Abstract
Obesity and its associated conditions, including type 2 diabetes and cardiovascular disease, have reached epidemic proportions. Gastrointestinal weight loss surgery (GIWLS) shows the most promise in achieving significant and sustained weight loss and diabetes resolution. However, a large mismatch exists between the magnitude of the obesity epidemic and the number of surgical procedures performed to produce a significant shift in the distribution of obesity on a population level. This mismatch is fueled by high surgical costs, morbidity and mortality associated with surgical interventions, and the fact that the greatest public health burden of obesity comes from those around the center of the population body mass index distribution with mild to moderate obesity, rather than those at the distribution tail with severe obesity that GIWLS targets. New endoscopic methods, capitalizing on advances in our understanding of the physiological mechanisms by which GIWLS works, are developing to provide viable alternatives in the treatment of bariatric surgical complications, and for the primary treatment of obesity. These methods may have the added advantage of reduced invasiveness, reversibility, cost-effectiveness, and applicability to a larger segment of the population with moderate obesity.
Keywords: obesity, endoscopy, surgical complications, weight loss surgery
Introduction
Obesity and its associated conditions, including type 2 diabetes and cardiovascular disease, have reached epidemic proportions. According to the National Health and Nutrition Survey (NHANES), about one third of the adult US population has obesity, and the prevalence of metabolic syndrome and diabetes in this population is 39.2% and 14.2%, respectively [Flegal et al. 2010; Nguyen et al. 2008].
Of the many therapeutic approaches for the treatment of obesity and its complications, gastrointestinal weight loss surgery (GIWLS) shows the most promise in achieving significant and sustained weight loss and diabetes resolution when compared with medications or dietary and behavioral modifications [Sjostrom et al. 2007]. Laparoscopic Roux-en-Y gastric bypass (RYGB), open RYGB, and adjustable gastric band comprise approximately 98% of all GIWLS, with vertical banded gastroplasty, duodenal switch, gastric sleeve and biliopancreatic diversion contributing the remaining 2%.
RYGB is currently the GIWLS of choice according to a meta-analysis of 136 studies including 22,094 subjects. The average excess body weight loss with RYGB is 62% with 84% full diabetes resolution, 68% full hypertension resolution, 97% hyperlipidemia improvement, and 81% complete resolution of obstructive sleep apnea. The 30 days operative mortality rate of the RYGB is 0.5% [Buchwald et al. 2009, 2004; Tice et al. 2008].
The proven efficacy of GIWLS coupled with an improved surgical safety profile afforded by the introduction of laparoscopic surgical techniques have led to a surge in the number of bariatric surgery procedures performed in the USA and worldwide, with an estimated 220,000 bariatric operations performed in the USA and Canada in 2008 [Buchwald and Oien, 2009; Kohn et al. 2009].
Despite this increase, a large mismatch exists between the magnitude of the obesity epidemic and the number of GIWLS performed to produce a significant shift in the distribution of obesity on a population level. This mismatch is fueled by high surgical costs, morbidity and mortality associated with surgical interventions, and the fact that the greatest public health burden of obesity comes from those around the center of the population body mass index distribution, with mild to moderate obesity, rather than those at the high distribution tail with severe obesity that GIWLS targets. Thus, minimally invasive and effective interventions that replicate some of the anatomical manipulations of GIWLS endoscopically are greatly needed.
Recently, our understanding of the mechanism by which RYGB surgery works has evolved from that of mechanical restriction and malabsorption [Fisher and Schauer. 2002], to that of anatomical surgical manipulations resulting in physiological alterations in the gut neuroendocrine signaling to the brain, pancreas, liver, adipose tissue, and muscles to regulate food intake through enhanced satiety, increased energy expenditure, and improved glucose homeostasis [Bueter et al. 2010; Stylopoulos et al. 2009; Vetter et al. 2009; Wang et al. 2008]. This coupled with rapidly evolving new endoscopic techniques and technologies have enabled endoscopy to not only assume a pivotal role in the effective management of post-RYGB complications, but also in the primary management of obesity.
In this review, we will discuss the advances in endoscopic techniques and technologies that have enabled the modern endoscopist to manage effectively the majority of post-RYGB surgery complications. We will also discuss the evolution of endoscopically placed devices that have the potential to replicate the physiological effects of the RYGB surgery in an effective and minimally invasive manner to allow their application to a larger subset of the population with moderate obesity.
