Abstract
In this article we provide a contemporary overview of available clinical data on certolizumab pegol, a pegylated anti-tumor necrosis factor (TNF) alpha agent that comprises a uniquely small protein, and its emerging role as a therapy for Crohn’s disease (CD). The results from a comprehensive clinical trial program suggest that certolizumab pegol offers rapid and sustained remission of moderate to severe CD. Certolizumab pegol is an effective and well-tolerated therapy both in patients who have already received biologics and in patients who are anti-TNF naïve. Benefits of therapy include a stable dosing regimen, which allows for rapid induction of a clinical response followed by long-term maintenance of response and remission under one fixed dose. Treatment with certolizumab pegol has been shown to improve function and quality of life in patients with CD, and insights into the potential mechanisms by which certolizumab pegol effects a response in CD suggest that this agent may have the potential to slow or even modify disease progression. Early therapy is particularly effective and could help control CD progression and lessen the burden of disease on patients.
Keywords: Anti-TNF, certolizumab pegol, Crohn’s disease, TNFα inhibitors
Introduction
Crohn’s disease (CD) can often be refractory to conventional treatments such as corticosteroids and oral immunosuppressants. In recent years, targeted biologic therapies against tumor necrosis factor (TNF) alpha have revolutionized the management of CD [Binion, 2010; Clark et al. 2007; Cominelli, 2004]. The era of biologic therapy began with the realization that TNF has an integral role in the pathogenesis of CD [Schreiber et al. 1999; Reinecker et al. 1993; MacDonald et al. 1990]. Monoclonal antibodies that bind to TNF have since become the cornerstone in the management of moderate to severe CD that is refractory to conventional treatment options [Bernstein et al. 2010; Binion, 2010; Lichtenstein et al. 2009; Sadowski et al. 2009]. The benefits of anti-TNF therapy include reduced reliance or dependence on corticosteroid-based therapies and the avoidance of corticosteroid-associated adverse effects [D’Haens et al. 2008; Colombel et al. 2007; Hanauer et al. 2002].
As evidence accumulates regarding the clinical efficacy of anti-TNF therapy in the long-term management of active CD, attention is now focused on understanding how to employ these agents in clinical practice to best meet the needs of patients with CD [Binion, 2010; Colombel et al. 2010; Van Assche et al. 2010; Rutgeerts et al. 2004]. CD is a chronic, progressive and disabling disease that requires long-term management. Optimizing outcomes in CD requires rapid control of inflammation [Cosnes et al. 2005]. Persistent inflammation in CD can lead to ongoing symptoms, organ damage and disease-related complications. The goals of biologic therapy are to induce rapid response, maintain disease remission without corticosteroids, achieve and maintain mucosal healing, prevent disease-related mortality and improve the patient’s health-related quality of life (HRQoL) [Lichtiger et al. 2010; Lichtenstein et al. 2009; Ng et al. 2009].
Complex moderate to severe CD, which corresponds to a CD Activity Index (CDAI) of more than 220 points, is characterized by failure to respond to treatment, with prominent symptoms of fever, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting, significant anemia or occurrence of complicating lesions such as deep colonic ulcer, perianal disease and/or fistulae [Lichtenstein et al. 2009]. Treatments for CD should offer both a rapid response and sustained disease remission, have a good benefit-to-risk profile and should, ideally, confer clinical benefits associated with improved HRQoL. Goals for the future include understanding whether earlier and more effective treatment of CD might modify disease progression and alter long-term outcomes for patients.
This review considers the most current data on the anti-TNF certolizumab pegol (Cimzia®, UCB Pharma) in the management of CD. It describes the unique physical properties of this anti-TNF and details the evidence and insights from a clinical trial program involving the study of certolizumab pegol in both patients new to biologic therapy and those who have received prior biologic therapy.
Certolizumab pegol: a unique anti-TNF
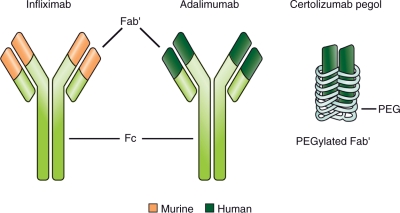
Certolizumab pegol is an anti-TNF with a unique structure (Figure 1) [Nesbitt et al. 2007]. Unlike other monoclonal antibodies indicated for the treatment of CD, such as infliximab [Centocor Ortho Biotech, 2009] and adalimumab [Abbott Laboratories, 2010], which are based on the human immunoglobulin 1 Fc, certolizumab pegol [UCB, 2009] does not contain an Fc portion and therefore does not display Fc-mediated effects (described below).
Figure 1.
Comparative structures of the anti-TNF agents infliximab, adalimumab and certolizumab pegol. Infliximab is a chimeric IgG1 monoclonal antibody, the Fc’ portion of which is human. Adalimumab is a human IgG1 monoclonal antibody. Certolizumab pegol is a human monoclonal antibody Fab′ conjugated with polyethylene glycol (PEG), an inert 40-kDa macromolecule used to enhance the pharmacokinetic properties of biologics. Adapted with permission from Adis Data Information BV [Bourne et al. 2008, 22(5): 331–337], copyright © 2008.
Certolizumab pegol is a PEGylated Fab′ of a human monoclonal antibody with high affinity for TNF. The PEGylation of a biological molecule changes its physical and chemical properties to improve its pharmacokinetic behavior, typically improving solubility, increasing drug stability and decreasing immunogenicity [Veronese and Mero, 2008]. In the case of certolizumab pegol, the Fab′ of human anti-TNF is conjugated with a 40-kDa polyethylene glycol molecule (PEG) [Nesbitt et al. 2007]. Certolizumab pegol is stable enough to be given by subcutaneous rather than intravenous injection. Studies in animal models have also shown that the PEGylation of certolizumab favors its distribution into inflamed tissue, a pharmacokinetic feature of importance for the effective treatment of chronic inflammation [Eddleston et al. 2009].
