Abstract
The prevalence of type 2 diabetes mellitus and insulin resistance are higher among people chronically infected with hepatitis C (CHC) when compared with the general population and people with other causes of chronic liver disease. Both insulin resistance and diabetes are associated with adverse outcomes across all stages of CHC, including the liver transplant population. CHC is also associated with the development of hepatic steatosis, a common histological feature present in approximately 55% (32–81%) of cases. There is a complex interrelationship between insulin resistance and hepatic steatosis and both are postulated to aggravate each other. The peroxisome proliferator-activated receptors (PPARs) are nuclear factors involved in the regulation of glucose, lipid homeostasis, inflammatory response, cell differentiation, and cell cycle. The relationship between hepatitis C virus replication and PPARs has been the focus of recent study. Given the availability of potent agonists, PPARs may represent a novel pharmacological target in the treatment of CHC.
Keywords: hepatitis C, insulin resistance, liver fibrosis, peroxisome proliferator-activated receptors, pioglitazone, steatosis
Introduction
Chronic hepatitis C virus (CHC) infects approximately 170 million people worldwide, and is a major cause of mortality and morbidity [WHO, 2009]. The natural history of HCV infection is characterized by a high rate of progression to chronic hepatitis leading to cirrhosis in approximately 20% of cases and possibly to hepatocellular carcinoma [Seeff, 2009]. In addition to progressive liver disease, CHC has been linked to dysregulated energy metabolism to include insulin resistance (IR), type 2 diabetes mellitus (T2DM), hepatic steatosis and increased risk of carotid atherosclerosis [Koike, 2009; Negro and Sanyal, 2009; Romero-Gómez, 2006]. This is due, in part, to a direct interference of HCV with lipid and glucose metabolism and a complex relationship between IR and steatosis in patients with CHC. The mechanisms involved seem to be HCV genotype specific [Moucari et al. 2008; Pazienza et al. 2007]. Since the peroxisome proliferator-activated receptors (PPARs) are nuclear factors involved in the regulation of glucose and lipid homeostasis, the relationship between HCV replication and protein expression and PPARs has been the focus of recent study. This review first describes the main function of PPARs within the liver, the interaction between PPARs and HCV, and then its potential role in HCV pathophysiology and therapy is discussed.
Peroxisome proliferator-activated receptors
PPARs belong to the nuclear receptor superfamily and require heterodimerization with receptor X for retinoids (RXR) in order to function [Desvergne and Wahli, 1999; Bardot et al. 1993]. The PPAR:RXR heterodimer, when bound to a ligand, changes conformation and binds to DNA at PPAR response elements, resulting in gene transcription [Bardot et al. 1993; Gearing et al. 1993]. PPARs play essential roles in the regulation of cellular differentiation, development, and metabolism (carbohydrate, lipid, protein), and tumorigenesis [Desvergne and Wahli, 1999; Bardot et al. 1993].
There are three isotypes of PPAR designated in mammals: PPARα (NR1C1), PPARδ (NR1C2) and PPARγ (NR1C3) [Nuclear Receptors Nomenclature Committee, 1999]. PPARα is activated by ligands termed peroxisome proliferators, which were named for their effects on peroxisomes in rodent livers [Svoboda and Azarnoff, 1966; Hess et al. 1965].
PPARα/γ, together with their obligate partner RXR, are the main nuclear receptors expressed in the liver. These receptors contribute to important physiological processes occurring in the liver to include control of lipid and glucose metabolism, inflammatory responses, and cellular differentiation and proliferation. The availability of new nonhepatotoxic ligands made it possible to evaluate certain PPARs as potential new therapeutic targets in liver disease.
PPARs and insulin resistance and diabetes
PPARs play an essential role in glucose homeostasis. PPARα upregulates glycerol-3-phosphate dehydrogenase, glycerol kinase and glycerol transport proteins, which allows for glucose synthesis during fasting states [Patsouris et al. 2004]. PPARα also stimulates pancreatic islet ß cells, increasing fatty acid oxidation and potentiating glucose-stimulated insulin secretion [Lefebvre et al. 2006]. In addition, PPARs possess insulin-sensitizing effects which lead to decreased ß-cell workload. This is particularly beneficial for ß-cell survival and prevention of pancreatic degradation.
In a recent randomized controlled trial the diabetic patients from the placebo group demonstrated a progressive decline in homeostatic indices of ß-cell function and an increase in IR was calculated according to the homeostasis model of assessment (HOMA) over 2 years of follow up. These longitudinal changes were attenuated by the PPARα ligand bezafibrate [Tenenbaum et al. 2007].
For PPARγ, clinical data demonstrated that the PPARγ agonists (thiazolidinediones such as pioglitazone) improve hyperglycaemia and hyperlipidaemia in obese and diabetic animals through reduction of both peripheral and hepatic IR [Jay and Ren, 2007].
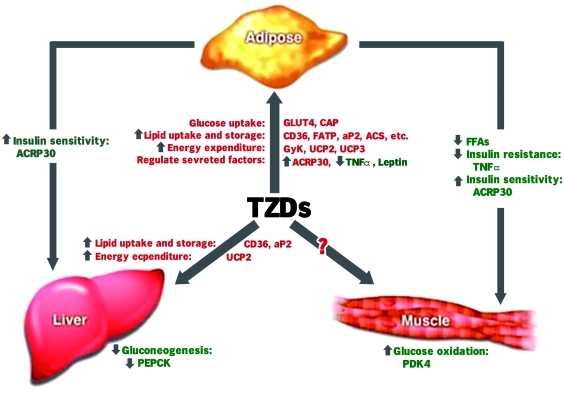
The mechanisms of PPARγ-mediated insulin sensitization are complex and are thought to involve specific effects on fat, skeletal muscle, and liver (Figure 1). Specifically, PPARγs:
Increase glucose catabolism and decrease hepatic glucose output [Nagashima et al. 2005; Sakamoto et al. 2005).
Increase GLUT4 expression and translocation in adipocytes [Armoni et al. 2003].
Lead to adipose remodeling. This phenomenon may explain the ‘fatty acid steal’ hypothesis [Berthiaume et al. 2004].
Increase expression of adipocytokines such as adiponectin, which may stimulate fatty acid oxidation in skeletal muscle and liver [Pajvani et al. 2004].
Increase glucose uptake in skeletal muscle resulting in lower blood glucose levels [Le Brasseur et al. 2006].