The role of endoscopy in the management of post-RYGB surgery complications
RYGB surgery is associated with a number of early and late complications that are associated with increased morbidity and mortality, and can result in re-operation or revision surgery [Kellogg et al. 2009; Almahmeed et al. 2007]. Early complications with their associated incidence are as follow: nausea, vomiting, and dehydration 5%, anastomotic leaks 4%, thromboembolic events 3.5%, bowel obstruction 2%, gastrointestinal hemorrhage 2%, wound complications 2%, and death 0.5% [Kellogg et al. 2009; Almahmeed et al. 2007; Gonzalez et al. 2006; Podnos et al. 2003]. Late complications include: marginal ulcers 27–52%, weight regain 30%, anastomotic stenosis 4–27%, incisional hernia 8%, gastrogastric fistula 1–2%, and biliary and nutritional complications [Lee et al. 2009; Diniz Mde et al. 2008; Carrodeguas et al. 2005; Podnos et al. 2003].
Marginal ulcers are the most frequently encountered post-RYGB complication that is usually diagnosed with an upper endoscopy. A thorough endoscopic examination for gastrogastric fistula and foreign material removal from the ulcer site by endoscopic scissors are important as this may aid in ulcer resolution [Frezza et al. 2007]. Further management of marginal ulcers consists of evaluating the pouch pH, testing and treating Helicobacter pylori, soluble proton-pump inhibitor therapy, liquid sucralfate, elimination of nonsteroidal anti-inflammatory drugs, smoking cessation, and optimizing diabetes control. Marginal ulcers that present with acute upper gastrointestinal bleeding are managed endoscopically with mechanical hemoclipping in a similar fashion to bleeding peptic ulcers [Guo et al. 2009], and those ulcers that re-bleed will often require surgical revisions.
Anastomotic strictures can result from tension on the anastomotic site and ischemia, or secondary to nonabsorbable suture material at the gastrojejunostomy site. Two options for the management of anastomotic strictures exist, that is, balloon dilation with a through the scope method, or Savary-Gilliard bougies. Both techniques are very effective and safe, with most patients responding to dilation after an average of two sessions [Fernandez-Esparrach et al. 2008; Ukleja et al. 2008]. Strictures refractory to dilation can be managed with endoscopic covered stents, such as Polyflex or Alveous, to avoid the high complication rate associated with re-operation. Although the short-term success rates of these stents is high at about 84%, the incidence of complications such as stent migration is also high, and data about their long-term benefit and risk profile are lacking [Eubanks et al. 2008].
Staple-line disruption and anastomotic leaks are devastating complications of RYGB surgery with high morbidity and mortality in the acute setting, and chronically may lead to fistula formations, such as gastrogastric fistulas that lead to weight regain. The incidence of staple-line disruption following RYGB surgery is highly dependent on the surgical technique utilized, with much higher rates reported when the pouch and bypassed stomach are stapled in continuity versus transected completely [Capella and Capella, 1996]. Although in continuity staple-line disruptions more commonly result in chronic gastrogastric fistula not leaks. Given the high morbidity and mortality associated with the operative management of staple-line complications, alternative endoscopic approaches have been explored. Covered stents are one method that has been developed as a means of primary endoscopic closure. In a meta-analysis of seven studies involving 77 subjects, in whom self-expanding stents were utilized for management of postbariatric surgery leaks, the pooled proportion of successful leak closure was 84.5%, with a successful stent removal after leak closure of 90%. Stent migration rate was 26%. Most migrations were minimal with no associated mortality reported [Puli et al. 2010]. Other means of primary closure have also been reported. Multiple case series demonstrated the feasibility of utilizing a variety of endoscopic techniques, such as fibrin glue, hemoclips, endoscopic suturing devices, sclerotherapy, Surgisis (an acellular matrix biomaterial derived from the porcine small intestine submucosa that stimulates proliferation of fibroblasts), and argon plasma coagulation for the repair of chronic fistula resulting from anastomotic leaks or in combination with covered self-expandable metallic stents for refractory fistulas cases [Fernandez-Esparrach et al. 2010; Toussaint et al. 2009; Papavramidis et al. 2008; Merrifield et al. 2006]. Although the above techniques appear feasible and promising, long-term data about their efficacy and durability are lacking.
Much of the focus on endoscopic revision of the RYGB has been on the gastrojejunal stoma and gastric pouch, as dilation of the gastrojejunal stoma, and/or enlargement of the gastric pouch, are thought to be risk factors for suboptimal weight loss and weight regain after the procedure. Although, the mechanisms and postsurgical physiology remain unclear, this has led to the development of a variety of less invasive endoscopic techniques and devices aimed at the reduction of the gastrojejunal stoma diameter and gastric pouch size. These include sclerotherapy techniques [Loewen and Barba, 2008; Catalano et al. 2007; Spaulding et al. 2007], endoluminal suturing devices [Ryou et al. 2009; Herron et al. 2008; Tang et al. 2008; Thompson et al. 2006], and tissue plication platforms [Mikami et al. 2010].