Studies to determine the mode of action of anti-TNF agents in CD suggest that certolizumab pegol differs from other anti-TNF agents in ways that do not affect its clinical efficacy in CD but that may affect its tolerability profile. In common with other agents, certolizumab pegol has been shown in vitro to bind to soluble and membrane-bound TNF—actions considered key to clinical efficacy. In the pathogenesis of CD, bacteria play a major part in inflammatory processes and, in in vitro studies, certolizumab pegol has been shown to inhibit monocyte cytokine production induced by bacterial lipopolysaccharide [Nesbitt et al. 2007]. However, unlike other anti-TNF agents, certolizumab pegol does not cause complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, apoptosis or necrosis of neutrophils [Bourne et al. 2008; Nesbitt et al. 2007]. As certolizumab pegol has been shown to be an effective agent for use in the clinical management of active CD, these latter mechanisms may not be required for efficacy in the treatment of CD but could be associated with some of the known undesirable effects of conventional anti-TNF therapy.
Additional modes of action for certolizumab pegol have also been identified as of potential importance to the observed clinical profile of this agent in CD. In vitro studies on mast cells suggest that the PEG component of certolizumab pegol can inhibit the degranulation response through pathways other than those mediated through immunologic processes [Lamour et al. 2009]. The concentrations at which this action is effected are similar to those expected transiently at the injection site (but not after dilution systemically in the blood stream) and may explain the low level of injection site pain observed with certolizumab pegol in clinical studies [Sandborn et al. 2010a, 2010b, 2007; Fleischmann et al. 2009; Lamour et al. 2009; Smolen et al. 2009; Keystone et al. 2008; Schreiber et al. 2007, 2005].
Approval status and prescriber information
Certolizumab pegol, in addition to being approved for the treatment of rheumatoid arthritis in the United States and several member states of the European Union, is currently approved in the United States, Switzerland and Russia for reducing the signs and symptoms of CD and maintaining clinical response in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy [UCB, 2009]. Certolizumab pegol is administered by subcutaneous injection at an initial dose of 400 mg (given as two injections of 200 mg) and repeated at weeks 2 and 4 (the induction dose). If a clinical response occurs, certolizumab pegol is then given at a dose of 400 mg every 4 weeks (the maintenance dose) [UCB, 2009]. In Canada and in some European countries, certolizumab pegol is not approved for the treatment of CD. The European Medicines Agency cited concerns about insufficient evidence of efficacy and the short duration of the maintenance phases in the PEGylated Antibody Fragment Evaluation in Crohn’s Disease: Safety and Efficacy (PRECiSE) 1 and 2 trials.
Efficacy of certolizumab pegol in CD
Induction therapy
Certolizumab pegol has been shown to be effective as induction therapy in patients with moderate to severe CD, offering a rapid treatment response and symptom relief [Schreiber et al. 2009, 2007, 2005; Sandborn et al. 2007].
A phase II placebo-controlled dose-ranging study of 292 patients with moderate to severe CD investigated the efficacy of certolizumab pegol 100, 200 or 400 mg versus placebo [Schreiber et al. 2005]. The primary endpoint was the percentage of patients with clinical response (defined as CDAI score reduction of at least 100 points [CDAI-100]) or clinical remission (CDAI equal to or less than 150 points) at week 12. All certolizumab pegol doses produced significant clinical benefit versus placebo as early as week 2. At week 2, the clinical benefit was higher for certolizumab pegol 400 mg (33%), compared with placebo (15%, p = 0.01).
The PRECiSE 1 study [Sandborn et al. 2007], a phase III double-blind randomized trial, compared efficacy and safety of certolizumab pegol against placebo in patients with moderate to severe CD. The induction phase of this study involved treatment with either certolizumab pegol 400 mg (n = 329) or placebo (n = 331) at weeks 0, 2 and 4, and the CDAI-100 response was determined. In the overall population, response rates at week 6 were 35% in the certolizumab group and 27% in the placebo group (p = 0.02); at both weeks 6 and 26, the response rates were 23% and 16%, respectively (p = 0.02). Rates of remission did not differ significantly between the two groups at week 6 (p = 0.17). However, among patients with a baseline C-reactive protein (CRP) concentration ≥10 mg/l, the week 6 response rate was 37% in the certolizumab pegol group compared with 26% in the placebo group (p = 0.04).
PRECiSE 2 is a second pivotal phase III trial of certolizumab pegol in patients with moderate to severe CD [Schreiber et al. 2007]. Both active treatment and placebo groups received open-label induction with certolizumab pegol 400 mg at weeks 0, 2 and 4. At week 6 (after three doses of certolizumab pegol), 64% (428/668) of patients had a response to induction therapy (defined as CDAI-100) and 43% (289/668) achieved remission (defined as a CDAI score ≤150) [Schreiber et al. 2009].
Maintenance therapy with certolizumab pegol: the PRECiSE trials
There is good evidence that, following successful induction of clinical response, certolizumab pegol, at a stable dose of 400 mg every 4 weeks, is an effective maintenance therapy in patients with active CD. The two pivotal phase III randomized clinical trials, PRECiSE 1 and PRECiSE 2 [Sandborn et al. 2007; Schreiber et al. 2007], have shown that maintenance treatment with certolizumab pegol 400 mg is associated with significantly greater rates of clinical response compared with placebo. Moreover, patients who responded to induction therapy were also more likely to have a sustained response and remission at 26 weeks with continued certolizumab pegol treatment compared with patients who switched to placebo.