Impact directly on factors involved in lipid and glucose homeostasis.
May have insulin-sensitizing effects via their anti-inflammatory activity.
Figure 1.
The complex mechanisms of peroxisome proliferator-activated receptor-γ agonist-mediated insulin sensitization. ACRP30, adipocyte complement related protein of 30 kDa; FATP, FA transport protein; FFA, free fatty acid; GLUT4, insulin-regulated glucose transporter isoform; PDK4, pyruvate dehydrogenase kinase 4; PEPCK, phosphoenolpyruvate carboxykinase; TNF, tumor necrosis factor; TZD, thiazolidinedione; UCP, uncoupling protein 2 and 3.
Thus, treatment with PPARγ agonists results in improved insulin sensitivity via diverse mechanisms, both direct and indirect, and at the level of the liver and other extrahepatic tissues.
PPARs and lipid metabolism
The liver is the central organ responsible for fat metabolism and lipid homeostasis [Everett et al. 2000]. It is involved in free fatty acid synthesis, esterification of triacylglycerols and their packaging into very low-density lipoproteins (VLDLs) for exportation during the fed state [Everett et al. 2000]. During the fasted state, the liver is responsible for controlling the rates of fatty acid ß-oxidation and ketogenesis. The liver maintains lipid homeostasis by balancing these processes.
PPARα is a key mediator in maintaining this balance [Mandard et al. 2004; Nakamura et al. 2004; Hertz et al. 1995] by acting as a sensor for the level of free fatty acids and modulating the responses of fat-oxidizing tissues [Everett et al. 2000].
PPARα is activated by dietary fatty acids and eicosanoids or by specific drugs such as the fibrates. Activation of PPARα results in an increase in the enzymes involved in lipid metabolism and fatty acid ß-oxidation. Loss of expression of the PPARα gene in mice results in hepatic steatosis under conditions of increased fatty acid metabolism in the liver, such as fasting or a high-fat diet [Kersten et al. 1999; Lee et al. 1995]. Administration of a potent PPARα agonist decreases hepatic steatosis in mice receiving a methionine and choline deficient diet [Nagasawa et al. 2006].
Intracellular fatty acid concentrations are controlled, in part, by regulation of the fatty acid import and export system. Activation of PPARα directly regulates genes involved in fatty acid uptake [Escher and Wahli, 2000]. Also, both PPARα and PPARγ prevent the efflux of fatty acids by promoting their activation into fatty acyl CoA thioesters by the acyl-CoA synthetase [Hsu et al. 2001].
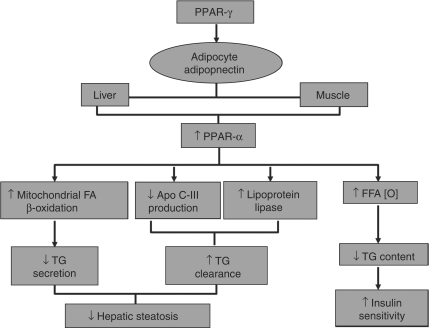
In addition, PPARα activation regulates hepatic triglyceride content by promoting fatty acid oxidation in peroxisomes and in the mitochondria, and reducing the fatty acid pool available to the liver for triglyceride synthesis [Latruffe et al. 2000]; enhancing lipoprotein lipase expression [Schoonjans et al. 1996]; and inhibiting apolipoprotein C-III in the liver [Staels et al. 1995] (Figure 2).
Figure 2.
The mechanism of peroxisome proliferator-activated receptor-α (PPARα) action in lipid and glucose metabolism and its connection with PPARγ through adiponectin. Apo, apolipoprotein; FA, fatty acid; FFA, free fatty acid; TG, triglyceride.
Adiponectin, an adipocyte produced peptide hormone, limits fat accumulation in the liver by a number of mechanisms including activation of PPARα to increase hepatic fatty acid oxidation [Yamauchi et al. 2003]. Adiponectin is upregulated by PPARγ, providing a connection between the two isotypes [Neschen et al. 2006] (Figure 2).
PPARγ is highly expressed in adipose tissue under two isoforms (PPARγ1 and PPARγ2) generated by the same gene through altered splicing [Fajas et al. 1997] and they have a complementary role in lipid homeostasis. PPARγ mediates its effect on lipid homeostasis by dual action: its role in increasing insulin sensitivity, and its effect on adipocytes.
PPARγ plays an important role in control of hepatic steatosis by increasing insulin sensitivity (see above). IR is integral to the development of nonalcoholic fatty liver disease (NAFLD), leading to increased fatty acid flux to the liver and increased hepatic fatty acid synthesis [Yu and Ginsberg, 2005].
Alternatively, PPARγ mediates its effect on adipocytes by promoting fatty acid uptake into adipocytes, adipocyte differentiation [Schoonjans et al. 1997] and by increasing the expression of adipocyte proteins involved in fatty acid uptake [Desvergne and Wahli, 1999], transport [Desvergne and Wahli, 1999] and synthesis [Frohnert et al. 1999]. The net effect of these processes is to increase triglyceride storage in adipocytes, reducing delivery of fatty acids to the liver. Patients with dominant negative mutations in PPARγ have NAFLD and metabolic syndrome, while lacking adipose tissue, suggesting increased triglyceride delivery to the liver [Savage et al. 2003].
PPARγ is also involved in the induction of uncoupling protein-2, which might decrease hepatic triglyceride accumulation by increasing energy expenditure [Castelein et al. 1994].
PPAR: anti-inflammatory and immunomodulatory properties in the liver
In addition to the lipid-lowering and insulin-sensitizing effects of the PPAR ligands, there are numerous experimental and clinical data in favor of the anti-inflammatory activity of PPARs in the liver.
Evidence has demonstrated the effects of an RXR agonist and a PPARγ agonist on tumor necrosis factor-α (TNF-α) production [Uchimura et al. 2001]. PPARγ:RXR activation suppresses nuclear factor kappa B, signal transducers and activators of transcription, activating protein 1 signalling pathways and TNF-α production in Kupffer cells in primary hepatocyte cultures and monocytes/macrophages.
PPARα can also control hepatic inflammation by regulating hemostatic factors, acute-phase response proteins in hepatocytes [Anderson et al. 2002], and regulating antioxidant enzyme activities, such as catalase. In addition, PPARα agonists may reduce the oxidative stress [Hashimoto et al. 2000].
