzioglu and Galter, 2008; Schober, 2004]. The NO radicals act mainly on the cell bodies of dopamine neurons while NO radicals derived from the neuronal isoform of NOS (nNOS) damage the dopaminergic fibers and terminals in the striatum. Hence, it has been suggested that agents which inhibit the degenerative effects of iNOS stimulation in the SN should be considered as potential candidates for treating PD [Zhang et al. 2000]. Inasmuch as melatonin can effectively downregulate iNOS and prevent NO radical formation in the brain, it may have the potential to become a therapeutic agent in PD. The neuroprotective action of melatonin in the 6-OHDA animal model of PD has also been documented in other studies [Thomas and Mohanakumar, 2004; Dabbeni-Sala et al. 2001]. In addition, administration of melatonin in various doses (10–30 mg/kg) attenuated the rotenone-induced reduced glutathione depletion and increased the activity of the antioxidant enzymes superoxide dismutase and catalase in the nigroestriatal pathway [Saravanan et al. 2007].
It has been suggested that 6-OHDA could be generated in the brain after increasing dopamine levels in excess and that the increased production of this neurotoxin could be the reason for selective degeneration of the SN in PD. Following levodopa administration, almost every cell in the brain having the aromatic amino acid decarboxylase enzyme will have an excess of dopamine, giving rise to high levels of 6-OHDA in the brain [Maharaj et al. 2005]. To examine the possibility that melatonin might prevent the formation of 6-OHDA in vitro, an experimental design using ferrous-ascorbate dopamine hydroxyl radical generating system, with addition of dopamine (1 mM) was employed [Borah and Mohanakumar, 2009]. The DA dependent production of 6-OHDA in vitro was decreased by melatonin in a dose-dependent manner. In vivo, daily administration of melatonin (30 mg/kg) along with levodopa was administered for seven consecutive days to mice and was found to reduce significantly the levodopa- or levodopa + MPTP-induced generation of striatal 6-OHDA [Borah and Mohanakumar, 2009].
Oxidative stress is known as one of the mechanisms of maneb- and paraquat-induced nigrostriatal dopaminergic neurodegeneration leading to PD phenotype. A recent study described melatonin efficacy to protect the SN of mice in this experimental model. Melatonin (30 mg/kg) for 9 weeks delayed degeneration, as indicated by changes in locomotor activity, striatal dopamine content, tyrosine hydroxylase immunoreactivity, number of degenerating neurons, lipid peroxidation, and nitrite content, as well as mRNA expression and catalytic activities of vesicular monoamine transporter, cytochrome P-450 2E1 and glutathione-S-transferase A4-4, and protein expressions of unphosphorylated and phosphorylated p53, Bax and caspase 9 [Singhal et al. 2011]. By downregulation of oxidative stress and the apoptotic pathway, melatonin displays nigrostriatal dopaminergic neuroprotection in mice.
It should be noted that contradictory results on the preventive role of melatonin in neurotoxin models of PD are also published. The chronic administration of pharmacological levels of melatonin did not ameliorate the MPTP-induced degeneration of the nigrostriatal pathway in mice [Morgan and Nelson, 2001; van der Schyf et al. 2000] and in one study [Willis and Armstrong, 1999] light exposure significantly reduced the severity of PD symptoms both under 6-OHDA and MPTP treatment of rats, whereas intracerebroventricular implants of slow release melatonin increased the severity of the symptoms.
The alteration of circadian rhythms has been documented in animals with experimental lesions of basal ganglia [Mena-Segovia et al. 2002; Ben and Bruguerolle, 2000]. In rats receiving striatal injections of 6-OHDA, the amplitude of circadian rhythms in body temperature, heart rate, and activity rhythm was not altered, but the acrophase (i.e. the time of the maximum values in the rhythm) was advanced as compared with sham-lesioned controls [Ben and Bruguerolle, 2000]. In another study, Mena-Segovia and colleagues reported altered sleep/wake rhythms in striatum-lesioned rats, with increased wakefulness and reduced SWS and little modification of the rhythm’s phase [Mena-Segovia et al. 2002].
Mitochondrial dysfunction in Parkinson’s disease and the neuroprotective effects of melatonin
PD is a neurodegenerative disorder of multifactorial etiology. Among the several putative causal factors, oxidative stress and inflammation have been claimed to play a role in the loss of dopaminergic neurons [Maguire-Zeiss and Federoff, 2010; Tansey et al. 2007; Zhang et al. 1999]. The principal mediators of inflammatory responses in PD are the microglial cells which upon activation release inflammatory cytokines and reactive oxygen species [Tansey and Goldberg, 2010]. In addition, a dysfunctional blood–brain barrier also appears to be involved in the progression of the disease [Monahan et al. 2008; Zlokovic, 2008; Kortekaas et al. 2005].
The MPTP model of PD is a valuable tool for studying not only the participation of various factors such as oxidative/nitrosative stress, excitotoxicity, and inflammation in the pathogenesis of PD, but also for studying the role of mitochondrial dysfunction. MPTP is metabolized into MPP+ (1-methyl-4-phenyl pyridinium) which is taken up into the dopaminergic neurons through dopamine transporter and accumulates in the mitochondria of SN [Terzioglu and Galter, 2008; Schober, 2004]. MPP+ binds to Complex I of the electron transport chain (ETC) to inhibit it, thereby causing increased generation of reactive oxygen species. This results in oxidative damage to ETC, decreased ATP production and nigral cell death [Terzioglu and Galter, 2008; Schober, 2004]. MPP+, by inducing microglial activation and iNOS expression in SN, has been shown to produce large amounts of NO and to cause neuronal cell death [Brown and Bal-Price, 2003]. NO, by reacting with O2- generates the highly toxic peroxynitrite, an agent that impairs mitochondrial function and causes irreversible inhibition of all ETC complexes [Brown and Borutaite, 2004] and neuronal cell death [Zhang et al. 2006].
Recently the participation of mitochondrial iNOS in the mitochondrial dysfunction and nigrostriatal degeneration was studied by using the MPTP model in mice [Tapias et al. 2009]. MPTP administration induced iNOS in the mitochondria of the dopamine neurons leading to high production of NO. Complex I inhibition, NO production and lipid peroxidation were all significantly higher after treatment with MPTP. Treatment with melatonin or AMK counteracted the effects of MPTP in brain nuclei, increasing Complex I activity above the control values in mitochondria. Both melatonin and AMK counteracted the effects of MPTP on lipid peroxidation and nitrite levels in the cytosol and the mitochondria of SN [Tapias et al. 2009]. A remarkable result of this study is that AMK, a brain metabolite of melatonin, was as efficient as melatonin treatment in counteracting i-mtNOS production, oxidative stress, and mitochondrial dysfunction induced by MPTP. The findings of this study raise the intriguing possibility of using kynuramines and related compounds from preventing Complex I dysfunction. Future directions of this study point out to the development of i-mtNOS antagonists such as AMT (2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine) or any other melatonin agonist may have significant value as therapeutic strategies for treating PD, and it is thus suggested that further research to explore this possibility should be undertaken.