In PRECiSE 1, following an induction phase of either certolizumab pegol 400 mg (n = 329) or placebo (n = 331) at weeks 0, 2 and 4, patients received maintenance treatment with certolizumab pegol 400 mg (n = 202) or placebo (n = 153) from week 8 every 4 weeks until week 26 [Sandborn et al. 2007]. Primary endpoints were the induction of a response at week 6 and a response at both weeks 6 and 26. Significantly greater rates of response (CDAI-100) to certolizumab pegol treatment compared with placebo treatment were observed at week 26 of maintenance therapy in the overall population (23% versus 16%; p = 0.02). Rates of remission at week 26 in the two groups did not differ significantly (p = 0.17); however, the proportion of responders in the certolizumab pegol and placebo groups remained similar between the start and completion of the maintenance phase: at week 26, 23% and 16% of patients achieved response in the certolizumab pegol and placebo groups, respectively (p = 0.02). Among patients whose baseline concentration of CRP was ≥10 mg/l, the proportion of certolizumab pegol responders to placebo responders was significantly greater at the end of the 26-week maintenance phase; 22% (31/144) and 12% (19/154) of patients in the certolizumab pegol and placebo groups, respectively, had a response at week 26 (p = 0.05) [Sandborn et al. 2007].
The PRECiSE 2 trial was designed specifically to evaluate the efficacy of certolizumab pegol 400 mg every 4 weeks as maintenance therapy [Schreiber et al. 2007]. The maintenance phase was randomized, double blind and placebo controlled, and only those patients with a clinical response (CDAI-100) following open-label induction (see above) at week 6 were assigned to either the active or placebo groups. Patients in either group were also stratified according to their baseline CRP concentration (either <10 mg/l or ≥10 mg/l) and concomitant use of immunosuppressants. Patients were randomly assigned to receive either 400 mg certolizumab pegol or placebo every 4 weeks through week 24, with follow up at week 26.
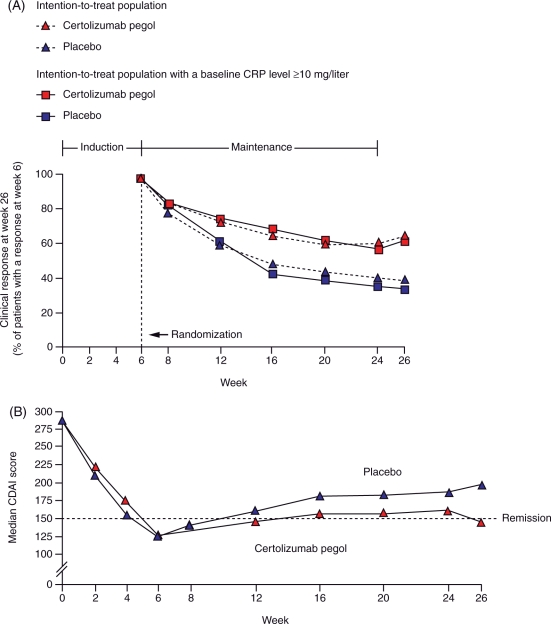
As in PRECiSE 1, clinical response in PRECiSE 2 was maintained through to week 26; response rates in the intention-to-treat population at week 26 were 63% (135/215) in certolizumab pegol-treated patients compared with 36% (76/210) for placebo (p < 0.001). Similar results were observed in patients with baseline CRP ≥10 mg/l; 62% (69/112) of patients receiving certolizumab pegol 400 mg every 4 weeks achieved a clinical response compared with 34% (34/101) of subjects randomized to placebo (p < 0.001) (Figure 2A). At week 26, 48% (103/215) of patients in the certolizumab pegol group achieved remission compared with 29% (60/210) of patients in the placebo group (p < 0.001) (Figure 2B) [Schreiber et al. 2007].
Figure 2.
(A) Response over time in patients in the intention-to-treat population who had responded to induction therapy at week 6 in PRECiSE 2. The percentage of patients with a response in the certolizumab pegol group was greater than in the placebo group in both the intention-to-treat population and in the high C-reactive protein (CRP) group (p < 0.001). Reprinted with permission from the Massachusetts Medical Society [Schreiber et al. 2007], copyright © 2007. (B) Median Crohn’s Disease Activity Index (CDAI) scores (last observation carried forward) over time in the intention-to-treat population in PRECiSE 2. Median CDAI remission scores were significantly lower in the certolizumab pegol group than in the placebo group at week 16 (p = 0.03), week 20 (p = 0.02), week 24 (p = 0.008) and week 26 (p < 0.001). Reprinted with permission from the Massachusetts Medical Society [Schreiber et al. 2007], copyright © 2007.
In a subanalysis of the PRECiSE 2 study, the superior efficacy of certolizumab pegol versus placebo treatment in maintaining disease response was shown in patients either taking immunosuppressants or naïve to immunosuppressants. At week 26, response rates were significantly greater among patients receiving maintenance therapy with certolizumab pegol 400 mg than among those receiving placebo, both for patients receiving concomitant immunosuppressive agents (61% [53/87] versus 33% [28/86], p < 0.001) and those not receiving concomitant immunosuppressive agents (64% [82/128] versus 39% [48/124], p < 0.001) [Schreiber et al. 2010b].
Data from PRECiSE 2 also suggest that early initiation of treatment (i.e. treatment early after first diagnosis of CD) may achieve improved outcomes. Maintenance of response to certolizumab pegol was achieved in 90% of patients in whom diagnosis of CD had been made within the previous year compared with rates of response of 75% in subjects with a diagnosis made between 1 and 2 years previously, 62% in cases where diagnosis was made 2–5 years previously and 57% when there had been a diagnosis of CD 5 or more years before study entry. Remission rates also showed this inverse relationship with disease duration. Other factors identified as predictors of maintenance of response were absence of prior resection, no prior use of biologic therapy and no corticosteroid use on study entry. These results support the concept that treatment with certolizumab pegol early in the course of disease progression can confer greater clinical benefit than when used as a second- or third-line treatment [Schreiber et al. 2010a].