Hepatitis C virus and insulin resistance
The prevalence of both IR and T2DM is significantly higher in patients with CHC when compared with other chronic liver diseases [Romero-Gómez et al. 2005; Hui et al. 2003; Caronia et al. 1999; Mason et al. 1999; Grimbert et al. 1996; Allison et al. 1994]. The prevalence of diabetes in patients with chronic hepatitis has ranged from 20% to 50% [Moucari et al. 2008; Caronia et al. 1999; Grimbert et al. 1996]. This is higher than that reported for patients with other chronic liver diseases such as chronic hepatitis B, independent of the stage of liver fibrosis [Romero-Gómez et al. 2005; Hui et al. 2003; Caronia et al. 1999; Mason et al. 1999; Grimbert et al. 1996; Allison et al. 1994].
This association between diabetes and CHC was first reported by Allison and colleagues [Allison et al. 1994], who observed that diabetes was significantly more prevalent in patients with hepatitis C-related cirrhosis than those with cirrhosis resulting from conditions other than CHC (50% versus 9%). Since then, a number of cross-sectional, case control, and longitudinal studies, performed in both large unselected cohorts and in patients with liver or kidney transplantation, have re-affirmed this association. Hui and colleagues [Hui et al. 2003] found that 121 patients with HCV and stage 0 or 1 liver fibrosis had higher HOMA scores compared with 137 healthy volunteers matched by sex, body mass index, and waist-to-hip ratio. This work proved that HCV may induce IR at early stages of liver disease. Another classic way to prove an association between infection and disease comes from the fact that curing HCV results in improvement in the HOMA score and a decreased incidence of T2D after cessation of therapy [Chehadeh et al. 2009; Romero-Gómez et al. 2008; Kawaguchi et al. 2007].
The mechanisms of IR in CHC are an area of intense study, and numerous molecular pathways have been implicated. IR in CHC arises both as a direct consequence of the virus and indirectly as a consequence of lipid accumulation and/or inflammation. HCV seems to lead to IR through interference of intracellular insulin signalling by HCV proteins, mainly the serine phosphorylation of IRS-1 and impairment of the downstream Akt signaling pathway. Also, HCV core protein inhibits PPARα and PPARγ expressed in hepatocytes and adipocytes promoting insulin receptor substrates-1 (IRS-1) degradation and IR. The HCV core protein interferes with in vitro insulin signaling by genotype-specific mechanisms [Moucari et al. 2008; Pazienza et al. 2007]. Moucari and colleagues found genotypes 1 and 4 to be correlated independently with IR [Moucari et al. 2008]. The rationale is unclear and may be based on the known differences in treatment response between these groups rather than interactions between viral proteins and host signaling pathways.
While CHC is independently associated with IR and T2DM, the presence of concomitant host-specific risk factors also contributes to both the prevalence and the degree of disturbance of glucose homeostasis in a complex interrelationship between these factors in CHC.
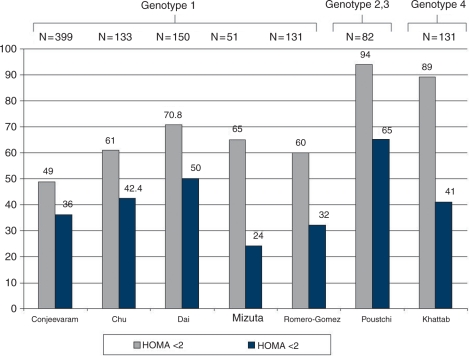
The association between HCV and IR has noteworthy consequences, clinically and conceptually. From the clinical standpoint, IR accelerates fibrogenesis [Hui et al. 2003], impairs early and sustained virologic response (SVR) to interferon-based antiviral therapy [Khattab et al. 2010; Mizuta et al. 2010; Chu et al. 2009; Dai et al. 2009; Poustchi et al. 2008; Conjeevaram et al. 2007; Romero-Gómez et al. 2005] (Figure 3), and increases the incidence of hepatocellular carcinoma [Pekow et al. 2007].
Figure 3.
Impact of insulin resistance on sustained virologic response (SVR) in patients with chronic hepatitis C virus genotype (1, 2, 3 and 4). HOMA, homeostasis model of assessment.
Hepatitis C virus and fatty liver
The prevalence of steatosis in patients with CHC is reported to be between 40% and 80% depending on the features of the population studied in terms of alcohol consumption, prevalence of overweight/obesity, diabetes and other risk factors of fatty liver [Asselah et al. 2005]. However, when all common factors of steatosis have been ruled out, the prevalence of steatosis in CHC remains about 40%. This figure represents an approximately twofold increase compared with the prevalence of steatosis in other common chronic liver diseases such as hepatitis B (20%) [Thomopoulos et al. 2006; Rubbia-Brandt et al. 2000]. This suggests that hepatitis C virus (HCV) may directly cause fatty liver, at least in subgroups of patients with CHC.
Steatosis appears to be a direct consequence of viral protein expression in genotype 3 infection, suggesting the presence of specific sequences across the genome of genotype 3 that are involved in fat accumulation within hepatocytes. With other genotypes, steatosis is associated with features of the metabolic syndrome to include IR and obesity [Negro and Sanyal, 2009].
This is further supported by two additional observations. First, the severity of steatosis correlates with the level of HCV RNA, both in liver [Rubbia-Brandt et al. 2000] and in serum [Adinolfi et al. 2001], especially in patients with genotype 3. Second, fatty liver may significantly decrease, if not disappear altogether, when patients are successfully treated with antiviral agents. Again, this is most pronounced in patients infected with genotype 3 virus [Poynard et al. 2003; Rubbia-Brandt et al. 2000]. Steatosis may persist in most patients with non-3 genotypes, even in the case of sustained virologic response [Poynard et al. 2003]. A relapse after the end of therapy may result in the reappearance of steatosis in patients in whom it had disappeared while on treatment [Rubbia-Brandt et al. 2001].
The composition of fatty acids that are accumulated in the liver of core gene transgenic mice is different from that in fatty liver due to simple obesity. Carbon-18 monounsaturated fatty acids (C18: 1) such as oleic or vaccenic acids are significantly increased. This is also the case in the comparison of liver tissues from patients with CV and those with simple fatty liver due to obesity [Moriya et al. 2001].
Recent studies have attempted to explain the potential mechanisms underlying the steatosis often seen in patients with CHC. In general, HCV may interfere with lipid metabolism at three levels: impaired secretion, impaired degradation and increased neolipogenesis.