The Randomized Evaluation of Endoscopic Suturing Transorally for Anastomotic Outlet Reduction (RESTORe) trial; a multicenter, prospective, double-blinded, sham-controlled trial, tested the use of the EndoCinch device (Bard, Murray Hill, NJ, USA) for treatment of weight regain after RYGB secondary to dilated gastrojejunal stoma. The RESTORe trial evaluated the 6 months absolute percentage weight loss outcome and safety of gastrojejunal anastomosis reduction with the EndoCinch device. On per-protocol completers analysis, the absolute weight loss in the intervention group was 4.7 ± 5.7% (n = 43), compared with 1.9 ± 5.2% (n = 26) in the sham group (p = 0.041). Using a last observation carried forward intent-to-treat analysis, the mean 6-month weight loss was 4.2 ± 5.4% (n = 50) and 1.9 ± 5.2% (n = 27) (p = 0.066). Technical success of reducing the gastrojejunal anastomosis to less than 10 mm was achieved in 89% of subjects. The percentage of adverse events were similar in both groups [Thompson et al. 2010].
Finally, rapid weight loss post-RYGB increases the risk of biliary complication, such as choledocholithiasis through increased risk of stone formation resulting from the weight loss. The RYGB anatomy, however, poses a particular challenge for the endoscopist to gain retrograde access to the biliary tree through the biliopancreatic limb utilizing endoscopic retrograde cholangiopancreatography (ERCP) to deal with these complications. Therefore, multiple endoscopic techniques and technologies have been developed to gain ERCP access. These can be divided into two categories: access through the RYGB anatomic route, or access through a gastrostomy or jejunostomy tract created in the gastric remnant or the small bowel.
Techniques that gain access to the biliopancreatic limb through the RYGB anatomic route either utilize a forward viewing pediatric colonoscope, balloon-assisted enteroscopy, or rotational overtube enteroscopy. A recent multicenter retrospective data collection from eight sites including 129 subjects, of which 64 had RYGB, reported an enteroscopy success rate of 88%, ERCP success rate of 79%, and successful native papilla cannulation in 46 out of 73 subjects. All techniques (i.e. single balloon, double balloon, and rotational overtube enteroscopy) achieved similar results [Shah et al. 2010].
Gastrostomy or jejunostomy access to the biliopancreatic limb can be gained endoscopically with the aid of balloon-assisted enteroscopy to create a retrograde percutaneous endoscopic gastrostomy (PEG) in the gastric remnant. The PEG tract can be dilated after maturation to allow access for the duodenoscope [Baron et al. 2008; Martinez et al. 2006]. Alternatively, laparoscopy-assisted ERCP may be performed in the operating room to gain immediate gastrostomy or jejunostomy access that can accommodate a sterilized duodenoscope [Lopes and Wilcox, 2010].
The role of endoscopy in the primary management of obesity and metabolic disease
In discussing endoscopic replication of RYGB anatomic manipulations, it is useful to divide the surgery into separate distinct components: (a) gastric volume restriction; (b) exclusion of the distal stomach from alimentary flow; (c) exclusion of the proximal intestine; (d) exposure of the jejunum to partially digested nutrients.
Gastric interventions
Endoscopically placed intragastric balloons (IGBs) for the treatment of obesity by reduction of gastric volume were first introduced to the US market in the mid 1880s with the Garren–Edwards gastric bubble (GEGB). The GEGB had multiple complications, mainly small bowel obstruction due to balloon deflation requiring endoscopic or surgical retrieval, and it failed to demonstrate efficacy in a prospective, double-blind, sham-operated, randomized trial of 59 obese patients with a 9-month follow-up period [Hogan et al. 1989; Benjamin, 1988]. Subsequently, newer generation IGBs with better safety profiles were developed. The BioEnterics (Allergan, Irvine, CA, USA) intragastric balloon (BIB) was introduced in the early 1990s, and the Heliosphere (Heliscopie, Vienne, France) balloon (Heliosphere BAG) in 2004. The BIB is an elastic spherical balloon made from silicone and filled with about 500 ml of saline; whereas, the Heliosphere is a double-layered polymer balloon covered with silicone, and filled with about 650–750 ml of air. Newer IGBs with more sophisticated migration-hindering and deployment/retrieval mechanisms, which also allow for endoscopic balloon volume adjustments, are now available. Data from a recent meta-analysis of 15 articles showed a mean weight loss of about 14.7 kg (12.2% of initial weight, and 32.1% of excess weight) at balloon removal with the BIB. This balloon was well tolerated with early removal rate of 4.2%. The long-term follow up of 50 subjects who had the BIB balloon for an average of 7 months, showed that 16 out of the 50 maintained a weight loss of ≥10% after about 5 years of removal [Ercan et al. 2010]. Similar experience was reported with the Heliosphere balloon with no long-term efficacy data available [Imaz et al. 2008; Trande et al. 2008].