Long-term efficacy of certolizumab pegol
The PRECiSE 1 and PRECiSE 2 studies provide evidence that certolizumab pegol offers both rapid response and sustained efficacy, and highlight that treatment is effective in both anti-TNF experienced and treatment-naïve subjects, with particular benefit conferred when therapy is started early after initial diagnosis. However, these studies were conducted over a short period (6 months) relative to the natural history of CD and its long-term clinical management. Open-label, long-term follow up of patients from these studies allows ongoing assessment of sustained response and remission rates in patients who continue to receive a stable dosing regimen of certolizumab pegol 400 mg every 4 weeks.
An open-label extension of the PRECiSE 1 and PRECiSE 2 studies, called PRECiSE 3, is ongoing to investigate the long-term efficacy and safety of certolizumab pegol 400 mg every 4 weeks in patients who completed the initial trials. PRECiSE 4 is an open-label study of patients who entered PRECiSE 1 and PRECiSE 2 and experienced a significant worsening of their CD symptoms, relapsed (defined as an increase in CDAI score of ≥70 points above baseline before week 26) and subsequently withdrew from PRECiSE 1 and PRECiSE 2. The PRECiSE 3 and PRECiSE 4 studies are planned for a patient follow-up duration of 7 years from initiation of certolizumab pegol treatment. At the time of writing, follow-up data are currently available for up to 4–5 years, allowing insights into sustained response and remission rates.
From PRECiSE 3 there are published reports for 18 months through 4-year follow up on the patients originally from PRECiSE 2. Within this PRECiSE 3 cohort, there are patients who have received continuous certolizumab pegol therapy (induction, then active treatment in PRECiSE 2) and patients who have had interrupted therapy (induction, then placebo in PRECiSE 2), and within each of these groups there were patients who responded to certolizumab pegol therapy and those who did not. At the start of PRECiSE 3, 141 of the original 215 PRECiSE 2 patients who received continuous certolizumab pegol 400 mg therapy and 100 (of the 200) who had received interrupted treatment were enrolled. Patients were well matched between the continuous and interrupted treatment groups in terms of their demographics, disease characteristics and previous and concomitant medications [Lichtenstein et al. 2010b].
An important aim of the PRECiSE 3 trial was to analyze outcomes in patients who received continuous certolizumab pegol therapy relative to outcomes in patients who received interrupted certolizumab pegol therapy. At the time of entry into PRECiSE 3, the CD response rate (defined as a reduction in the Harvey–Bradshaw Index ≥3 points from baseline) for the patients from PRECiSE 2 in the continuous treatment group was 56% compared with 38% for the drug interruption group, with corresponding remission rates of 48% (103/215 patients) and 32% (68/210 patients), respectively [Lichtenstein et al. 2010b]. For patients who were in response at the end of PRECiSE 2, the response rates at 80 weeks after the initial dose of certolizumab pegol for the continuous and drug interruption groups in PRECiSE 3 were 66% (80/121) and 63% (50/79), respectively. For patients who were in remission at the end of PRECiSE 2, the remission rates at 80 weeks after the initial dose of certolizumab pegol were 62% (64/103) and 63% (43/68), respectively, for the continuous and the drug interruption groups in PRECiSE 3. In addition, more patients in the drug interruption group had developed antibodies against certolizumab pegol than patients who had received continuous therapy. The 18-month PRECiSE 3 data therefore indicate that certolizumab pegol is effective in maintaining response and remission rates during long-term treatment. Moreover, data from PRECiSE 3 suggest that clinical outcomes are superior with continuous therapy compared with interrupted therapy.
Impact of certolizumab pegol on fistulizing disease
Data from the PRECiSE 3 study provide insights into the efficacy of certolizumab pegol in a subpopulation of patients who responded to induction therapy and had fistulizing CD at week 0 of PRECiSE 2. Patients with draining fistulas at baseline from PRECiSE 2 (n = 108) received open-label induction with certolizumab pegol 400 mg at weeks 0 (baseline), 2 and 4. The majority of these patients (55/58) had perianal fistula. At week 6, responders (CDAI-100) with draining fistulas (n = 58) were randomized to certolizumab pegol 400 mg (n = 28) or placebo (n = 30) every 4 weeks across weeks 8–24. At week 26, 36% of patients in the certolizumab pegol group had 100% fistula closure compared with 17% of patients receiving placebo (p = 0.038), although the protocol-defined fistula closure ( ≥ 50% closure at two consecutive postbaseline visits ≥3 weeks apart) was not statistically significant (p = 0.069) [Schreiber et al. 2011]. In patients who achieved the prespecified definition of fistula closure during the study and 100% fistula closure at week 26, CDAI remission rates were 60% (6/10) in the certolizumab pegol and 50% (2/4) in the placebo group. In contrast, CDAI remission rates in patients who did not achieve the prespecified definition of fistula closure during the study and achieved 100% fistula closure at week 26 were 50% (9/18) and 15% (4/26), in the certolizumab pegol and placebo groups, respectively [Schreiber et al. 2011].
Impact of previous TNF inhibitor therapy on certolizumab pegol efficacy
Certolizumab pegol has been shown to be an effective induction/maintenance therapy regardless of prior anti-TNF use. A post hoc analysis of data from PRECiSE 2 assessing covariates such as previous anti-TNF use as predictors of sustained response (CDAI-100) and remission (CDAI ≤150) confirmed that maintenance therapy with certolizumab pegol is effective regardless of prior therapy with the anti-TNF agent infliximab. At week 26, 44% of infliximab-experienced patients benefited from second-line certolizumab pegol therapy compared with 26% in the placebo group (p = 0.018). In infliximab-naïve patients, a group thought to represent individuals with CD of shorter duration and, consequently, less disease progression, the response rate was 69% in the certolizumab pegol group, compared with 40% in the placebo group (p < 0.001) [Hanauer et al. 2010].