Impaired secretion of lipoproteins from the infected hepatocyte was the first proposed mechanism of HCV-induced steatosis. Data suggest that HCV core protein may cause hepatic steatosis through inhibition of microsomal triglyceride transfer protein (MTP) activity [Perlemuter et al. 2002] because this enzyme plays a key rate-limiting role in very-low-density lipoprotein assembly. This inhibits the secretion of VLDL consisting of apolipoprotein B, cholesterol and triglycerides from the liver. The findings of impaired MTP functioning as a result of HCV core protein expression have more recently been extended to humans. In a study of 58 patients infected with various HCV genotypes, a highly significant (p = 0.0017), inverse correlation was found for liver MTP mRNA levels and the degree of hepatic steatosis that was independent of genotype, suggesting an important role for MTP in hepatic steatosis [Mirandola et al. 2006].
HCV may also cause steatosis by decreasing fatty acid oxidation through impairment of the expression and transcriptional activity of PPARα which regulates several genes responsible for fatty acid degradation [Cheng et al. 2005] (see below).
HCV may induce fatty liver by stimulating de novo synthesis of fatty acids. During primary infection in chimpanzees, HCV activates genes involved in lipid metabolism via upregulation of sterol regulatory element binding protein-1c (SREBP) [Waris et al. 2007; Su et al. 2002]. SREBP activity is stimulated in vitro by several viral proteins, including core [Waris et al. 2007] and nonstructural proteins 2 [Oem et al. 2008] and 4B [Park et al. 2009; Oem et al. 2008; Waris et al. 2007]. Activation of SREBP and several enzymes involved in lipidogenesis has also been reported in transgenic mice expressing different HCV proteins [Lerat et al. 2009; Chang et al. 2008]. In addition to activating SREBP, the HCV core protein may also bind to and activate the DNA-binding domain of the retinoid receptor α, a transcriptional regulator that controls many cellular functions including cellular lipid synthesis [Fukasawa et al. 2006].
Finally, an additional mechanism leading to fatty liver may be related to the increased prevalence of IR among patients with CHC. IR increases the peripheral release and hepatic uptake of fatty acids, resulting in an accumulation of lipid in the liver [Browning and Horton, 2004]. Although HCV may be associated with extrahepatic IR [Milner et al. 2010; Vanni et al. 2009], this seems to involve striated muscles rather than the adipose tissue. Thus, there is no evidence as yet that HCV may induce fatty liver via increased lipolysis in adipocytes.
Insulin resistance and steatosis: the chicken and the egg relationship
Although both IR and steatosis are common findings in patients with CHC, what comes first is not entirely clear. In the absence of HCV infection, much of the cardiovascular and endocrine literature has attributed IR to the presence of excessive triglyceride in organs such as the liver and muscle [den Boer et al. 2004; Ryysy et al. 2000]. By contrast, accumulating data seem to indicate that rather than causing IR, the presence of visible hepatocyte triglyceride droplets is a consequence of IR, hyperinsulinemia, and the result of excessive flux of free fatty acids through the liver [Monetti et al. 2007; Buettner et al. 2004].
Moreover, it is still unclear if hepatic steatosis represents the first hit to proinflammatory cytokine production which then leads to the development of IR or vice versa. Recent evidence, discriminating between ‘systemic’ and ‘hepatic’ IR, shows that in young, lean, patients with IR there was a low prevalence of hepatic steatosis and no cytokine/adipocytokine changes. This suggests that steatosis and cytokines interact without assuming a primary and independent role in the early stage of IR [Petersen et al. 2007]. Alternatively, other authors support the idea that hyperinsulinemia is likely to be the consequence rather than the cause of a fatty liver, as suggested by the fact that fatty liver is associated with both hepatic IR and impaired insulin clearance [Harsha and Bray, 2008; Hickman et al. 2004].
In patients with CHC, the HCV virus seems to play an additive role in this complex and reciprocal relationship as evidenced by the accumulating evidence of a direct ‘metabolic’ effect of the HCV virus on a large number of molecular pathways that lead to hepatic steatosis, IR and metabolic syndrome [Sheikh et al. 2008; Eckel et al. 2005]. Whereas core proteins from all HCV genotypes appear to influence this relationship, genotype-specific mechanisms may account for these effects, and although selected genotypes (e.g. 3a) may be more efficient in promoting hepatic steatosis, triglyceride droplets may be inert with respect to promoting injury and altered cellular homeostasis. In this case fat accumulation does not in itself precipitate IR and those patients who are IR typically have other causes such as obesity [Hui et al. 2003].
Preexisting metabolic conditions such as metabolic syndrome can also contribute to IR and steatosis in patients with HCV. In a study of 271 patients without diabetes, liver fat was four times higher in patients with metabolic syndrome (median 8.2%) compared with those without metabolic syndrome (median 2.0%; p = 0.0001), and this difference remained significant when adjusted for age, gender and body mass index (BMI) (p = 0.011) [Kotronen et al. 2007]. Among patients with metabolic syndrome in this study, liver fat was progressively increased (p = 0.0001 for trend) in patients as the number of components of metabolic syndrome increased. Patients with metabolic syndrome also had significantly higher fasting serum insulin and C-peptide concentrations than those without metabolic syndrome independent of age, gender and BMI (fasting serum insulin adjusted r = 0.39, p = 0.0001; C-peptide adjusted r = 0.36, p = 0.0001) [Kotronen et al. 2007]. Thus, this study suggests that liver fat content is an important component of the metabolic syndrome and increases proportionally with the number of components of the metabolic syndrome. Moreover, of all measures of IR, fasting serum insulin and C-peptide were the best correlates of liver fat [Kotronen et al. 2007]. These findings, taken together, suggest a definite genotypic association between the IR and steatosis/steatohepatitis with HCV as the ‘third player’. However, to further refine the nature of this interaction more investigation of the genotypic specificity of the virus–host interaction is needed.
Interaction between hepatitis C and PPARs
The interaction between HCV products and PPAR expression has been an area of recent focus. Liver inflammation, hepatocyte fat accumulation, and diabetes are three pivotal hallmarks in the natural history of CHC infection which are at least in part controlled by PPARs. Expression of PPARα appears to be impaired with HCV infection [de Gottardi et al. 2006; Dharancy et al. 2005]. In patients with CHC, expression of the PPARα gene in the liver was reduced by 86% compared with controls, and the expression of its target gene, CPT1A, was coordinately reduced by 85%. Thus, hepatocytes infected with HCV display abnormally low levels of PPARα. Alternatively, PPARγ, RXR and liver X receptor were not different. Similarly, expression of the core protein in hepatoma cells was also found to reduce PPARα levels, but not the expression of the aforementioned receptors, and the induced expression of the CPT1A target gene by fenofibric acid was inhibited in core protein-expressing cells but not control cells [Dharancy et al. 2005].