Mitochondrial dysfunction is a cell biological characteristic of numerous neurodegenerative diseases and of PD, in particular [Acuña Castroviejo et al. 2011]. This has been demonstrated in detail using the cybrid (cytoplasmic hybrid) technique [Esteves et al. 2010; Borland et al. 2009; Keeney et al. 2009a, 2009b]. These studies convincingly showed defects in Complex I functioning of mitochondria obtained from PD patients. Improvements of Complex I activity by melatonin or AMK are, thus, not only relevant to models based on mitochondrial oxidotoxins, but potentially also to patients. Melatonin’s property of enhancing Complex I activity has been also demonstrated in otherwise untreated, normally aging and senescence-accelerated mouse strains [Carretero et al. 2009; Rodriguez et al. 2008; Okatani et al. 2003]. These data show that it would be a misconception to interpret melatonin’s antioxidant actions only in terms of radical scavenging. Therefore, it is clearly different from other antioxidants, such as tocopherols, which are poorly effective in PD. Apart from its various additional effects on antioxidant enzymes and glutathione levels, melatonin reduces radical formation, e.g. by preventing excessive electron leakage at Complex I [Hardeland et al. 2011].
Melatonin as a therapeutic agent in Parkinson’s disease
The finding that a reduced expression of melatonin MT1 and MT2 receptors occurs in amygdala and SN in patients with PD [Adi et al. 2010] indicates that there is a possibility that the melatonergic system is involved in the abnormal sleep mechanisms seen in PD as well as in its overall pathophysiology. Melatonin has been used for treating sleep problems, insomnia, and daytime sleepiness in PD patients. In a study undertaken on 40 PD patients (11 women, 29 men; range 43–76 years) melatonin was administered for a treatment period of 2 weeks, in doses ranging from 5 to 50 mg/day [Dowling et al. 2005]. To avoid the possibility of producing a circadian shift melatonin was administered 30 min before bedtime (circadian shifts can occur if melatonin is administered at any other time). All subjects were taking stable doses of antiparkinsonian medications during the course of the study. Relative to placebo, treatment with 50 mg of melatonin significantly increased night time sleep, as revealed by actigraphy. As compared with 50 mg or placebo, administration of 5 mg of melatonin was associated with significant improvement of sleep in the subjective reports. The study also found that the high dose of melatonin (50 mg) was well tolerated [Dowling et al. 2005].
In another study 18 patients were randomized after performing a basal PSG examination to receive melatonin (3 mg) or placebo 1 hour before bedtime for 4 weeks [Medeiros et al. 2007]. Subjective sleep quality was assessed by the Pittsburgh Sleep Quality Index and daytime somnolence by the Epworth Sleepiness Scale. All measures were repeated at the end of treatment. On initial assessment, 14 patients (70%) showed poor quality sleep and 8 patients (40%) showed EDS. Increased sleep latency (50%), REM sleep without atonia (66%), and reduced sleep efficiency (72%) were found in PSG. Sleep fragmentation tended to be more severe in patients on lower doses of levodopa. Although melatonin significantly improved subjective quality of sleep PSG abnormalities remained unchanged. Motor dysfunction was not improved by the use of melatonin [Medeiros et al. 2007]. Although undetected differences in motor scores and PSG findings may have been due to the small sample size and a type II error, it must be noted that a significant divergence between PSG and subjective sleep evaluation had been reported in PD patients [Happe et al. 2005]. The findings that subjective more than objective findings of impaired sleep quality have a greater negative impact on life quality [Means et al. 2003] further suggest that PSG procedures may not be the best method for evaluating sleep disturbance in PD.
Melatonin 3–12 mg at bedtime has been shown to be effective in the treatment of RBD. This benefit has been reported in one case report [Kunz and Bes, 1997], two open-label prospective case series of patients with RBD [Takeuchi et al. 2001; Kunz and Bes, 1999] and two retrospective case series [Anderson and Shneerson, 2009; Boeve et al. 2003].Taken together, these reports include a total of 38 patients. Out of these, 31 were noted to experience improvement with melatonin, 2 more experienced transient improvements and 1 seemed to worsen. Follow up as far as 25 months was reported. PSG showed statistically significant decreases in number of R epochs without atonia and in movement time in R. This contrasted with the persistence of tonic muscle tone in R sleep seen with patients treated with clonazepam. Owing to these data a recent clinical consensus recommended melatonin use in RBD at Level B, i.e. ‘assessment supported by sparse high-grade data or a substantial amount of low-grade data and/or clinical consensus by the task force’ [Aurora et al. 2010].
Exposure to light of 1000–1500 lux intensity for 1–1.5 h, 1 h prior to bedtime for 2–5 weeks has been found to improve the bradykinesia and rigidity observed in 12 PD patients [Willis and Turner, 2007]. A reduction in agitation and psychiatric side effects were also reported in this study. The authors suggested that activation of the circadian system by antagonizing melatonin secretion with bright light has a therapeutic value for treating the symptoms of PD [Willis, 2008]. At least one study performed in rats receiving 6-OHDA and MPTP treatment indicated that light exposure significantly reduced the severity of PD symptoms while intracerebroventricular melatonin implants increased the severity of the symptoms [Willis and Armstrong, 1999].
However, a number of observations contradict the conclusion that melatonin promotes PD. Bright light has been employed in a number of studies for treating depressive symptoms and the view has been advanced that suppression of melatonin secretion is not the likely mechanism by which artificial light exerts its therapeutic effect [Rosenthal et al. 1984]. Two possible mechanisms have been proposed for the therapeutic effect of bright light. First bright light could reset the phase of abnormal circadian rhythms seen in depressed patients [Lewy et al. 1984]. Secondly, although evening bright light exposure produces a momentary suppression of melatonin, it actually causes a rebound increase in melatonin secretion late in the night [Beck-Friis et al. 1985]. The fact that bright light exposure ultimately facilitates melatonin secretion rather than suppressing it is said to be responsible for the therapeutic efficacy of bright light in affective disorders. Hence, in the case of PD, bright light may improve the symptoms of PD, not by antagonizing melatonin secretion but by increasing it through a rebound effect.
The bright light effect may be indicative of circadian changes in PD. This may be supported by the reduced Bmal1 mRNA expression in leukocytes [Cai et al. 2010b], although effects in peripheral oscillators do not necessarily allow conclusions on changes in the hypothalamic master clock. The recent finding that the mouse striatal dopamine receptors D1R and D2R are under circadian control [Cai et al. 2010a], can be seen as an interesting facet in this context, although circadian variations in receptor expression are by no means exceptional features. However, the conclusion that PD is a ‘melatonin hyperplasia’ disorder [Willis, 2008], is not supported by these findings. In this case, one would expect to find substantial elevations of melatonin in PD patients relative to age-matched controls, what has not been observed [Bordet et al. 2003; Sandyk, 1997; Fertl et al. 1993, 1991] and a decrease in the amplitude of circulating melatonin rhythm was also observed [Bordet et al. 2003]. Instead, melatonin receptor expression has been found to be reduced in the PD SN [Adi et al. 2010] rather indicating a weakened melatonin signaling in this relevant area. If PD should turn out to be a circadian disorder, one would expect readjustments by melatonin rather than detrimental effects. However, in this case, this would be no longer a matter of dosage only, but even more that of appropriate circadian timing. In a disturbed circadian system, this may differ from that of healthy subjects and, therefore, require specific determinations of phase shifting effects by melatonin in PD patients.