The PRECiSE 3 and PRECiSE 4 studies, which provide longer term follow-up outcome data on the efficacy of certolizumab pegol, also provide insights into the sustained efficacy of certolizumab pegol regardless of previous treatment history. In PRECiSE 3, 114 of the 141 patients enrolled were infliximab naïve and in PRECiSE 4, 84 of 124 subjects were infliximab naïve [Lichtenstein et al. 2010a; Sandborn et al. 2010c]. In PRECiSE 3, 78% of the infliximab-naïve patients were in remission at study start. The remission rates after 1, 2, 3 and 4 years for infliximab-naïve patients who were in remission at the start of PRECiSE 3 (nonresponder imputation [NRI] analysis) were 59%, 41%, 32% and 22%, respectively. In contrast, less than 5% (10/208) of infliximab-naïve patients in PRECiSE 4 were in remission at the start of the study. However, remission rates after 1, 2, 3 and 4 years for these infliximab-naïve patients recaptured from relapse in PRECiSE 4 (NRI analysis) were 41%, 30%, 13% and 12%, respectively. These rates of remission were similar to those achieved for the overall study cohort. Thus, continuous certolizumab pegol therapy in patients who experience CD relapse is associated with long-term remission, regardless of the patients’ previous exposure to infliximab.
Strategies for loss of response to infliximab or adalimumab as the first anti-TNF agent has been to increase the dose (infliximab) and/or increase the dosing frequency (infliximab and adalimumab) [Sandborn et al. 2009, 2007; Hanauer et al. 2002]. Strategies for loss of response to an anti-TNF agent for patients who do not have therapeutic serum trough levels may be to (1) increase the dose and/or increase the dosing frequency or (2) switch to a different anti-TNF agent. Patients with loss of response to an anti-TNF agent who have therapeutic serum trough levels may benefit from switching to a different drug class, however this strategy has not been tested in a blinded, prospective clinical trial. Switching to a different drug class may also be considered for patients who have undetectable trough levels and do not respond to a different anti-TNF agent. Patients with loss of response to an anti-TNF agent who have detectable antibodies to the original agent may benefit from switching to a different anti-TNF agent, whereas patients with loss of response to an anti-TNF agent who do not have detectable antibodies to the original agent may benefit from increasing the dose and/or an increased dosing frequency.
The WELCOME study: certolizumab pegol in patients failing on infliximab
As many as 40–50% of patients who respond to induction therapy with conventional anti-TNF therapy develop secondary failure, defined as a loss of response and/or development of hypersensitivity reactions or injection site reactions within 6–12 months [Colombel et al. 2007; Schreiber et al. 2007; Hanauer et al. 2002]. The WELCOME (26-Week open-label trial Evaluating the clinical benefit and tolerability of certoLizumab pegol induCtiOn and Maintenance in patients suffering from Crohn's disease with prior loss of response or intolErance to infliximab) was a 26-week randomized, double-blind trial that prospectively evaluated the efficacy of certolizumab pegol given either every 2 weeks or every 4 weeks at a dose of 400 mg in patients (N = 539) with moderate to severe CD with secondary failure to infliximab [Sandborn et al. 2010a]. WELCOME involved an open-label induction phase of treatment with certolizumab pegol 400 mg given at weeks 0, 2 and 4, with patients in response at week 6 eligible for randomization to one of the active maintenance regimens. At week 6, 62% (334/539) of patients achieved response, 39% achieved remission and 329 were randomized. By week 26, 40% of patients receiving certolizumab pegol therapy every 4 weeks and 37% of those receiving treatment every 2 weeks were in clinical response (p = 0.55), and 29% and 30%, respectively, were in remission (p = 0.81). The efficacy of certolizumab pegol in the WELCOME patients was not affected by concomitant use of either corticosteroids or immunosuppressants [Sandborn et al. 2009]. Certolizumab pegol therefore appears to be an effective treatment option for many patients failing on first-line infliximab therapy.
PRECiSE 4: reinduction with certolizumab pegol in patients with disease relapse
There is a report on the 12-month follow up of PRECiSE 2 patients from the PRECiSE 4 study, which was specifically designed to examine a recapture/reinduction protocol for patients who lost response following induction therapy [Sandborn et al. 2010b]. Loss of response was defined as an increase in CDAI of at least 70 points above baseline or an increase in the CDAI score to ≥350 points. Patients in PRECiSE 2 who had disease relapse during weeks 6–26 of continuous certolizumab pegol therapy underwent recapture with a one-time supplemental dose of certolizumab pegol 400 mg. Patients with loss of response in the PRECiSE 2 drug interruption arm could enter PRECiSE 4 and be reinduced with certolizumab pegol 400 mg administered at weeks 0, 2 and 4 followed by maintenance therapy at 400 mg every 4 weeks thereafter. A total of 124 patients with loss of response during PRECiSE 2 enrolled in PRECiSE 4, 49 of whom had been randomized to certolizumab pegol and 75 who had been randomized to placebo. At week 4 of PRECiSE 4, the response rates were 63% for patients who had relapsed in the continuous treatment group and 65% for the drug interruption group. Response and remission rates were maintained through week 52. At week 52, a clinical response was maintained in 55% and 59% of responders in the continuous treatment and drug interruption group, respectively, suggesting that recapture/reinduction with certolizumab pegol without the need for dose escalation in patients with relapsed CD is an effective management strategy.
Attenuation of response among patients who respond to anti-TNF therapy with certolizumab pegol, infliximab and adalimumab, and then continue the same agent as maintenance therapy, occurs in approximately 60% of patients within 6 months [Colombel et al. 2007; Schreiber et al. 2007; Hanauer et al. 2002]. Disease relapse with all three anti-TNF agents among patients with active CD has been reported in clinical trials. In the PRECiSE 2 trial, 37% of patients in the certolizumab pegol group lost response at week 26 [Schreiber et al. 2007]. In PRECiSE 3, the response rate at 80 weeks for patients receiving continuous maintenance therapy with certolizumab pegol was 66% (80/121) [Lichtenstein et al. 2010b]. Loss of response was demonstrated from week 30 to week 54 in approximately 42% (160/385) of patients during maintenance therapy with infliximab [Hanauer et al. 2002]. Similar rates of loss of response were reported for adalimumab maintenance therapy (approximately 45–55% by weeks 26 and 56, respectively) [Colombel et al. 2007]. As stated above, factors identified as predictors of maintenance of response for certolizumab pegol include shorter disease duration (<1 year), absence of prior resection, no prior use of biologic therapy and no corticosteroid use on study entry [Schreiber et al. 2010a].