Recent evidence of a direct role for PPARα activation in the pathogenesis of steatosis and HCC has been obtained from studies that combined core gene transgenic mice with PPARα knockout (KO) mice. The results of these experiments found that PPARα KO mice have reduced expression of target genes of PPARα, and have mild steatosis in the liver as expected [Tanaka et al. 2008]. Steatosis and hepatocellular carcinoma developed in PPARα intact but not in PPARα heterozygous or PPARα null core gene transgenic mice, indicating that not the presence but the persistent activation of PPARα would be important in hepatocarcinogenesis by HCV core protein. In general, PPARα acts to ameliorate steatosis, but with the presence of mitochondrial dysfunction, which is also provoked by the core protein, the core activated PPARα may exacerbate steatosis. This study showed that expression of the core protein in and of itself was insufficient to cause steatosis, as evidenced by the lack of steatosis in mice heterozygous or homozygous for the deletion of PPARα. A persistent activation of PPARα with ‘strong’ ligands such as the core protein of HCV could be carcinogenic in humans, although the low-affinity fibrate ligands are not likely associated with human cancers [Tanaka et al. 2008].
Others have shown that PPARγ expression is significantly lower in genotype 3 compared with genotype 1 HCV infection and that steatosis is associated with decreased levels of PPARγ in genotype 1 HCV infection [de Gottardi et al. 2006]. In this study, there was no significant relationship between the PPARs mRNA levels and liver activity or fibrosis. The presence of steatosis and HCV genotype 3 were both associated with a significant downregulation of PPARs.
Perspectives for treatment
Although, increasing insulin sensitivity may be a rationale option in patients with CHC, especially those with metabolic syndrome, the modalities for this intervention have not been established yet. The primary data on the use of pioglitazone (PIO) was from a prospective, multicenter study aimed at investigating the efficacy and safety of PIO, 15 mg daily, added to pegylated interferon-2α (Peg-IFN-2α), 180 g every week and ribavirin (RBV) 1000–1200 mg daily combination therapy in retreatment of patients with CHC who were previously nonresponders to a Peg-IFN-α/RBV combination. All patients were IR and had a baseline HOMA > 2 [Overbeck et al. 2008]. The study was prematurely terminated as none of the first five patients enrolled into the trial had a sufficient virological response after 12 weeks. The dose of PIO used in this study was quite low, however, and may have impacted on the results [Negro, 2009]. The following emerging data from the four available studies evaluating the use of PIO in addition to the Peg-IFN α/RBV combination is provocative [Khattab et al. 2010; Vierling et al. 2010; Conjeevaram et al. 2008; Elgouhari et al. 2008]. In one study of treatment-naïve, non-diabetic, genotype 1 patients, 30 mg/day of PIO was given for 4 weeks as monotherapy and then added for the first 4 weeks of a standard therapy. The authors showed that the triple regimen increased the rate of virological response significantly after 4 weeks of therapy compared with the standard of care. Long-term data from this study is keenly awaited [Elgouhari et al. 2008]. In another randomized, double-blind, placebo-controlled study in genotype 1 patients, adding PIO 30 mg/day simultaneously to the standard of care (i.e. without preceding administration as monotherapy) clearly improved IR, steatosis and increased the on-treatment virological response but failed to increase the SVR [Conjeevaram et al. 2008]. The third trial was conducted conclusively in patients infected with HCV genotype 4. The use of PIO 30 mg/day simultaneously with the standard antiviral therapy increased rapid virologic response (RVR) and SVR rates with improvement in all parameters of IR [Khattab et al. 2010].
Results from another study were previously presented at the 2010 meeting of the American Association for the Study of Liver Disease. The sequential use of PIO for 16 weeks (30 mg/day × 8 weeks then 45 mg/day × 8 weeks) prior to and concordant with 48 weeks of standard of care therapy revealed improvements in several glycemic variables, but no improvement in RVR or early virologic response (EVR) was seen. Data on SVR were not available [Vierling et al. 2010].
The final answer as to whether improving IR with thiazolidinedione therapy such as PIO equates to improved virologic response remains unknown at the current time. It is possible that there may be genotypic variations in response. In addition, genetic and epigenetic influences, such as IL28B single nucleotide polymorphisms, may be associated with IR and despite improvement in IR enhancement in SVR may not be seen. Clinical trials are underway seeking answers to these questions and the data are eagerly anticipated.
Acknowledgements
All authors contributed equally to this article. The opinion or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the view of the US Department of the Army or the US Department of Defense.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Advisory Board for Bristol Myers Squibb and Merck. Speaker’s bureau for Bristol Myers Squibb and Merck. Research support from Genentech, Merck, and Rottapharm. No potential competing interests.