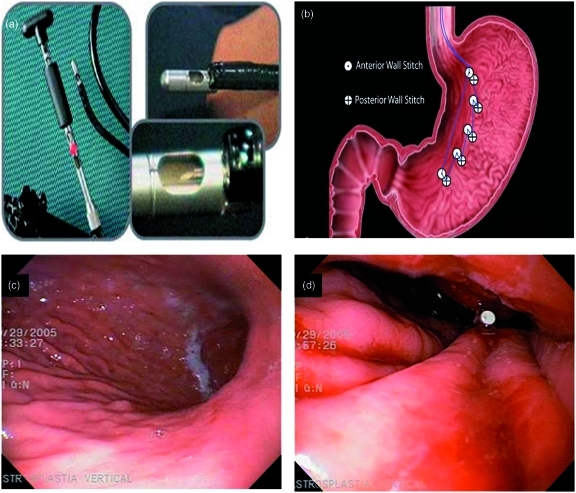
Several endoscopic devices have been developed for the primary treatment of obesity by creating multiple tissue plications to reduce gastric volume. Fogel and colleagues first described the use of the EndoCinch device for the creation of an endoluminal vertical gastroplasty as a primary treatment of obesity in 64 subjects (Figure 1). This study was a single-center, uncontrolled study with a 1-year follow up. The procedure was performed in roughly 60 min under general anesthesia. The percentage of excess weight lost (EWL) reported was 58.1 +/− 19.9% with a favorable safety profile [Fogel et al. 2008]. However, the durability of the tissue plications was not adequately assessed in this study, and randomized, sham-controlled trials are needed to validate these results.
Figure 1.
Endoscopic vertical gastroplasty technique using the EndoCinch endoscopic suturing device: (a) the EndoCinch device; (b) endoscopic suturing pattern for the creation of a vertical gastroplasty by the EndoCinch device; (c) and (d) endoscopic views before and after the creation of the endoscopic vertical gastroplasty. (Reprinted with permission from Fogel et al. [2008].)
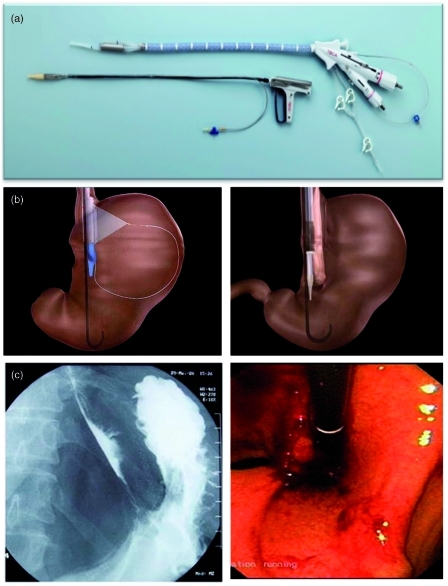
A transoral endoscopically guided stapling system (TOGA system) (Satiety Inc., Palo Alto, CA, USA) has been used to create an endoluminal vertical gastroplasty in two separate, prospective, uncontrolled pilot human trials (Figure 2). The procedure takes about 131 min to perform under general anesthesia. The mean percentage EWL reported in these two trials was 24% and 46% at 6 months. An intact staple line at 6 months was documented by endoscopy in most subjects [Deviere et al. 2008; Moreno et al. 2008]. A multicenter, randomized, sham-controlled trial of this technique is currently underway.
Figure 2.
Transoral endoscopically guided stapling system (TOGA) for the creation of an endoscopic vertical gastroplasty, (a) The TOGA sleeve stapler that is used to create a gastric sleeve with a luminal diameter of approximately 20 mm parallel to the lesser curvature of the stomach, and the TOGA restrictor used to staple gastric folds together and restrict the distal end of the sleeve to approximately 12 mm. (b) The TOGA sleeve stapler with the extendable wire fully deployed for optimal alignment of the stapler, and a diagram showing the TOGA restrictor in place. (c) Radiographic and endoscopic views of the vertical gastroplasty created by the TOGA system. (Reprinted with permission from Deviere et al. [2008].)