Patient outcome benefits of certolizumab pegol
The clinical trial program for certolizumab pegol, including the findings of the long-term, open-label follow ups to the PRECiSE studies, show that this therapy offers rapid response and sustained response and remission for patients with moderate to severe CD, regardless of their prior exposure to anti-TNF therapy and concomitant medication use. In addition to reducing the burden posed by symptoms of active CD, there is growing evidence that treatment with certolizumab pegol can have sustained benefits that impact positively on patient QoL and everyday functional ability.
Health-related quality of life
The morbidity of CD often limits patients’ physical functioning, emotional wellbeing and social interactions. While clinical efficacy and safety studies typically assess changes in severity of CD according to clinical indices such as the CDAI, the effect of certolizumab pegol maintenance therapy on patient-reported outcomes (PROs) within the PRECiSE 2 cohort has also been evaluated in order to determine the effects of treatment on HRQoL [Feagan et al. 2009b].
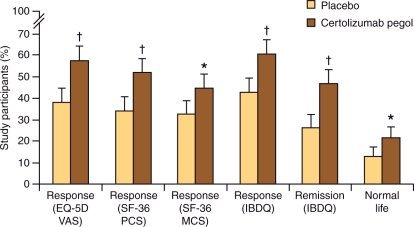
A study on the return to ‘normal life’ was conducted on patients from PRECiSE 2 using multiple PRO instruments. Maintenance therapy with certolizumab pegol resulted in statistically significant and clinically meaningful improvements in HRQoL, as assessed by multiple PRO instruments (Figure 3). Patients receiving continuous certolizumab pegol therapy (n = 215) compared with those in the placebo group (n = 210) reported improved inflammatory bowel disease questionnaire (IBDQ) scores (60% versus 43%; p < 0.001) and significantly higher Short-Form 36 (SF-36) physical component (51% versus 34%; p < 0.001) and mental component (44% versus 32%; p = 0.016) summary responses. There were also significantly more subjects receiving certolizumab pegol (57%) who achieved a clinically meaningful improvement in EQ-5D Visual Analog Scale scores compared with placebo treatment (38%; p < 0.001) (Figure 3) [Feagan et al. 2009b]. In this same analysis, treatment with certolizumab pegol was also associated with a greater gain in quality-adjusted life years than placebo (0.25 ± 0.10 versus 0.21 ± –0.11; p = 0.001) [Feagan et al. 2009b]. The proportion of patients satisfying the normal life criteria increased from 0% at baseline to 26% (p < 0.001) at the end of the 6-week open-label induction period with certolizumab pegol. Moreover, 21% of patients receiving certolizumab pegol versus 13% of patients in the placebo group reported living a normal life at week 26 (p = 0.019) [Feagan et al. 2009b].
Figure 3.
Improvement in various health-related quality of life measures with certolizumab pegol in PRECiSE 2. PCS, physical component summary; MCS, mental component summary; VAS, visual analog scale; IBDQ, inflammatory bowel disease questionnaire. *p < 0.05 (logistic regression). †p < 0.001 (logistic regression). Error bars represent the 95% confidence interval. Reprinted with permission from Macmillan Publishers Ltd [Feagan et al. 2009b, 104(8)], copyright © 2009.
Work productivity and daily activities
Data from the PRECiSE and WELCOME trials have also been useful in determining the impact of certolizumab pegol treatment on patients’ ability to remain productive and undertake normal daily living activities. The Work Productivity and Activities Index in CD (WPAI:CD) was validated using data from PRECiSE 1. This health outcomes instrument measures the impact of CD on productivity in both the home and the workplace, during the 7-day period prior to an evaluation, by assessing absenteeism, presenteeism (time at work during which there is reduced productivity), overall work impairment and daily living impairment [Reilly et al. 2008].
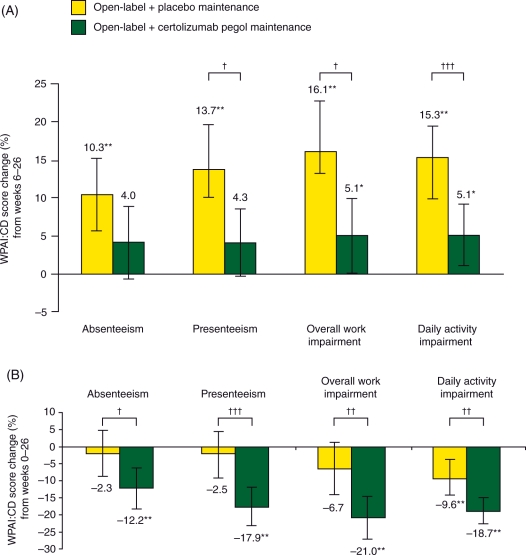
PRECiSE 1 data were used to estimate the smallest changes in WPAI:CD scores that patients or clinicians would perceive as beneficial following a treatment intervention, that is, changes that would constitute a minimally important difference (MID). Changes in MID scores of more than 7–8% can be regarded as sizeable changes in work productivity for patients with active CD [Reilly et al. 2007]. At weeks 6, 16 and 26 of PRECiSE 1, there was a significantly larger improvement in overall work impairment in patients treated with certolizumab pegol compared with those assigned to placebo (p < 0.05) [Feagan et al. 2007]. Improvements in work productivity and employment status have also been noted in analyses of PRECiSE 2 data [Feagan et al. 2010] (Figure 4). Induction and maintenance with certolizumab pegol was associated with significantly improved work productivity compared with placebo. Significant improvements were seen by week 6 following certolizumab pegol induction therapy. Improvements in scores for absenteeism, presenteeism, overall work impairment and daily activity impairment were sustained during maintenance therapy, showing significant advantages of continuous treatment over placebo (Figure 4). When both induction and maintenance phases were considered together, certolizumab pegol treatment was associated with a 14% lower overall work impairment rating, equivalent to 5.7 hours per week increased work productivity at week 26 when compared with placebo (p = 0.004).