References
- Adinolfi L.E., Gambardella M., Andreana A., Tripodi M.F., Utili R., Ruggiero G. (2001) Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 33: 1358e64–1358e64 [DOI] [PubMed] [Google Scholar]
- Anderson S.P., Yoon L., Richard E.B., Dunn C.S., Cattley R.C., Corton J.C. (2002) Delayed liver regeneration in peroxisome proliferator-activated receptor-α-null mice. Hepatology 36: 544–554 [DOI] [PubMed] [Google Scholar]
- Allison M.E., Wreghitt T., Palmer C.R., Alexander G.J. (1994) Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol 21: 1135–1139 [DOI] [PubMed] [Google Scholar]
- Armoni M., Kritz N., Harel C., Bar-Yoseph F., Chen H., Quon M.J., et al. (2003) Peroxisome proliferators activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J Biol Chem 278: 30614–30623 [DOI] [PubMed] [Google Scholar]
- Asselah T., Rubbia-Brandt L., Marcellin P., Negro F. (2006) Steatosis in chronic hepatitis C: why does it really matter? Gut 55: 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot O., Aldridge T.C., Latruffe N., Green S. (1993) PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochem Biophys Res Commun 192: 37–45 [DOI] [PubMed] [Google Scholar]
- Berthiaume M., Sell H., Lalonde J., Gélinas Y., Tchernof A., Richard D., et al. (2004) Actions of PPAR gamma agonism on adipose tissue remodeling, insulin sensitivity, and lipemia in absence of glucocorticoids. Am J Physiol Regul Integr Comp Physiol 287: R1116–R1123 [DOI] [PubMed] [Google Scholar]
- Browning J.D., Horton J.D. (2004) Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147e52–147e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Ottinger I., Scholmerich J., Bollheimer L.C. (2004) Preserved direct hepatic insulin action in rats with diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 286: E828–E833 [DOI] [PubMed] [Google Scholar]
- Caronia S., Taylor K., Pagliaro L., Carr C., Palazzo U., Petrik J., et al. (1999) Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology 30: 1059–1063 [DOI] [PubMed] [Google Scholar]
- Castelein H., Gulick T., Declercq P.E., Mannaerts G.P., Moore D.D., Baes M.I. (1994) The peroxisome proliferator activated receptor regulates malic enzyme gene expression. J Biol Chem 269: 26754–26758 [PubMed] [Google Scholar]
- Chang M.L., Yeh C.T., Chen J.C., Huang C.C., Lin S.M., Sheen I.S., et al. (2008) Altered expression patterns of lipid metabolism genes in an animal model of HCV core related, non obese, modest hepatic steatosis. BMC Genomics 9: 109–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehadeh W., Abdella N., Ben-Nakhi A., Al-Arouj M., Al-Nakib W. (2009) Risk factors for the development of diabetes mellitus in chronic hepatitis C virus genotype 4 infection. J Gastroenterol Hepatol 24: 42–48 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Dharancy S., Malapel M., Desreumaux P. (2005) Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol 11: 7591–7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.J., Lee S.D., Hung T.H., Lin H.C., Hwang S.J., Lee F.Y., et al. (2009) Insulin resistance is a major determinant of sustained virological response in genotype 1 chronic hepatitis C patients receiving peginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther 29: 46–54 [DOI] [PubMed] [Google Scholar]
- Conjeevaram H., Burant C.F., McKenna, Harsh D., Kang H., Das A.K., et al. (2008) A randomized, double-blind, placebo-controlled study of PPAR gamma agonist pioglitazone given in combination with peginterferon and ribavirin in patients with genotype-1 chronic hepatitis C. Hepatology 48: 384A–384A [Google Scholar]
- Conjeevaram H.S., Kleiner D.E., Everhart J.E., Hoofnagle J.H., Zacks S., Afdhal N.H., et al. (2007) Race, insulin resistance and hepatic steatosis in chronic hepatitis C. Hepatology 45: 80–87 [DOI] [PubMed] [Google Scholar]
- Dai C.Y., Huang J.F., Hsieh M.Y., Hou N.J., Lin Z.Y., Chen S.C., et al. (2009) Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol 50: 712–718 [DOI] [PubMed] [Google Scholar]
- de Gottardi A., Pazienza V., Pugnale P., Bruttin F., Rubbia-Brandt L., Juge-Aubry C.E., et al. (2006) Peroxisome proliferator-activated receptor-alpha and-gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther 23: 107–114 [DOI] [PubMed] [Google Scholar]
- den Boer M., Voshol P.J., Kuipers F., Havekes L.M., Romijn J.A. (2004) Hepatic steatosis: A mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol 24: 644–649 [DOI] [PubMed] [Google Scholar]
- Desvergne B., Wahli W. (1999) Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev 20: 649–688 [DOI] [PubMed] [Google Scholar]
- Dharancy S., Malapel M., Perlemuter G., Roskams T., Cheng Y., Dubuquoy L., et al. (2005) Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology 128: 334–342 [DOI] [PubMed] [Google Scholar]
- Eckel R.H., Grundy S.M., Zimmet P.Z. (2005) The metabolic syndrome. Lancet 365: 1415–1428 [DOI] [PubMed] [Google Scholar]
- Elgouhari H.M., Cesario K.B., Lopez R., Zein N. (2008) Pioglitazone improves early virologic kinetic response to PEG IFN/RBV combination therapy in hepatitis C genotype 1 naïve pts. Hepatology 48: 383A–383A [Google Scholar]
- Escher P., Wahli W. (2000) Peroxisome proliferator-activated receptors: Insight into multiple cellular functions. Mutat Res 448: 121–138 [DOI] [PubMed] [Google Scholar]
- Eslam, M., Khattab, M.A., Harrison, S.A. (2011) Insulin resistance and hepatitis C: an evolving story. Gut Jan 19. [DOI] [PubMed]
- Everett L., Galli A., Crabb D. (2000) The role of hepatic peroxisome proliferator-activated receptors (PPARs) in health and disease. Liver 20: 191–199 [DOI] [PubMed] [Google Scholar]
- Fajas L., Auboeuf D., Raspé E., Schoonjans K., Lefebvre A.M., Saladin R., et al. (1997) The organization, promoter analysis, and expression of the human PPARγ gene. J Biol Chem 272: 18779–18789 [DOI] [PubMed] [Google Scholar]
- Frohnert B.I., Hui T.Y., Bernlohr D.A. (1999) Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem 274: 3970–3977 [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Tanaka Y., Sato S., Ono Y., Nitahara-Kasahara Y., Suzuki T., et al. (2006) Enhancement of de novo fatty acid biosynthesis in hepatic cell line Huh7 expressing hepatitis C virus core protein. Biol Pharm Bull 29: 1958e61–1958e61 [DOI] [PubMed] [Google Scholar]
- Gearing K.L., Gottlicher M., Teboul M., Widmark E., Gustafsson J.A. (1993) Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A 90: 1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbert S., Valensi P., Levy-Marchal C., Perret G., Richardet J.P., Raffoux C., et al. (1996) High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case–control study. Gastroenterol Clin Biol 20: 544–548 [PubMed] [Google Scholar]