Potential use of melatonin agonists in the treatment of Parkinson’s disease
The available evidence indicates that both sleep induction as well as sleep maintenance at appropriate circadian phases are affected in PD patients. Moreover, the onset and timing of REM sleep is also very much impaired in PD patients [Naismith et al. 2010]. RBD seen in PD patients occurs much earlier and is predictive of dementia [Marion et al. 2008; Vendette et al. 2007]. Treatment of sleep disturbances in PD patients with appropriate drugs may help not only in solving sleep problems but may also help to prevent the progression of PD as well.
Since conventional drugs such as benzodiazepines, which are used for treating insomnia, may worsen the cognitive and memory impairment associated with PD, a hypnotic drug without any of these side effects merits consideration as a therapeutic alternative. Moreover, the finding that melatonin exerts its hypnotic and chronobiotic effects by acting through MT1 and MT2 receptors located in the SCN further supports the conclusion that it may have value as a therapy in PD. Although melatonin significantly improved subjective quality of sleep, PSG sleep abnormalities persisted in PD [Medeiros et al. 2008, 2007]. Since melatonin has a short half life (less than 30 minutes), a melatonin agonist with a longer duration of action and enhanced bioavailability might be of a greater benefit than melatonin in promoting sleep initiation.
Ramelteon is a novel melatonin receptor agonist that has been shown to act on MT1 and MT2 receptors, and has longer duration of action than melatonin [Kato et al. 2005]. The efficacy and safety of ramelteon in treating insomnia have been proven in a number of clinical studies conducted on elderly insomniacs [Richardson et al. 2009; Roth et al. 2007; Zammit et al. 2007; Erman et al. 2006]. Ramelteon may have considerable therapeutic potential in treating the sleep problems including RBD seen in PD. Apart from treating sleep disturbances, ramelteon can alter the sleep/wake rhythm and can correct REM rhythm abnormality. Tasimelteon, another MT1/MT2 agonist in the process of evaluation, has also been shown to be effective for sleep resynchronization [Rajaratnam et al. 2009].
Depression is a common nonmotor symptom in PD. A review of 104 prevalence studies of depression in PD reported a prevalence of major depression of 17% and a prevalence of minor depression and dysthymia of 22 and 13%, respectively [Reijnders et al. 2008]. Depressive disorders may represent the first manifestation of PD years before the onset of motor symptoms [Postuma et al. 2010]. Almost two thirds of subjects meeting the criteria for depression are not treated with a clear impact on disability and on the increased need for symptomatic therapy of PD [Ravina et al. 2009].
PET studies have revealed a dysfunction of dopaminergic and noradrenergic central pathways in depressive Parkinsonian patients [Remy et al. 2005]. In addition, the involvement of the serotoninergic system has been proposed, as shown by postmortem [Paulus and Jellinger, 1991] and PET [Boileau et al. 2008] studies. To what extent depression and sleep disorders are causally linked in PD patients is not known. Evidence from epidemiological and electroencephalographic studies implicate sleep disturbances as key factors in the pathogenesis of depressive illness [Lustberg and Reynolds, 2000]. Other evidence consistent with the circadian disruption hypothesis of depression comes from the observation that more than 80% of depressed patients have complaints of sleep disturbances and demonstrate a variety of PSG abnormalities [Riemann et al. 2001] and further that antidepressant therapies that also improve sleep efficiency are especially effective in reducing depressive symptomatology [Srinivasan et al. 2009a]. Moreover, detailed analyses have shown that currently used antidepressants such as selective serotonin reuptake inhibitors (SSRIs) exert adverse effects on sleep, and that the antidepressant effect may be counteracted by their effects on sleep [Lam, 2006; Moltzen and Bang-Andersen, 2006]. Hence, an ideal antidepressant in PD should improve sleep efficiency and reduce depressive symptomatology. Drugs such as nortrypine and other SSRIs, which have been employed to treat depressive symptoms in PD, have not shown great effectiveness against the associated mood disturbances [Dobkin et al. 2010; Weintraub et al. 2010].
In this context it is worthwhile to mention the newly introduced melatonergic antidepressant, agomelatine. This drug has been tried in major depression disorders and has shown significant antidepressant activity in a number of clinical trials [Eser et al. 2010; Hale et al. 2010; Kasper et al. 2010; Zupancic and Guilleminault, 2006; Loo et al. 2002]. Agomelatine has a dually phased mechanism of action. At night its sleep-promoting melatonergic effects prevail over its potentially antihypnotic 5-HT2c antagonism, while during the day, its antidepressant action via 5-HT2c is uncoupled from melatonin’s nocturnal actions. The sequential mode of action of agomelatine is its major therapeutic advantage over other agents in the antidepressant class [Pandi-Perumal et al. 2006a]. The use of agomelatine in PD may have considerable therapeutic potential because of its dual action for treating both the symptoms of depression and disturbed sleep. Since it also shares melatonin’s chronobiotic properties, it may thus represent an ideal drug for treating PD.
Concluding remarks
At this time, there is no treatment to delay or stop the progression of PD. Rather, the medications currently available aim more towards the alleviation of PD symptoms. New surgical strategies may reversibly switch on the functionally damaged circuits through the electrical stimulation of deep brain structures. Nevertheless, while deep brain stimulation is a major advance in therapy, it is not suitable for all PD patients. New cell therapy approaches may be helpful in the future but are in a starting phase of design. Therefore, symptomatic medication will prevail for a while as a major approach for ameliorating the clinical signs of PD.
Nonmotor symptoms of PD such as RBD, occur in many PD patients, and predate the manifestation of motor symptoms. Their early diagnosis and treatment are essential for improving the quality of life in PD patients. Melatonin and melatonin agonists can be useful tools in treating sleep and associated disorders in PD. Although some of the suggestions put forward in the present manuscript are speculative, the available evidence supports the inference that melatonin activity in PD can be substantial, and, further, that clinical investigations into the nature of this activity are warranted. This review comes at a critical time, as both preclinical and clinical studies strongly suggest melatonin’s efficacy but further analysis is obviously needed. To what extent the therapeutic application of melatonin receptor agonists such as ramelteon or tasimelteon, or the melatonergic antidepressant agomelatine, is useful in PD deserves to be explored.