Figure 4.
Improvement in work productivity with certolizumab pegol in PRECiSE 2. Mean changes in Work Productivity and Activities Index in Crohn’s disease (WPAI:CD) scores and 95% confidence interval (within-treatment) (A) at week 26 from week 6 (maintenance phase) and (B) at week 26 from baseline (induction and maintenance). *p < 0.05. **p < 0.001; comparisons within treatment groups for week 6 versus week 26 (a) and week 0 versus week 26 (b) are by paired t-test (change significantly different from 0). †p < 0.05, ††p < 0.01, †††p < 0.001; comparisons between the two treatment arms are by Student’s t-test. A larger negative change means greater improvement for each parameter. Reprinted with permission from Blackwell Publishing Ltd [Feagan et al. 2010, pp. 1276–1285], copyright © 2010.
Recently, certolizumab pegol treatment was shown to result in meaningful improvements in work productivity, daily activities and HRQoL in patients with active CD who previously responded to but either lost response or could not tolerate infliximab [Feagan et al. 2011, 2009a]. In the WELCOME Health Outcomes study, work productivity and HRQoL were assessed using the WPAI:CD questionnaire and the IBDQ, respectively [Feagan et al. 2011]. Analyses were conducted in all randomized patients who had a clinical response at week 6 in the WELCOME study and received at least one dose of study medication after randomization. The baseline HRQoL burden (as indicated by a baseline IBDQ score of approximately 120 points) was representative of moderate to severe CD. HRQoL, daily activity and work productivity improved in both certolizumab pegol 400 mg every 2 weeks and every 4 weeks treatment groups as early as week 6. These improvements were maintained through week 26. Moreover, treatment benefits to HRQoL, daily activity and work productivity were similar between the certolizumab pegol every 2 weeks versus every 4 weeks groups.
Insights into potential disease modification
There is growing interest and emerging evidence to support the disease-modifying potential of a number of therapeutic agents currently used in CD management [Van Assche et al. 2010]. Anti-TNF therapies are reported to hold potential for mucosal healing and actions that may prevent fibrotic wall thickening. Data from clinical studies are therefore increasingly assessed for evidence that anti-TNF treatment may modify disease progression and natural history. It is commonly felt that the ability to induce mucosal healing is associated with the disease-modifying potential of a drug.
The MUSIC trial
The MUSIC trial [Colombel et al. 2010] assessed changes in the intestinal mucosa of patients with active CD during long-term treatment with certolizumab pegol 400 mg every 4 weeks. The study enrolled 89 patients with endoscopically active disease. Among the 78 patients who completed 10 weeks of certolizumab pegol therapy, 62% and 42% achieved endoscopic response and remission, respectively. Among the 53 patients who completed 54 weeks of certolizumab pegol therapy, 62% and 28% achieved endoscopic response and remission, respectively.
Certolizumab pegol safety overview
Data from the PRECiSE and WELCOME trials support the safety and tolerability of certolizumab pegol as induction therapy and long-term maintenance treatment of active CD. While a comprehensive safety overview is beyond the remit of this review, the adverse event profile of certolizumab pegol demonstrates that this anti-TNF is safe and well tolerated. In the 26-week PRECiSE 2 study, the most frequently reported adverse events were similar in certolizumab pegol and placebo groups. Serious adverse events occurred in 6% (12/216) and 7% (14/212) of patients in the certolizumab pegol and placebo group, respectively. Serious infection occurred in 3% of patients in the certolizumab group and in less than 1% of patients in the placebo group. One patient in the certolizumab pegol group had pulmonary tuberculosis, which responded to standard combination therapy with antibiotics. One or more local reactions to injection occurred in 3% of patients in the certolizumab group and 15% of patients in the placebo group [Schreiber et al. 2007] In PRECiSE 1, detectable anticertolizumab antibodies developed in 8% (26/331) of patients in the certolizumab pegol group [Sandborn et al. 2007]. In PRECiSE 2, of the 668 patients entered the induction phase, 58 (9%) had detectable antibodies against certolizumab pegol at some point during the study [Schreiber et al. 2007]. Three of the four patients in whom antibodies developed during the induction phase were randomly assigned to receive maintenance therapy, one with certolizumab pegol and two with placebo. In the remaining 54 patients, antibodies developed during the maintenance phase, 80% of which were neutralizing antibodies in vitro. There was no statistically significant reduction in efficacy. Injection site pain and injection site erythema were reported at an incidence of at least 3% higher in antibody-positive patients compared with antibody-negative patients [UCB, 2009].
In the 18-month PRECiSE 3 analysis, similar rates of adverse events and serious adverse events were reported by patients in the continuous treatment (n = 141) and drug interruption (n = 100) groups. Exacerbation of CD was the most commonly reported adverse event [Lichtenstein et al. 2010b]. Most treatment-emergent adverse events in the 26-week PRECiSE 4 analysis were of mild or moderate intensity and were considered to be unrelated to the study drug. There was a low incidence (≤2%) of injection site reactions [Sandborn et al. 2010c]. In the WELCOME study, the most commonly occurring adverse events were headache, nausea and arthralgia. The percentage of patients experiencing injection site pain was low (1.9% in the induction phase and 0% and 0.5% in the certolizumab pegol every 4 weeks and every 2 weeks groups during the double-blind maintenance phase, respectively). Serious drug-related adverse events occurred in 15 (2.8%) patients during induction and 12 (3.2%) patients during the maintenance phase. Serious infections were reported in nine (1.7%) patients during the induction phase and 12 (3.2%) patients in the maintenance phase, with no cases of tuberculosis reported. A single malignancy (squamous cell carcinoma of the skin) occurred in the certolizumab pegol every 4 weeks group [Sandborn et al. 2010a]. In support of these safety observations, certolizumab pegol 400 mg every 2 weeks was found to be well tolerated in patients with rheumatoid arthritis [Smolen et al. 2009].