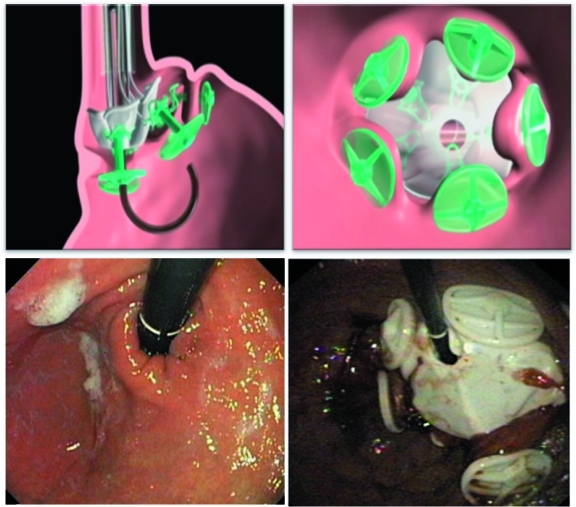
A new transoral endoscopic restrictive system (TERIS) (BaroSense, Redwood, CA, USA) that endoscopically places a restrictive silicone device with a 10 mm orifice anchored by five silicone anchors through five transmural plications at the gastric side of the gastroesophageal junction to replicate the effects of a laparoscopic gastric band has been developed (Figure 3). De Jong and colleagues have recently reported their experience in 13 subjects followed for 3 months. The median procedure time was 142 min under general anesthesia. Serious complications were reported in three subjects (two pneumoperitoneum requiring percutaneous intervention, and one gastric perforation). The safety profile of the procedure improved after adjusting the stapling device and performing the procedure with carbon dioxide insufflation. The median reported EWL at 3 months was 28% [De Jong et al. 2010].
Figure 3.
TERIS showing an endoscopically placed restrictive silicone device with a 10 mm orifice anchored by five silicone anchors through five transmural plications at the gastric side of the gastroesophageal junction. (Reprinted with permission from De Jong et al. [2010].) TERIS, transoral endoscopic restrictive system.
Small bowel interventions
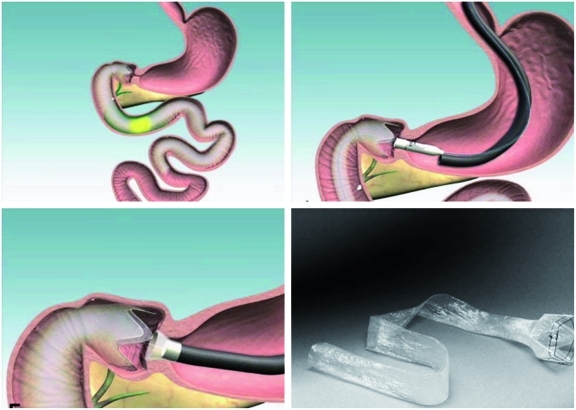
EndoBarrier (GI Dynamics, Lexington, MA, USA), a new duodenal-jejunal bypass sleeve (DJBS) made from a Teflon liner, and delivered endoscopically to the duodenal bulb, shows promise and efficacy in the management of obesity and associated diabetes (Figure 4). When deployed, the DJBS mimics the mechanical manipulation of RYGB surgery by creating a mechanical barrier that allows food to bypass the duodenum and proximal jejunum without mixing with bile and pancreatic enzymes until later in the gut, thus potentially manipulating the enteroinsular system. In rats with diet-induced diabetes, this device showed a 20% weight reduction compared with sham-operated rats with no malabsorption and substantial improvement in insulin resistance [Aguirre et al. 2008].
Figure 4.
A duodenal-jejunal bypass sleeve (EndoBarrier) made from a Teflon liner and delivered endoscopically to the duodenal bulb. (Reprinted with permission from Coté and Edmundowicz [2009].)
Results from two recent multicenter, prospective, randomized, open-label, sham-controlled trials enrolling 21 DJBS subjects and 26 sham controls in one, and 30 DJBS subjects with 11 diet controls in the other were recently reported [Gersin et al. 2010; Schouten et al. 2010]. Percentage EWL at 12 weeks was 11.9% versus 2.0% in the sham controls in one, and 19% versus 6.9% in the diet controls in the other. Eight DJBS subjects required early termination in one trial and four in the other. Reasons for the early termination included device migration, obstruction, bleeding, epigastric pain, nausea, and vomiting. Average implantation time was 35 min [range 12–102], and average explantation time was 17 min [range 5–99]. No acute procedural complications were reported. Diabetic subjects had an impressive 3.4 point improvement in their HbA1c within the 12-week study period, compared with 0.7 point in the diet-controlled diabetic group [Schouten et al. 2010].
Natural orifice transluminal endoscopic surgery
Although in its infancy, the field of endoscopic surgery shows promise in reproducing different components of bariatric surgical procedures endoscopically. Recently, a hybrid natural orifice transluminal endoscopic surgery (NOTES) transvaginal approach was used to create a sleeve gastrectomy in obese women in Brazil [Ramos et al. 2008]. Furthermore, work to create a gastrojejunal anastomosis through a NOTES approach is underway.
Others
Other endoscopically delivered devices, endoscopic techniques, and endoscopic suturing and endoplication platforms that can be utilized in the primary treatment of obesity are at different stages of development.
Conclusion
New endoscopic methods are being developed to provide viable alternatives in the treatment of bariatric surgical complications. In addition, understanding the physiological effects of the different gastrointestinal mechanical manipulations produced by RYGB surgery may enable modern endoscopy to replicate different components of this surgery, with the potential added advantages of reduced invasiveness, reversibility, and cost effectiveness. Additionally, these features may allow endoscopic procedures to be applied to the larger segment of the population with moderate obesity. Although, it is unlikely for a single endoscopic approach to be as effective as traditional surgery for severe obesity, the application of a combination of such devices in tandem or in sequence, potentially with new selective drugs, will likely enable the treatment of a range of patients with varying severity of disease.