Acknowledgments
Daniel P. Cardinali is a Research Career Awardee from the Argentine Research Council (CONICET) and Professor Emeritus, University of Buenos Aires.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
S.R. Pandi-Perumal is a stockholder and the President and Chief Executive Officer of Somnogen Inc., a New York Corporation. He declared no competing interests that might be perceived to influence the content of this article. All remaining authors declare that they have no proprietary, financial, professional, nor any other personal interest of any kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
References
- Acuña-Castroviejo D., Coto-Montes A., Gaia M.M., Ortiz G.G., Reiter R.J. (1997) Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci 60: L23–L29 [DOI] [PubMed] [Google Scholar]
- Acuña Castroviejo D., Lopez L.C., Escames G., Lopez A., Garcia J.A., Reiter R.J. (2011) Melatonin-mitochondria Interplay in health and disease. Curr Top Med Chem 11: 221–240 [DOI] [PubMed] [Google Scholar]
- Adi N., Mash D.C., Ali Y., Singer C., Shehadeh L., Papapetropoulos S. (2010) Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med Sci Monit 16: BR61–BR67 [PubMed] [Google Scholar]
- Adler C.H. (2005) Nonmotor complications in Parkinson's disease. Mov Disord 20(Suppl. 11): S23–S29 [DOI] [PubMed] [Google Scholar]
- Adler C.H., Thorpy M.J. (2005) Sleep issues in Parkinson's disease. Neurology 64: S12–S20 [DOI] [PubMed] [Google Scholar]
- Al Ghoul W.M., Herman M.D., Dubocovich M.L. (1998) Melatonin receptor subtype expression in human cerebellum. Neuroreport 9: 4063–4068 [DOI] [PubMed] [Google Scholar]
- Anderson K.N., Shneerson J.M. (2009) Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med 5: 235–239 [PMC free article] [PubMed] [Google Scholar]
- Antolin I., Mayo J.C., Sainz R.M., del Brio M.L., Herrera F., Martin V., et al. (2002) Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Res 943: 163–173 [DOI] [PubMed] [Google Scholar]
- Arendt J., Skene D.J. (2005) Melatonin as a chronobiotic. Sleep Med Rev 9: 25–39 [DOI] [PubMed] [Google Scholar]
- Armstrong S.M. (1989) Melatonin and circadian control in mammals. Experientia 45: 932–938 [DOI] [PubMed] [Google Scholar]
- Arnulf I., Konofal E., Merino-Andreu M., Houeto J.L., Mesnage V., Welter M.L., et al. (2002) Parkinson's disease and sleepiness: an integral part of PD. Neurology 58: 1019–1024 [DOI] [PubMed] [Google Scholar]
- Aurora R.N., Zak R.S., Maganti R.K., Auerbach S.H., Casey K.R., Chowdhuri S., et al. (2010) Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med 6: 85–95 [PMC free article] [PubMed] [Google Scholar]
- Beach T.G., Adler C.H., Lue L., Sue L.I., Bachalakuri J., Henry-Watson J., et al. (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: 613–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Friis J., Kjellman B.F., Aperia B., Unden F., von Rosen D., Ljunggren J.G., et al. (1985) Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand 71: 319–330 [DOI] [PubMed] [Google Scholar]
- Ben V., Bruguerolle B. (2000) Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in the rat: a radiotelemetric study. Life Sci 67: 1549–1558 [DOI] [PubMed] [Google Scholar]
- Benitez-King G. (2006) Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res 40: 1–9 [DOI] [PubMed] [Google Scholar]
- Benitez-King G., Anton-Tay F. (1993) Calmodulin mediates melatonin cytoskeletal effects. Experientia 49: 635–641 [DOI] [PubMed] [Google Scholar]
- Berson D.M., Dunn F.A., Takao M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073 [DOI] [PubMed] [Google Scholar]
- Blask D.E., Dauchy R.T., Sauer L.A. (2005) Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 27: 179–188 [DOI] [PubMed] [Google Scholar]
- Boeve B.F., Dickson D.W., Olson E.J., Shepard J.W., Silber M.H., Ferman T.J., et al. (2007a) Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med 8: 60–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve B.F., Silber M.H., Ferman T.J. (2003) Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med 4: 281–284 [DOI] [PubMed] [Google Scholar]
- Boeve B.F., Silber M.H., Saper C.B., Ferman T.J., Dickson D.W., Parisi J.E., et al. (2007b) Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130: 2770–2788 [DOI] [PubMed] [Google Scholar]
- Boileau I., Warsh J.J., Guttman M., Saint-Cyr J.A., McCluskey T., Rusjan P., et al. (2008) Elevated serotonin transporter binding in depressed patients with Parkinson's disease: a preliminary PET study with [11C]DASB. Mov Disord 23: 1776–1780 [DOI] [PubMed] [Google Scholar]
- Borah A., Mohanakumar K.P. (2009) Melatonin inhibits 6-hydroxydopamine production in the brain to protect against experimental parkinsonism in rodents. J Pineal Res 47: 293–300 [DOI] [PubMed] [Google Scholar]
- Borbely A.A. (1982) A two process model of sleep regulation. Hum Neurobiol 1: 195–204 [PubMed] [Google Scholar]
- Bordet R., Devos D., Brique S., Touitou Y., Guieu J.D., Libersa C., et al. (2003) Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin Neuropharmacol 26: 65–72 [DOI] [PubMed] [Google Scholar]
- Borland M.K., Mohanakumar K.P., Rubinstein J.D., Keeney P.M., Xie J., Capaldi R., et al. (2009) Relationships among molecular genetic and respiratory properties of Parkinson's disease cybrid cells show similarities to Parkinson's brain tissues. Biochim Biophys Acta 1792: 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211 [DOI] [PubMed] [Google Scholar]
- Braak H., Sastre M., Bohl J.R., de Vos R.A., Del Tredici K. (2007) Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113: 421–429 [DOI] [PubMed] [Google Scholar]
- Brainard G.C., Hanifin J.P., Rollag M.D., Greeson J., Byrne B., Glickman G., et al. (2001) Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab 86: 433–436 [DOI] [PubMed] [Google Scholar]
- Brown G.C., Bal-Price A. (2003) Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 27: 325–355 [DOI] [PubMed] [Google Scholar]
- Brown G.C., Borutaite V. (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658: 44–49 [DOI] [PubMed] [Google Scholar]
- Bruguerolle B., Simon N. (2002) Biologic rhythms and Parkinson's disease: a chronopharmacologic approach to considering fluctuations in function. Clin Neuropharmacol 25: 194–201 [DOI] [PubMed] [Google Scholar]
- Cai Y., Ding H., Li N., Chai Y., Zhang Y., Chan P. (2010a) Oscillation development for neurotransmitter-related genes in the mouse striatum. Neuroreport 21: 79–83 [DOI] [PubMed] [Google Scholar]
- Cai Y., Liu S., Sothern R.B., Xu S., Chan P. (2010b) Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. Eur J Neurol 17: 550–554 [DOI] [PubMed] [Google Scholar]
- Cardinali D.P., Furio A.M., Reyes M.P., Brusco L.I. (2006) The use of chronobiotics in the resynchronization of the sleep-wake cycle. Cancer Causes Control 17: 601–609 [DOI] [PubMed] [Google Scholar]
- Cardinali D.P., Lynch H.J., Wurtman R.J. (1972) Binding of melatonin to human and rat plasma proteins. Endocrinology 91: 1213–1218 [DOI] [PubMed] [Google Scholar]
- Carretero M., Escames G., Lopez L.C., Venegas C., Dayoub J.C., Garcia L., et al. (2009) Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res in press [DOI] [PubMed] [Google Scholar]
- Catala M.D., Canete-Nicolas C., Iradi A., Tarazona P.J., Tormos J.M., Pascual-Leone A. (1997) Melatonin levels in Parkinson's disease: drug therapy versus electrical stimulation of the internal globus pallidus. Exp Gerontol 32: 553–558 [DOI] [PubMed] [Google Scholar]