- Harsha D.W., Bray G.A. (2008) Weight loss and blood pressure control (Pro). Hypertension 51: 1420–1425 discussion 1425 [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Cook W.S., Qi C., Yeldandi A.V., Reddy J.K., Rao M.S. (2000) Defect in peroxisome proliferators activated receptor α-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem 275: 28918–28928 [DOI] [PubMed] [Google Scholar]
- Hertz R., Bishara-Shieban J., Bar-Tana J. (1995) Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem 270: 13470–13475 [DOI] [PubMed] [Google Scholar]
- Hess R., Staubli W., Riess W. (1965) Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature 208: 856–858 [DOI] [PubMed] [Google Scholar]
- Hickman I.J., Jonsson J.R., Prins J.B., Ash S., Purdie D.M., Clouston A.D., et al. (2004) Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M.H., Savas U., Griffin K.J., Johnson E.F. (2001) Identification of peroxisome proliferators responsive human genes by elevated expression of the peroxisome proliferators activated receptor α in HepG2 cells. J Biol Chem 276: 27950–27958 [DOI] [PubMed] [Google Scholar]
- Hui J.M., Sud A., Farrell G.C., Bandara P., Byth K., Kench J.G., et al. (2003) Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology 125: 1695–1704 [DOI] [PubMed] [Google Scholar]
- Jay M.A., Ren J. (2007) Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev 3: 33–39 [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Ide T., Taniguchi E., Hirano E., Itou M., Sumie S., et al. (2007) Clearance of HCV improves insulin resistance, betacell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol 102: 570–576 [DOI] [PubMed] [Google Scholar]
- Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. (1999) Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 103: 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab M., Emad M., Abdelaleem A., Eslam M., Atef R., Shaker Y., et al. (2010) Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int 30: 447–454 [DOI] [PubMed] [Google Scholar]
- Khattab M., Eslam M., Sharwae M.A., Shatat M., Ali A., Hamdy L. (2010) Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol 105: 1970–1977 [DOI] [PubMed] [Google Scholar]
- Koike K. (2009) Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol 44(Suppl. 19)82–88 [DOI] [PubMed] [Google Scholar]
- Kotronen A., Westerbacka J., Bergholm R., Westerbacka J., Cornér A., Bergholm R., et al. (2007) Liver fat in the metabolic syndrome. J Clin Endocrinol Metab 92: 3490–3497 [DOI] [PubMed] [Google Scholar]
- Latruffe N., Cherkaoui Malki M., Nicolas-Frances V., Clemencet M.C., Jannin B., Berlot J.P. (2000) Regulation of the peroxisomal β-oxidation-dependent pathway by peroxisome proliferator-activated receptor α and kinases. Biochem Pharmacol 60: 1027–1032 [DOI] [PubMed] [Google Scholar]
- Le Brasseur N.K., Kelly M., Tsao T.S., Tsu-Shuen Tsao S.R., Asish K., et al. (2006) Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab 291: E175–E181 [DOI] [PubMed] [Google Scholar]
- Lee S.S., Pineau T., Drago J., Lee E.J., Owens J.W., Kroetz D.L., et al. (1995) Targeted disruption of the alpha isoform of the peroxisome proliferator activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15: 3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P., Chinetti G., Fruchart J.C., Staels B. (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerat H., Kammoun H.L., Hainault I., Mérour E., Higgs M.R., Callens C., et al. (2009) Hepatitis C virus (HCV) proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem 284: 33466e74–33466e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandard S., Müller M., Kersten S. (2004) Peroxisome proliferator-activated receptor a target genes. Cell Mol Life Sci 61: 393–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A.L., Lau J.Y., Hoang N., Qian K., Alexander G.J., Xu L., et al. (1999) Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology 29: 328–333 [DOI] [PubMed] [Google Scholar]
- Milner K.L., van der Poorten D., Trenell M., Jenkins A.B., Xu A., Smythe G., et al. (2010) Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology 138: 932–941 e1–e3 [DOI] [PubMed] [Google Scholar]
- Mirandola S., Realdon S., Iqbal J., Gerotto M., Dal Pero F., Bortoletto G., et al. (2006) Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology 130: 1661–1669 [DOI] [PubMed] [Google Scholar]
- Mizuta T., Kawaguchi Y., Eguchi Y., Takahashi H., Ario K., Akiyama T., et al. (2010) Whole-body insulin sensitivity index is a highly specific predictive marker for virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients with genotype 1b and high viral load. Dig Dis Sci 55: 183–189 [DOI] [PubMed] [Google Scholar]
- Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K., et al. (2007) Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78 [DOI] [PubMed] [Google Scholar]
- Moriya K., Todoroki T., Tsutsumi T., Fujie H., Shintani Y., Miyoshi H., et al. (2001) Increase in the concentration of carbon-18 monounsaturated fatty acids in the liver with hepatitis C: Analysis in transgenic mice and humans. Biophys Biochem Res Commun 281: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Moucari R., Asselah T., Cazals-Hatem D., Voitot H., Boyer N., Ripault M.P., et al. (2008) Insulin resistance in chronic hepatitis C: Association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 134: 416–423 [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Inada Y., Nakano S., Tamura T., Takahashi T., Maruyama K., et al. (2006) Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPAR delta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol 536: 182–191 [DOI] [PubMed] [Google Scholar]
- Nagashima K., Lopez C., Donovan D., Ngai C., Fontanez N., Bensadoun A., et al. (2005) Effects of the PPAR gamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest 115: 1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M.T., Cheon Y., Li Y., Nara T.Y. (2004) Mechanisms of regulation of gene expression by fatty acids. Lipids 39: 1077–1083 [DOI] [PubMed] [Google Scholar]
- Negro F. (2009) Correction of insulin resistance in chronic hepatitis C patients not responding to the standard of care: More questions than answers. J Hepatol 50: 1271–1272 [DOI] [PubMed] [Google Scholar]
- Negro F., Sanyal A.J. (2009) Hepatitis C virus, steatosis and lipid abnormalities: Clinical and pathogenic data. Liver Int 29(Suppl. 2)26–37 [DOI] [PubMed] [Google Scholar]
- Neschen S., Morino K., Rossbacher J.C., Pongratz R.L., Cline G.W., Sono S., et al. (2006) Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptorgamma-dependent mechanism in mice. Diabetes 55: 924–928 [DOI] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell 97: 161–163 [DOI] [PubMed] [Google Scholar]