Conflict of interest statement
Christopher C. Thompson is a consultant for USGI medical, BARD medical, Covidien, Boston Scientific, and ValenTx. No conflict of interest exists for Barham K. Abu Dayyeh.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Aguirre V., Stylopoulos N., Grinbaum R., Kaplan L.M. (2008) An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity (Silver Spring) 16: 2585–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahmeed T., Gonzalez R., Nelson L.G., Haines K., Gallagher S.F., Murr M.M. (2007) Morbidity of anastomotic leaks in patients undergoing Roux-en-Y gastric bypass. Arch Surg 142: 954–957 [DOI] [PubMed] [Google Scholar]
- Baron T.H., Chahal P., Ferreira L.E. (2008) ERCP via mature feeding jejunostomy tube tract in a patient with Roux-en-Y anatomy (with video). Gastrointest Endosc 68: 189–191 [DOI] [PubMed] [Google Scholar]
- Benjamin S.B. (1988) Small bowel obstruction and the Garren–Edwards gastric bubble: An iatrogenic bezoar. Gastrointest Endosc 34: 463–467 [DOI] [PubMed] [Google Scholar]
- Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K., et al. (2004) Bariatric surgery: A systematic review and meta-analysis. JAMA 292: 1724–1737 [DOI] [PubMed] [Google Scholar]
- Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J., et al. (2009) Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med 122: 248–256 e245 [DOI] [PubMed] [Google Scholar]
- Buchwald H., Oien D.M. (2009) Metabolic/bariatric surgery worldwide 2008. Obes Surg 19: 1605–1611 [DOI] [PubMed] [Google Scholar]
- Bueter M., Lowenstein C., Olbers T., Wang M., Cluny N.L., Bloom S.R., et al. (2010) Gastric bypass increases energy expenditure in rats. Gastroenterology 138: 1845–1853 [DOI] [PubMed] [Google Scholar]
- Capella J.F., Capella R.F. (1996) Staple disruption and marginal ulceration in gastric bypass procedures for weight reduction. Obes Surg 6: 44–49 [DOI] [PubMed] [Google Scholar]
- Carrodeguas L., Szomstein S., Soto F., Whipple O., Simpfendorfer C., Gonzalvo J.P., et al. (2005) Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: Analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis 1: 467–474 [DOI] [PubMed] [Google Scholar]
- Catalano M.F., Rudic G., Anderson A.J., Chua T.Y. (2007) Weight gain after bariatric surgery as a result of a large gastric stoma: Endotherapy with sodium morrhuate may prevent the need for surgical revision. Gastrointest Endosc 66: 240–245 [DOI] [PubMed] [Google Scholar]
- Coté G.A., Edmundowicz S.A. (2009) Emerging technology: endoluminal treatment of obesity. Gastrointest Endosc 70: 991–999 [DOI] [PubMed] [Google Scholar]
- De Jong K., Mathus-Vliegen E.M., Veldhuyzen E.A., Eshuis J.H., Fockens P. (2010) Short-term safety and efficacy of the trans-oral endoscopic restrictive implant system for the treatment of obesity. Gastrointest Endosc 72: 497–504 [DOI] [PubMed] [Google Scholar]
- Deviere J., Ojeda Valdes G., Cuevas Herrera L., Closset J., Le Moine O., Eisendrath P., et al. (2008) Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc 22: 589–598 [DOI] [PubMed] [Google Scholar]
- Diniz Mde F., Passos V.M., Barreto S.M., Diniz M.T., Linares D.B., Simil Fde S. (2008) Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg 247: 205–206 [DOI] [PubMed] [Google Scholar]
- Ercan C., Hélène T., Marleen D., Davy V., Christl T., Thierry O. (2010) M1363 weight reduction by means of an intragastric balloon in daily routine practice: Results at removal and in long-term. Gastroenterology 138: S–389 [Google Scholar]
- Eubanks S., Edwards C.A., Fearing N.M., Ramaswamy A., De La Torre R.A., Thaler K.J., et al. (2008) Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg 206: 935–939 [DOI] [PubMed] [Google Scholar]
- Fernandez-Esparrach G., Bordas J.M., Llach J., Lacy A., Delgado S., Vidal J., et al. (2008) Endoscopic dilation with Savary–Gilliard bougies of stomal strictures after laparosocopic gastric bypass in morbidly obese patients. Obes Surg 18: 155–161 [DOI] [PubMed] [Google Scholar]
- Fernandez-Esparrach G., Lautz D.B., Thompson C.C. (2010) Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: A less-invasive approach. Surg Obes Relat Dis 6: 282–288 [DOI] [PubMed] [Google Scholar]