- Ceravolo R., Rossi C., Kiferle L., Bonuccelli U. (2010) Nonmotor symptoms in Parkinson's disease: the dark side of the moon. Future Neurology 5: 851–871 [Google Scholar]
- Cermakian N., Monaco L., Pando M.P., Dierich A., Sassone-Corsi P. (2001) Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J 20: 3967–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri K.R., Healy D.G., Schapira A.H. (2006) Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 5: 235–245 [DOI] [PubMed] [Google Scholar]
- Chaudhuri K.R., Pal S., DiMarco A., Whately-Smith C., Bridgman K., Mathew R., et al. (2002) The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry 73: 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comella C.L. (2006) Sleep disturbances and excessive daytime sleepiness in Parkinson disease: an overview. J Neural Transm Suppl 349–355 [DOI] [PubMed] [Google Scholar]
- Comella C.L. (2007) Sleep disorders in Parkinson's disease: an overview. Mov Disord 22(Suppl. 17): S367–S373 [DOI] [PubMed] [Google Scholar]
- Compta Y., Santamaria J., Ratti L., Tolosa E., Iranzo A., Munoz E., et al. (2009) Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson's disease dementia. Brain 132: 3308–3317 [DOI] [PubMed] [Google Scholar]
- Dabbeni-Sala F., Di Santo S., Franceschini D., Skaper S.D., Giusti P. (2001) Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J 15: 164–170 [DOI] [PubMed] [Google Scholar]
- Dawson D., Armstrong S.M. (1996) Chronobiotics—drugs that shift rhythms. Pharmacol Ther 69: 15–36 [DOI] [PubMed] [Google Scholar]
- Deacon S., Arendt J. (1995) Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res 688: 77–85 [DOI] [PubMed] [Google Scholar]
- Dhawan V., Dhoat S., Williams A.J., DiMarco A., Pal S., Forbes A., et al. (2006a) The range and nature of sleep dysfunction in untreated Parkinson's disease (PD). A comparative controlled clinical study using the Parkinson's disease sleep scale and selective polysomnography. J Neurol Sci 248: 158–162 [DOI] [PubMed] [Google Scholar]
- Dhawan V., Healy D.G., Pal S., Chaudhuri K.R. (2006b) Sleep-related problems of Parkinson's disease. Age Ageing 35: 220–228 [DOI] [PubMed] [Google Scholar]
- Dibner C., Schibler U., Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549 [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Shanahan T.L., Duffy J.F., Ronda J.M., Czeisler C.A. (1997) Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol 505: 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin R.D., Menza M., Bienfait K.L., Gara M., Marin H., Mark M.H., et al. (2010) Depression in Parkinson's disease: symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatry in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling G.A., Mastick J., Colling E., Carter J.H., Singer C.M., Aminoff M.J. (2005) Melatonin for sleep disturbances in Parkinson's disease. Sleep Med 6: 459–466 [DOI] [PubMed] [Google Scholar]
- Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62: 343–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D.M., Dement W.C., Fuller C.A. (1993) Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep–wake regulation. J Neurosci 13: 1065–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A., Moisan F. (2008) Update in the epidemiology of Parkinson's disease. Curr Opin Neurol 21: 454–460 [DOI] [PubMed] [Google Scholar]
- Erman M., Seiden D., Zammit G., Sainati S., Zhang J. (2006) An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med 7: 17–24 [DOI] [PubMed] [Google Scholar]
- Erro M.E., Moreno M.P., Zandio B. (2010) [Pathophysiological bases of the non-motor symptoms in Parkinson's disease]. Rev Neurol 50(Suppl. 2): S7–S13 [PubMed] [Google Scholar]
- Eser D., Baghai T.C., Moller H.J. (2010) Agomelatine: The evidence for its place in the treatment of depression. Core Evid 4: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves A.R., Lu J., Rodova M., Onyango I., Lezi E., Dubinsky R., et al. (2010) Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson's subject mitochondrial transfer. J Neurochem 113: 674–682 [DOI] [PubMed] [Google Scholar]
- Fahn S., Cohen G. (1992) The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol 32: 804–812 [DOI] [PubMed] [Google Scholar]
- Fertl E., Auff E., Doppelbauer A., Waldhauser F. (1991) Circadian secretion pattern of melatonin in Parkinson's disease. J Neural Transm Park Dis Dement Sect 3: 41–47 [DOI] [PubMed] [Google Scholar]
- Fertl E., Auff E., Doppelbauer A., Waldhauser F. (1993) Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect 5: 227–234 [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Chou K.L. (2004) Sleep and fatigue in Parkinson's disease. Parkinsonism Relat Disord 10(Suppl. 1): S27–S35 [DOI] [PubMed] [Google Scholar]
- Fukuya H., Emoto N., Nonaka H., Yagita K., Okamura H., Yokoyama M. (2007) Circadian expression of clock genes in human peripheral leukocytes. Biochem Biophys Res Commun 354: 924–928 [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D., Larrosa O., Bravo M. (2003) Parkinson's disease and sleep. Sleep Med Rev 7: 115–129 [DOI] [PubMed] [Google Scholar]
- Gibson G.E., Starkov A., Blass J.P., Ratan R.R., Beal M.F. (2010) Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta 1802: 122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad E., Zisapel N. (1995) High-affinity binding of melatonin to hemoglobin. Biochem Mol Med 56: 115–120 [DOI] [PubMed] [Google Scholar]
- Grandas F., Iranzo A. (2004) Nocturnal problems occurring in Parkinson's disease. Neurology 63: S8–S11 [DOI] [PubMed] [Google Scholar]
- Guerrero J.M., Reiter R.J. (2002) Melatonin-immune system relationships. Curr Top Med Chem 2: 167–179 [DOI] [PubMed] [Google Scholar]
- Hale A., Corral R.M., Mencacci C., Ruiz J.S., Severo C.A., Gentil V. (2010) Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double-blind study. Int Clin Psychopharmacol 25: 305–314 [DOI] [PubMed] [Google Scholar]
- Happe S., Klosch G., Lorenzo J., Kunz D., Penzel T., Roschke J., et al. (2005) Perception of sleep: subjective versus objective sleep parameters in patients with Parkinson's disease in comparison with healthy elderly controls. Sleep perception in Parkinson's disease and controls. J Neurol 252: 936–943 [DOI] [PubMed] [Google Scholar]
- Happe S., Ludemann P., Berger K. (2002) The association between disease severity and sleep-related problems in patients with Parkinson's disease. Neuropsychobiology 46: 90–96 [DOI] [PubMed] [Google Scholar]
- Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Progr Neurobiol 93: 350–384 [DOI] [PubMed] [Google Scholar]
- Hardeland R., Tan D.X., Reiter R.J. (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 47: 109–116 [DOI] [PubMed] [Google Scholar]
- Hilker R., Razai N., Ghaemi M., Weisenbach S., Rudolf J., Szelies B., et al. (2003) [18F]fluorodopa uptake in the upper brainstem measured with positron emission tomography correlates with decreased REM sleep duration in early Parkinson's disease. Clin Neurol Neurosurg 105: 262–269 [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O., Tokuyama T., Seno S. (1974) In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem 249: 1311–1313 [PubMed] [Google Scholar]