- Oem J.K., Jackel-Cram C., Li Y.P., Zhou Y., Zhong J., Shimano H., et al. (2008) Activation of sterol regulatory element-binding protein 1c and fatty acid synthase transcription by hepatitis C virus non-structural protein 2. J Gen Virol 89: 1225e30–1225e30 [DOI] [PubMed] [Google Scholar]
- Overbeck K., Genné D., Golay A., Negro F. (2008) Pioglitazone in chronic hepatitis C not responding to pegylated interferon alpha and ribavirin. J Hepatol 49: 295–298 [DOI] [PubMed] [Google Scholar]
- Pajvani U.B., Hawkins M., Combs T.P., Rajala M.W., Doebber T., Berger J.P., et al. (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162 [DOI] [PubMed] [Google Scholar]
- Park C.Y., Jun H.J., Wakita T., Cheong J.H., Hwang S.B. (2009) Hepatitis C virus nonstructural 4B protein modulates sterol regulatory element-binding protein signaling via the AKT pathway. J Biol Chem 284: 9237e46–9237e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D., Mandard S., Voshol P.J., Escher P., Tan N.S., Havekes L.M., et al. (2004) PPAR alpha governs glycerol metabolism. J Clin Invest 114: 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazienza V., Clément S., Pugnale P., Conzelman S., Foti M., Mangia A., et al. (2007) The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology 45: 1164–1171 [DOI] [PubMed] [Google Scholar]
- Pekow J.R., Bhan A.K., Zheng H., Chung R.T. (2007) Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer 109: 2490–2496 [DOI] [PubMed] [Google Scholar]
- Perlemuter G., Sabile A., Letteron P., Vona G., Topilco A., Chrétien Y., et al. (2002) Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J 16: 185–194 [DOI] [PubMed] [Google Scholar]
- Petersen K.F., Dufour S., Savage D.B., Bilz S., Solomon G., Yonemitsu S., et al. (2007) The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 104: 12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustchi H., Negro F., Hui J., Cua I.H., Brandt L.R., Kench J.G., et al. (2008) Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol 48: 28–34 [DOI] [PubMed] [Google Scholar]
- Poynard T., Ratziu V., McHutchison J., Manns M., Goodman Z., Zeuzem S., et al. (2003) Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 38: 75e85–75e85 [DOI] [PubMed] [Google Scholar]
- Romero-Gómez M., Fernández-Rodríguez C.M., Andrade R.J., Diago M., Alonso S., Planas R., et al. (2008) Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol 48: 721–727 [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Giostra E., Mentha G., Quadri R., Negro F. (2001) Expression of liver steatosis in hepatitis C virus infection and pattern of response to alpha-interferon. J Hepatol 35: 307–307 [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Quadri R., Abid K., Giostra E., Malé P.J., Mentha G., et al. (2000) Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol 33: 106–115 [DOI] [PubMed] [Google Scholar]
- Ryysy L., Häkkinen A.M., Goto T., Vehkavaara S., Westerbacka J., Halavaara J., et al. (2000) Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49: 749–758 [DOI] [PubMed] [Google Scholar]
- Sakamoto J., Kimura H., Moriyama S., Odaka H., Momose Y., Sugiyama Y., et al. (2000) Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun 278: 704–711 [DOI] [PubMed] [Google Scholar]
- Savage D.B., Tan G.D., Acerini C.L., Jebb S.A., Agostini M., Gurnell M., et al. (2003) Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes 52: 910–917 [DOI] [PubMed] [Google Scholar]
- Schoonjans K., Martin G., Staels B., Auwerx J. (1997) Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol 8: 159–166 [DOI] [PubMed] [Google Scholar]
- Schoonjans K., Peinado-Onsurbe J., Lefebvre A.M., Heyman R.A., Briggs M., Deeb S., et al. (1996) PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15: 5336–5348 [PMC free article] [PubMed] [Google Scholar]
- Seeff L.B. (2002) Natural history of chronic hepatitis C. Hepatology 36: S35–S46 [DOI] [PubMed] [Google Scholar]
- Sheikh M.Y., Choi J., Qadri I., Friedman J.E., Sanyal A.J. (2008) Hepatitis C virus infection: Molecular pathways to metabolic syndrome. Hepatology 47: 2127–2133 [DOI] [PubMed] [Google Scholar]
- Staels B., Vu-Dac N., Kosykh V.A., Saladin R., Fruchart J.C., Dallongeville J., et al. (1995) Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest 95: 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A.I., Pezacki J.P., Wodicka L., Brideau A.D., Supekova L., Thimme R., et al. (2002) Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A 99: 15669–15674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D.J., Azarnoff D.L. (1966) Response of hepatic microbodies to a hypolipidemic agent, ethyl chlorophenoxyisobutyrate (CPIB). J Cell Biol 30: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Moriyam K., Kiyosawa K., Koike K., Gonzalez F.J., Aoyama T. (2008) PPAR alpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest 118: 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum H., Behar S., Boyko V., Adler Y., Fisman E.Z., Tanne D., et al. (2007) Long-term effect of bezafibrate on pancreatic beta-cell function and insulin resistance in patients with diabetes. Atherosclerosis 194: 265–271 [DOI] [PubMed] [Google Scholar]
- Thomopoulos K.C., Arvaniti V., Tsamantas A.C., Dimitropoulou D., Gogos C.A., Siagris D., et al. (2006) Prevalence of liver steatosis in patients with chronic hepatitis B: A study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol 18: 233–237 [DOI] [PubMed] [Google Scholar]
- Uchimura K., Nakamuta M., Enjoji M., Irie T., Sugimoto R., Muta T., et al. (2001) Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology 33: 91–99 [DOI] [PubMed] [Google Scholar]
- Vanni E., Abate M.L., Gentilcore E., Hickman I., Gambino R., Cassader M., et al. (2009) Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology 50: 697e706–697e706 [DOI] [PubMed] [Google Scholar]
- Vierling J.M., Prbhaker A., Han J., Harrison S.A., et al. (2010) Virologic and metabolic responses in chronic hepatitis C (CHC) patients with insulin resistance (IR) treated with pioglitazone and peginterferon alpha-2 A plus ribavirin. Final results of week 12 early virologic response. Hepatology 52: 707A–707A [Google Scholar]
- Waris G., Felmlee D.J., Negro F., Siddiqui A. (2007) Hepatitis C virus induces the proteolytic cleavage of sterol regulatory element binding proteins (SREBPs) and stimulates the phosphorylation of SREBPs via oxidative stress. J Virol 81: 8122–8130 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- World Health Organization (2000) Hepatitis C. Fact sheet number 164, revised October 2000. Available at: www.who.int/mediacentre/factssheets/fs164len [accessed 6 March 2009]
- Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769 [DOI] [PubMed] [Google Scholar]
- Yu Y.H., Ginsberg H.N. (2005) Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ Res 2005 96: 1042–1052 [DOI] [PubMed] [Google Scholar]