- Fisher B.L., Schauer P. (2002) Medical and surgical options in the treatment of severe obesity. Am J Surg 184: 9S–16S [DOI] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241 [DOI] [PubMed] [Google Scholar]
- Fogel R., De Fogel J., Bonilla Y., De La Fuente R. (2008) Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 68: 51–58 [DOI] [PubMed] [Google Scholar]
- Frezza E.E., Herbert H., Ford R., Wachtel M.S. (2007) Endoscopic suture removal at gastrojejunal anastomosis after Roux-en-Y gastric bypass to prevent marginal ulceration. Surg Obes Relat Dis 3: 619–622 [DOI] [PubMed] [Google Scholar]
- Gersin K.S., Rothstein R.I., Rosenthal R.J., Stefanidis D., Deal S.E., Kuwada T.S., et al. (2010) Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc 71: 976–982 [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Haines K., Nelson L.G., Gallagher S.F., Murr M.M. (2006) Predictive factors of thromboembolic events in patients undergoing Roux-en-Y gastric bypass. Surg Obes Relat Dis 2: 30–36 [DOI] [PubMed] [Google Scholar]
- Guo S.B., Gong A.X., Leng J., Ma J., Ge L.M. (2009) Application of endoscopic hemoclips for nonvariceal bleeding in the upper gastrointestinal tract. World J Gastroenterol 15: 4322–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron D.M., Birkett D.H., Thompson C.C., Bessler M., Swanstrom L.L. (2008) Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: A feasibility study. Surg Endosc 22: 1093–1099 [DOI] [PubMed] [Google Scholar]
- Hogan R.B., Johnston J.H., Long B.W., Sones J.Q., Hinton L.A., Bunge J., et al. (1989) A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc 35: 381–385 [DOI] [PubMed] [Google Scholar]
- Imaz I., Martinez-Cervell C., Garcia-Alvarez E.E., Sendra-Gutierrez J.M., Gonzalez-Enriquez J. (2008) Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 18: 841–846 [DOI] [PubMed] [Google Scholar]
- Kellogg T.A., Swan T., Leslie D.A., Buchwald H., Ikramuddin S. (2009) Patterns of readmission and reoperation within 90 days after Roux-en-Y gastric bypass. Surg Obes Relat Dis 5: 416–423 [DOI] [PubMed] [Google Scholar]
- Kohn G.P., Galanko J.A., Overby D.W., Farrell T.M. (2009) Recent trends in bariatric surgery case volume in the United States. Surgery 146: 375–380 [DOI] [PubMed] [Google Scholar]
- Lee J.K., Van Dam J., Morton J.M., Curet M., Banerjee S. (2009) Endoscopy is accurate, safe, and effective in the assessment and management of complications following gastric bypass surgery. Am J Gastroenterol 104: 575–582 [DOI] [PubMed] [Google Scholar]
- Loewen M., Barba C. (2008) Endoscopic sclerotherapy for dilated gastrojejunostomy of failed gastric bypass. Surg Obes Relat Dis 4: 539–542 [DOI] [PubMed] [Google Scholar]
- Lopes T.L., Wilcox C.M. (2010) Endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y anatomy. Gastroenterol Clin N Am 39: 99–107 [DOI] [PubMed] [Google Scholar]
- Martinez J., Guerrero L., Byers P., Lopez P., Scagnelli T., Azuaje R., et al. (2006) Endoscopic retrograde cholangiopancreatography and gastroduodenoscopy after Roux-en-Y gastric bypass. Surg Endosc 20: 1548–1550 [DOI] [PubMed] [Google Scholar]
- Merrifield B.F., Lautz D., Thompson C.C. (2006) Endoscopic repair of gastric leaks after Roux-en-Y gastric bypass: A less invasive approach. Gastrointest Endosc 63: 710–714 [DOI] [PubMed] [Google Scholar]
- Mikami D., Needleman B., Narula V., Durant J., Melvin W.S. (2010) Natural orifice surgery: initial US experience utilizing the stomaphyx device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc 24: 223–228 [DOI] [PubMed] [Google Scholar]
- Moreno C., Closset J., Dugardeyn S., Barea M., Mehdi A., Collignon L., et al. (2008) Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: Results of the Second Human Pilot Study. Endoscopy 40: 406–413 [DOI] [PubMed] [Google Scholar]
- Nguyen N.T., Magno C.P., Lane K.T., Hinojosa M.W., Lane J.S. (2008) Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg 207: 928–934 [DOI] [PubMed] [Google Scholar]
- Papavramidis T.S., Kotzampassi K., Kotidis E., Eleftheriadis E.E., Papavramidis S.T. (2008) Endoscopic fibrin sealing of gastrocutaneous fistulas after sleeve gastrectomy and biliopancreatic diversion with duodenal switch. J Gastroenterol Hepatol 23: 1802–1805 [DOI] [PubMed] [Google Scholar]