- Hobson D.E., Lang A.E., Martin W.R., Razmy A., Rivest J., Fleming J. (2002) Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 287: 455–463 [DOI] [PubMed] [Google Scholar]
- Itoh N., Masuo Y., Yoshida Y., Cynshi O., Jishage K., Niki E. (2006) γ-Tocopherol attenuates MPTP-induced dopamine loss more efficiently than α-tocopherol in mouse brain. Neurosci Lett 403: 136–140 [DOI] [PubMed] [Google Scholar]
- Jahan I., Hauser R.A., Sullivan K.L., Miller A., Zesiewicz T.A. (2009) Sleep disorders in Parkinson's disease. Neuropsychiatr Dis Treat 5: 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S., Hajak G., Wulff K., Hoogendijk W.J., Montejo A.L., Smeraldi E., et al. (2010) Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry 71: 109–120 [DOI] [PubMed] [Google Scholar]
- Kato K., Hirai K., Nishiyama K., Uchikawa O., Fukatsu K., Ohkawa S., et al. (2005) Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48: 301–310 [DOI] [PubMed] [Google Scholar]
- Kaynak D., Kiziltan G., Kaynak H., Benbir G., Uysal O. (2005) Sleep and sleepiness in patients with Parkinson's disease before and after dopaminergic treatment. Eur J Neurol 12: 199–207 [DOI] [PubMed] [Google Scholar]
- Keeney P.M., Dunham L.D., Quigley C.K., Morton S.L., Bergquist K.E., Bennett J.P., Jr (2009a) Cybrid models of Parkinson's disease show variable mitochondrial biogenesis and genotype-respiration relationships. Exp Neurol 220: 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney P.M., Quigley C.K., Dunham L.D., Papageorge C.M., Iyer S., Thomas R.R., et al. (2009b) Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther 20: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.C., Weller J.L., Moore R.Y. (1971) Melatonin metabolism: neural regulation of pineal serotonin: acetyl coenzyme A N-acetyltransferase activity. Proc Natl Acad Sci U S A 68: 3107–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov R.V., Kondratova A.A., Gorbacheva V.Y., Vykhovanets O.V., Antoch M.P. (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas R., Leenders K.L., van Oostrom J.C., Vaalburg W., Bart J., Willemsen A.T., et al. (2005) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57: 176–179 [DOI] [PubMed] [Google Scholar]
- Kunz D., Bes F. (1997) Melatonin effects in a patient with severe REM sleep behavior disorder: case report and theoretical considerations. Neuropsychobiology 36: 211–214 [DOI] [PubMed] [Google Scholar]
- Kunz D., Bes F. (1999) Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord 14: 507–511 [DOI] [PubMed] [Google Scholar]
- Lam R.W. (2006) Sleep disturbances and depression: a challenge for antidepressants. Int Clin Psychopharmacol 21(Suppl. 1): S25–S29 [DOI] [PubMed] [Google Scholar]
- Larsen J.P. (2003) Sleep disorders in Parkinson's disease. Adv Neurol 91: 329–334 [PubMed] [Google Scholar]
- Lavie P., Wajsbort J., Youdim M.B. (1980) Deprenyl does not cause insomnia in parkinsonian patients. Commun Psychopharmacol 4: 303–307 [PubMed] [Google Scholar]
- Lees A.J., Blackburn N.A., Campbell V.L. (1988) The nighttime problems of Parkinson's disease. Clin Neuropharmacol 11: 512–519 [DOI] [PubMed] [Google Scholar]
- Leston J., Harthe C., Brun J., Mottolese C., Mertens P., Sindou M., et al. (2010) Melatonin is released in the third ventricle in humans. A study in movement disorders. Neurosci Lett 469: 294–297 [DOI] [PubMed] [Google Scholar]
- Lewy A.J., Sack R.A., Singer C.L. (1984) Assessment and treatment of chronobiologic disorders using plasma melatonin levels and bright light exposure: the clock-gate model and the phase response curve. Psychopharmacol Bull 20: 561–565 [PubMed] [Google Scholar]
- Liu C., Weaver D.R., Jin X., Shearman L.P., Pieschl R.L., Gribkoff V.K., et al. (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102 [DOI] [PubMed] [Google Scholar]
- Liu S., Cai Y., Sothern R.B., Guan Y., Chan P. (2007) Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice. Chronobiol Int 24: 793–820 [DOI] [PubMed] [Google Scholar]
- Loo H., Hale A., D'haenen H. (2002) Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2C antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int Clin Psychopharmacol 17: 239–247 [DOI] [PubMed] [Google Scholar]
- Lustberg L., Reynolds C.F. (2000) Depression and insomnia: questions of cause and effect. Sleep Med Rev 4: 253–262 [DOI] [PubMed] [Google Scholar]
- Maguire-Zeiss K.A., Federoff H.J. (2010) Future directions for immune modulation in neurodegenerative disorders: focus on Parkinson's disease. J Neural Transm 117: 1019–1025 [DOI] [PubMed] [Google Scholar]
- Maharaj H., Sukhdev M.D., Scheepers M., Mokokong R., Daya S. (2005) l-DOPA administration enhances 6-hydroxydopamine generation. Brain Res 1063: 180–186 [DOI] [PubMed] [Google Scholar]
- Marion M.H., Qurashi M., Marshall G., Foster O. (2008) Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson's disease? J Neurol 255: 192–196 [DOI] [PubMed] [Google Scholar]
- Means M.K., Edinger J.D., Glenn D.M., Fins A.I. (2003) Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med 4: 285–296 [DOI] [PubMed] [Google Scholar]
- Medeiros C.A., Carvalhedo de Bruin P.F., Lopes L.A., Magalhaes M.C., de Lourdes S.M., Sales de Bruin V. (2007) Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease: A randomized, double blind, placebo-controlled study. J Neurol 254: 459–464 [DOI] [PubMed] [Google Scholar]
- Medeiros C.A., Carvalhedo de Bruin P.F., Lopes L.A., Magalhaes M.C., de Lourdes S.M., Sales de Bruin V. (2008) Melatonin in Parkinson's disease. J Neurol 255: 758–758 [DOI] [PubMed] [Google Scholar]
- Mehta S.H., Morgan J.C., Sethi K.D. (2008) Sleep disorders associated with Parkinson's disease: role of dopamine, epidemiology, and clinical scales of assessment. CNS Spectr 13: 6–11 [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J., Cintra L., Prospero-Garcia O., Giordano M. (2002) Changes in sleep-waking cycle after striatal excitotoxic lesions. Behav Brain Res 136: 475–481 [DOI] [PubMed] [Google Scholar]
- Moltzen E.K., Bang-Andersen B. (2006) Serotonin reuptake inhibitors: the corner stone in treatment of depression for half a century—a medicinal chemistry survey. Curr Top Med Chem 6: 1801–1823 [DOI] [PubMed] [Google Scholar]
- Monahan A.J., Warren M., Carvey P.M. (2008) Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant 17: 363–372 [PubMed] [Google Scholar]
- Monderer R., Thorpy M. (2009) Sleep disorders and daytime sleepiness in Parkinson's disease. Curr Neurol Neurosci Rep 9: 173–180 [DOI] [PubMed] [Google Scholar]
- Morgan W.W., Nelson J.F. (2001) Chronic administration of pharmacological levels of melatonin does not ameliorate the MPTP-induced degeneration of the nigrostriatal pathway. Brain Res 921: 115–121 [DOI] [PubMed] [Google Scholar]
- Naismith S.L., Rogers N.L., Mackenzie J., Hickie I.B., Lewis S.J. (2010) The relationship between actigraphically defined sleep disturbance and REM sleep behaviour disorder in Parkinson's Disease. Clin Neurol Neurosurg 112: 420–423 [DOI] [PubMed] [Google Scholar]
- Nomura T., Inoue Y., Miyake M., Yasui K., Nakashima K. (2006) Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson's disease. Mov Disord 21: 380–384 [DOI] [PubMed] [Google Scholar]
- Okatani Y., Wakatsuki A., Reiter R.J., Miyahara Y. (2003) Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int J Biochem Cell Biol 35: 367–375 [DOI] [PubMed] [Google Scholar]