- Podnos Y.D., Jimenez J.C., Wilson S.E., Stevens C.M., Nguyen N.T. (2003) Complications after laparoscopic gastric bypass: A review of 3464 cases. Arch Surg 138: 957–961 [DOI] [PubMed] [Google Scholar]
- Puli S.R., Spofford I.S., Thompson C.C. (2010) Use of self expanding stents in the treatment of bariatric surgery leaks: A meta-analysis and systematic review. Gastrointest Endosc 71: AB140–AB140 [DOI] [PubMed] [Google Scholar]
- Ramos A.C., Zundel N., Neto M.G., Maalouf M. (2008) Human Hybrid Notes Transvaginal Sleeve Gastrectomy: Initial Experience. Surg Obes Relat Dis 5: 660–663 [DOI] [PubMed] [Google Scholar]
- Ryou M., Mullady D.K., Lautz D.B., Thompson C.C. (2009) Pilot study evaluating technical feasibility and early outcomes of second-generation endosurgical platform for treatment of weight regain after gastric bypass surgery. Surg Obes Relat Dis 5: 450–454 [DOI] [PubMed] [Google Scholar]
- Schouten R., Rijs C.S., Bouvy N.D., Hameeteman W., Koek G.H., Janssen I.M., et al. (2010) A multicenter, randomized efficacy study of the endobarrier gastrointestinal liner for presurgical weight loss prior to bariatric surgery. Ann Surg 251: 236–243 [DOI] [PubMed] [Google Scholar]
- Shah R.J., Smolkin M., Ross A.S., Kozarek R.A., Howel D.A., Bakis G., et al. (2010) 788e: A multi-center, US experience of single balloon, double balloon, and rotational overtube enteroscopy-assisted ERCP in long limb surgical bypass patients. Gastrointest Endosc 71: AB134–AB135 [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C.D., Karason K., Larsson B., Wedel H., et al. (2007) Effects of bariatric surgery on mortality in swedish obese subjects. N Engl J Med 357: 741–752 [DOI] [PubMed] [Google Scholar]
- Spaulding L., Osler T., Patlak J. (2007) Long-term results of sclerotherapy for dilated gastrojejunostomy after gastric bypass. Surg Obes Relat Dis 3: 623–626 [DOI] [PubMed] [Google Scholar]
- Stylopoulos N., Hoppin A.G., Kaplan L.M. (2009) Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 17: 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.J., Olukoga C.O., Provost D.A., Hogg D., Livingston E., Scott D.J. (2008) Gastrojejunal stomal reduction with the t-tag device in porcine models (with videos). Gastrointest Endosc 68: 132–138 [DOI] [PubMed] [Google Scholar]
- Thompson C.C., Roslin M.S., Chand B., Chen Y.K., Demarco D.C., Miller L.S., et al. (2010) M1359 RESTORE: Randomized Evaluation of Endoscopic Suturing Transorally for Anastomotic Outlet Reduction: A double-blind, sham-controlled multicenter study for treatment of inadequate weight loss or weight regain following Roux-en-Y gastric bypass. Gastroenterology 138: S–388 [Google Scholar]
- Thompson C.C., Slattery J., Bundga M.E., Lautz D.B. (2006) Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: A possible new option for patients with weight regain. Surg Endosc 20: 1744–1748 [DOI] [PubMed] [Google Scholar]
- Tice J.A., Karliner L., Walsh J., Petersen A.J., Feldman M.D. (2008) Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med 121: 885–893 [DOI] [PubMed] [Google Scholar]
- Toussaint E., Eisendrath P., Kwan V., Dugardeyn S., Deviere J., Le Moine O. (2009) Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy 41: 560–563 [DOI] [PubMed] [Google Scholar]
- Trande P., Mussetto A., Mirante V.G., De Martinis E., Olivetti G., Conigliaro R.L., et al. (2008) Efficacy, tolerance and safety of new intragastric air-filled balloon (Heliosphere bag) for obesity: The experience of 17 cases. Obes Surg 20: 1227–1230 [DOI] [PubMed] [Google Scholar]
- Ukleja A., Afonso B.B., Pimentel R., Szomstein S., Rosenthal R. (2008) Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc 22: 1746–1750 [DOI] [PubMed] [Google Scholar]
- Vetter M.L., Cardillo S., Rickels M.R., Iqbal N. (2009) Narrative review: Effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med 150: 94–103 [DOI] [PubMed] [Google Scholar]
- Wang P.Y., Caspi L., Lam C.K., Chari M., Li X., Light P.E., et al. (2008) Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452: 1012–1016 [DOI] [PubMed] [Google Scholar]