- Olanow C.W. (1992) An introduction to the free radical hypothesis in Parkinson's disease. Ann Neurol 32(Suppl): S2–S9 [DOI] [PubMed] [Google Scholar]
- Olson E.J., Boeve B.F., Silber M.H. (2000) Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain 123: 331–339 [DOI] [PubMed] [Google Scholar]
- Ondo W.G., Vuong K.D., Jankovic J. (2002) Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 59: 421–424 [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal S.R., Srinivasan V., Cardinali D.P., Monti M.J. (2006a) Could agomelatine be the ideal antidepressant? Expert Rev Neurother 6: 1595–1608 [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal S.R., Srinivasan V., Maestroni G.J.M., Cardinali D.P., Poeggeler B., Hardeland R. (2006b) Melatonin: Nature's most versatile biological signal? FEBS J 273: 2813–2838 [DOI] [PubMed] [Google Scholar]
- Paulus W., Jellinger K. (1991) The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol 50: 743–755 [DOI] [PubMed] [Google Scholar]
- Pérez Lloret S., Rossi M., Cardinali D.P., Merello M. (2010) Activity-rest rhythm abnormalities in Parkinson's disease patients are related to dopaminergic therapy. Int J Neurosci 120: 11–16 [DOI] [PubMed] [Google Scholar]
- Pérez Lloret S., Rossi M., Nouzeilles M.I., Trenkwalder C., Carreras L.O., Merello M. (2009) Parkinson's disease sleep scale, sleep logs, and actigraphy in the evaluation of sleep in parkinsonian patients. J Neurol 256: 1480–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pévet P., Bothorel B., Slotten H., Saboureau M. (2002) The chronobiotic properties of melatonin. Cell Tissue Res 309: 183–191 [DOI] [PubMed] [Google Scholar]
- Postuma R.B., Gagnon J.F., Montplaisir J. (2010) Clinical prediction of Parkinson's disease: planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry 81: 1008–1013 [DOI] [PubMed] [Google Scholar]
- Rajaratnam S.M., Middleton B., Stone B.M., Arendt J., Dijk D.J. (2004) Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol 561: 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam S.M., Polymeropoulos M.H., Fisher D.M., Roth T., Scott C., Birznieks G., et al. (2009) Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet 373: 482–491 [DOI] [PubMed] [Google Scholar]
- Ravina B., Elm J., Camicioli R., Como P.G., Marsh L., Jankovic J., et al. (2009) The course of depressive symptoms in early Parkinson's disease. Mov Disord 24: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders J.S., Ehrt U., Weber W.E., Aarsland D., Leentjens A.F. (2008) A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 23: 183–189 [DOI] [PubMed] [Google Scholar]
- Reiter R.J. (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1: 109–131 [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.X., Fuentes-Broto L. (2010) Melatonin: a multitasking molecule. Prog Brain Res 181: 127–151 [DOI] [PubMed] [Google Scholar]
- Remy P., Doder M., Lees A., Turjanski N., Brooks D. (2005) Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128: 1314–1322 [DOI] [PubMed] [Google Scholar]
- Richardson G.S., Zammit G., Wang-Weigand S., Zhang J. (2009) Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry 70: 467–476 [DOI] [PubMed] [Google Scholar]
- Riemann D., Berger M., Voderholzer U. (2001) Sleep and depression—results from psychobiological studies: an overview. Biol Psychol 57: 67–103 [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Crews L., Masliah E. (2007) Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev 59: 1093–1102 [DOI] [PubMed] [Google Scholar]
- Rod N.H., Hansen J., Schernhammer E., Ritz B. (2010) Major life events and risk of Parkinson's disease. Mov Disord 25: 1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.I., Escames G., Lopez L.C., Lopez A., Garcia J.A., Ortiz F., et al. (2008) Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol 43: 749–756 [DOI] [PubMed] [Google Scholar]
- Rosenthal N.E., Sack D.A., Gillin J.C., Lewy A.J., Goodwin F.K., Davenport Y., et al. (1984) Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41: 72–80 [DOI] [PubMed] [Google Scholar]
- Roth T., Seiden D., Wang-Weigand S., Zhang J. (2007) A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin 23: 1005–1014 [DOI] [PubMed] [Google Scholar]
- Sack R.L., Lewy A.J., Blood M.L., Keith L.D., Nakagawa H. (1992) Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab 75: 127–134 [DOI] [PubMed] [Google Scholar]
- Sandyk R. (1997) The accelerated aging hypothesis of Parkinson's disease is not supported by the pattern of circadian melatonin secretion. Int J Neurosci 90: 271–275 [DOI] [PubMed] [Google Scholar]
- Saper C.B., Lu J., Chou T.C., Gooley J. (2005) The hypothalamic integrator for circadian rhythms. Trends Neurosci 28: 152–157 [DOI] [PubMed] [Google Scholar]
- Saravanan K.S., Sindhu K.M., Mohanakumar K.P. (2007) Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res 42: 247–253 [DOI] [PubMed] [Google Scholar]
- Savaskan E., Olivieri G., Meier F., Brydon L., Jockers R., Ravid R., et al. (2002) Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer's disease patients. J Pineal Res 32: 59–62 [DOI] [PubMed] [Google Scholar]
- Savica R., Rocca W.A., Ahlskog J.E. (2010) When does Parkinson disease start? Arch Neurol 67: 798–801 [DOI] [PubMed] [Google Scholar]
- Scaravilli T., Gasparoli E., Rinaldi F., Polesello G., Bracco F. (2003) Health-related quality of life and sleep disorders in Parkinson's disease. Neurol Sci 24: 209–210 [DOI] [PubMed] [Google Scholar]
- Scheer F.A., Wright K.P., Jr, Kronauer R.E., Czeisler C.A. (2007) Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE 2: e721–e721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck C.H., Bundlie S.R., Mahowald M.W. (1996) Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46: 388–393 [DOI] [PubMed] [Google Scholar]
- Schernhammer E., Chen H., Ritz B. (2006) Circulating melatonin levels: possible link between Parkinson's disease and cancer risk? Cancer Causes Control 17: 577–582 [DOI] [PubMed] [Google Scholar]
- Schober A. (2004) Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res 318: 215–224 [DOI] [PubMed] [Google Scholar]
- Shiba M., Bower J.H., Maraganore D.M., McDonnell S.K., Peterson B.J., Ahlskog J.E., et al. (2000) Anxiety disorders and depressive disorders preceding Parkinson's disease: a case–control study. Mov Disord 15: 669–677 [DOI] [PubMed] [Google Scholar]
- Silva S.O., Ximenes V.F., Livramento J.A., Catalani L.H., Campa A. (2005) High concentrations of the melatonin metabolite, N1-acetyl-N2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. J Pineal Res 39: 302–306 [DOI] [PubMed] [Google Scholar]
- Simuni T. (2004) Somnolence and other sleep disorders in Parkinson's disease: the challenge for the practicing neurologist. Neurol Clin 22: S107–S126 [DOI] [PubMed] [Google Scholar]
- Singhal N.K., Srivastava G., Patel D.K., Jain S.K., Singh M.P. (2011) Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res 50: 97–109 [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Maestroni G., Cardinali D., Esquifino A., Perumal S.P., Miller S. (2005) Melatonin, immune function and aging. Immun Ageing 2: 17–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V., Pandi-Perumal S.R., Spence D.W., Moscovitch A., Trakht I., Brown G.M., et al. (2010) Potential use of melatonergic drugs in analgesia: mechanisms of action. Brain Res Bull 81: 362–371 [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Pandi-Perumal S.R., Trakht I., Spence D.W., Hardeland R., Poeggeler B., et al. (2009a) Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Res 165: 201–214 [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Spence D.W., Pandi-Perumal S.R., Trakht I., Cardinali D.P. (2008) Therapeutic actions of melatonin in cancer: possible mechanisms. Integrative Cancer Therapies 7: 189–203 [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Spence W.D., Pandi-Perumal S.R., Zakharia R., Bhatnagar K.P., Brzezinski A. (2009b) Melatonin and human reproduction: shedding light on the darkness hormone. Gynecol Endocrinol 25: 779–785 [DOI] [PubMed] [Google Scholar]
- Stevens R.G., Hansen J., Costa G., Haus E., Kauppinen T., Aronson K.J., et al. (2011) Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occup Environ Med 68: 154–162 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Okuma Y., Hattori N., Kamei S., Yoshii F., Utsumi H., et al. (2007) Characteristics of sleep disturbances in Japanese patients with Parkinson's disease. A study using Parkinson's disease sleep scale. Mov Disord 22: 1245–1251 [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Uchimura N., Hashizume Y., Mukai M., Etoh Y., Yamamoto K., et al. (2001) Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci 55: 267–269 [DOI] [PubMed] [Google Scholar]
- Tan D.X., Manchester L.C., Burkhardt S., Sainz R.M., Mayo J.C., Kohen R., et al. (2001) N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J 15: 2294–2296 [DOI] [PubMed] [Google Scholar]
- Tan D.X., Manchester L.C., Reiter R.J., Plummer B.F., Hardies L.J., Weintraub S.T., et al. (1998) A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun 253: 614–620 [DOI] [PubMed] [Google Scholar]
- Tandberg E., Larsen J.P., Karlsen K. (1999) Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community-based study. Mov Disord 14: 922–927 [DOI] [PubMed] [Google Scholar]
- Tansey M.G., Goldberg M.S. (2010) Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37: 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey M.G., McCoy M.K., Frank-Cannon T.C. (2007) Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 208: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V., Escames G., Lopez L.C., Lopez A., Camacho E., Carrion M.D., et al. (2009) Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res 87: 3002–3010 [DOI] [PubMed] [Google Scholar]
- Terzioglu M., Galter D. (2008) Parkinson's disease: genetic versus toxin-induced rodent models. FEBS J 275: 1384–1391 [DOI] [PubMed] [Google Scholar]
- Thannickal T.C., Lai Y.Y., Siegel J.M. (2007) Hypocretin (orexin) cell loss in Parkinson's disease. Brain 130: 1586–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal T.C., Lai Y.Y., Siegel J.M. (2008) Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson's disease. Brain 131: e87–e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Parkinson Study Group (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med 328: 176–183 [DOI] [PubMed] [Google Scholar]
- Thomas B., Mohanakumar K.P. (2004) Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J Pineal Res 36: 25–32 [DOI] [PubMed] [Google Scholar]
- Trenkwalder C. (1998) Sleep dysfunction in Parkinson's disease. Clin Neurosci 5: 107–114 [PubMed] [Google Scholar]
- Tricoire H., Moller M., Chemineau P., Malpaux B. (2003) Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod Suppl 61: 311–321 [PubMed] [Google Scholar]
- Ubhi K., Rockenstein E., Mante M., Inglis C., Adame A., Patrick C., et al. (2010) Alpha-synuclein deficient mice are resistant to toxin-induced multiple system atrophy. Neuroreport 21: 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T., Arslan A.D., Kurtuncu M., Imbesi M., Akhisaroglu M., Dwivedi Y., et al. (2005) The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res 136: 45–53 [DOI] [PubMed] [Google Scholar]
- van der Schyf C.J., Castagnoli K., Palmer S., Hazelwood L., Castagnoli N., Jr (2000) Melatonin fails to protect against long-term MPTP-induced dopamine depletion in mouse striatum. Neurotox Res 1: 261–269 [DOI] [PubMed] [Google Scholar]
- van Hilten B., Hoff J.I., Middelkoop H.A., van der Velde E.A., Kerkhof G.A., Wauquier A., et al. (1994) Sleep disruption in Parkinson's disease. Assessment by continuous activity monitoring. Arch Neurol 51: 922–928 [DOI] [PubMed] [Google Scholar]
- Vendette M., Gagnon J.F., Decary A., Massicotte-Marquez J., Postuma R.B., Doyon J., et al. (2007) REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 69: 1843–1849 [DOI] [PubMed] [Google Scholar]
- Wehr T.A., Aeschbach D., Duncan W.C., Jr (2001) Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol 535: 937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D., Mavandadi S., Mamikonyan E., Siderowf A.D., Duda J.E., Hurtig H.I., et al. (2010) Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 75: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D., Moberg P.J., Duda J.E., Katz I.R., Stern M.B. (2004) Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc 52: 784–788 [DOI] [PubMed] [Google Scholar]
- Weisskopf M.G., Chen H., Schwarzschild M.A., Kawachi I., Ascherio A. (2003) Prospective study of phobic anxiety and risk of Parkinson's disease. Mov Disord 18: 646–651 [DOI] [PubMed] [Google Scholar]
- Wiesenberg I., Missbach M., Carlberg C. (1998) The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci 12: 143–150 [PubMed] [Google Scholar]
- Willis G.L. (2008) Parkinson's disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci 19: 245–316 [DOI] [PubMed] [Google Scholar]
- Willis G.L., Armstrong S.M. (1999) A therapeutic role for melatonin antagonism in experimental models of Parkinson's disease. Physiol Behav 66: 785–795 [DOI] [PubMed] [Google Scholar]
- Willis G.L., Turner E.J. (2007) Primary and secondary features of Parkinson's disease improve with strategic exposure to bright light: a case series study. Chronobiol Int 24: 521–537 [DOI] [PubMed] [Google Scholar]
- Wu Y., Le W., Jankovic J. (2011) Preclinical biomarkers of Parkinson disease. Arch Neurol 68: 22–30 [DOI] [PubMed] [Google Scholar]
- Wurtman R.J., Zhdanova I. (1995) Improvement of sleep quality by melatonin. Lancet 346: 1491–1491 [DOI] [PubMed] [Google Scholar]
- Young A., Home M., Churchward T., Freezer N., Holmes P., Ho M. (2002) Comparison of sleep disturbance in mild versus severe Parkinson's disease. Sleep 25: 573–577 [PubMed] [Google Scholar]
- Zammit G., Erman M., Wang-Weigand S., Sainati S., Zhang J., Roth T. (2007) Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med 3: 495–504 [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Perry G., Smith M.A., Robertson D., Olson S.J., Graham D.G., et al. (1999) Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol 154: 1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Dawson V.L., Dawson T.M. (2006) Role of nitric oxide in Parkinson's disease. Pharmacol Ther 109: 33–41 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Dawson V.L., Dawson T.M. (2000) Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis 7: 240–250 [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201 [DOI] [PubMed] [Google Scholar]
- Zupancic M., Guilleminault C. (2006) Agomelatine: a preliminary review of a new antidepressant. CNS Drugs 20: 981–992 [DOI] [PubMed] [Google Scholar]