The FDA pregnancy category for certolizumab pegol is category B. Ex vivo studies on human placentas indicate no measurable transfer of certolizumab pegol across the placenta, an effect probably due to the lack of the Fc region [Porter et al. 2010]. Reproduction studies in rats at drug doses up to 100 mg/kg revealed no evidence of impaired fertility or harm to the fetus due to the antimurine TNFα PEGylated Fab′ [UCB, 2009]. However, there are no well-controlled studies of certolizumab pegol in pregnant women, and it is not yet known whether certolizumab pegol is excreted in human milk [UCB, 2009].
Conclusions
A growing body of evidence supports anti-TNF therapy with certolizumab pegol 400 mg as an efficacious treatment for induction and long-term maintenance of clinically meaningful response and remission in patients with moderate to severe CD. This physically unique anti-TNF has a favorable benefit-to-risk profile. Experience to date suggests that long-term maintenance of remission is achievable using a stable, subcutaneous, monthly dosing regimen, without an apparent need to resort to dose escalation. If relapse occurs, clinical response can be recaptured in a proportion of patients by the administration of one additional certolizumab pegol dose to the regular 4-weekly maintenance dosing regimen. Treatment is effective in patients regardless of their prior exposure to other anti-TNF therapies.
Results from the PRECiSE program demonstrate the efficacy and safety of a maintenance dosing schedule of certolizumab pegol 400 mg every 4 weeks [Lichtenstein et al. 2010b; Sandborn et al. 2010b, 2007; Schreiber et al. 2007]. In a small case series, five of six patients losing response during maintenance therapy with certolizumab pegol 400 mg every 4 weeks subsequently achieved remission when the dose was administered at 200 mg every 2 weeks [Fernandez-Blanco and Hinojosa, 2008]. However, as discussed above, the WELCOME trial showed that treatment benefits were similar between certolizumab pegol dosing schedules of every 2 weeks versus every 4 weeks [Sandborn et al. 2010a; Feagan et al. 2009b].
Greater treatment effects with anti-TNF agents have been reported in patients with higher baseline CRP concentrations [Schreiber et al. 2005], suggesting that clinical response may be associated with a high inflammatory burden. Thus, patient selection for future clinical trials should emphasize the treatment of patients who have objective evidence of inflammation in addition to symptoms of active disease.
Currently, anti-TNF agents are reserved for the treatment of patients with moderate to severe CD in whom conventional therapy has failed. Thus, they tend to be used later in the course of disease as part of a ‘step-up’ approach. However, a recent article suggests that a ‘top-down’ treatment strategy, in which immunosuppressants and anti-TNF agents are used earlier in the course of the disease, may alter the natural history of the disease and prevent late complications [Krygier et al. 2009]. In PRECiSE 2, certolizumab pegol was found to be more efficacious when used in patients with a shorter disease duration than in patients who have had CD for longer, which probably reflects the development of complications (e.g. strictures or penetrating disease) that are more refractory to treatment. Therefore, a ‘top-down’ approach where certolizumab pegol is given earlier as a second-line therapy after other conventional CD therapies have failed may be particularly valuable in patients at risk of developing aggressive disease, such as a history of surgery, fistulas or the need for corticosteroids at initial presentation.
The clinical benefits of certolizumab pegol are reflected in marked improvements in patient HRQoL, work productivity and daily function, with benefits seen both early in treatment and sustained during long-term maintenance therapy. The therapeutic profile of certolizumab pegol offers the clinician a new paradigm for the control of CD symptoms and potential for CD modification.
Acknowledgments
Editorial assistance was provided by contract medical writers (Ann P. Tighe, PhD, and C. Grantham, PhD, of PPSI, a PAREXEL company, Stamford, CT, USA) supported by UCB. Stefan Schreiber, MD, was primarily responsible for the content of the manuscript, including development of the first draft, writing or approving all subsequent revisions, and/or critically reviewing it for intellectual content.
Funding
This work was supported by UCB.
Conflict of interest statement
Stefan Schreiber, MD, has served as a speaker, a consultant and/or an advisory board member for Abbott Laboratories, Bayer Schering, Centocor, Dr. Falk Pharma, Essex/Schering Plough, Ferring, Genentech, GlaxoSmithKline, Novartis, Novo Nordisk, Pfizer, Schering-Plough and its subsidiary Essex Pharma and UCB, and owns stocks and shares in Conaris Research Institute.
References
- Abbott Laboratories (2010) Humira [prescribing information]. North Chicago, IL: Abbott Laboratories [Google Scholar]
- Bernstein C.N., Fried M., Krabshuis J.H., Cohen H., Eliakim R., Fedail S., et al. (2010) World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 16: 112–124 [DOI] [PubMed] [Google Scholar]
- Binion D.G. (2010) Biologic therapies for Crohn’s disease: update from the 2009 ACG meeting. Gastroenterol Hepatol (N Y) 6(1 Suppl. 1)4–16 [PMC free article] [PubMed] [Google Scholar]
- Bourne T., Fossati G., Nesbitt A. (2008) A PEGylated Fab' fragment against tumor necrosis factor for the treatment of Crohn’s disease: exploring a new mechanism of action. BioDrugs 22: 331–337 [DOI] [PubMed] [Google Scholar]
- Centocor Ortho Biotech (2009) Remicade [prescribing information]. Malvern, PA: Centocor Ortho Biotech, Inc [Google Scholar]
- Clark M., Colombel J.-F., Feagan B.C., Fedorak R.N., Hanauer S.B., Kamm M.A., et al. (2007) American Gastroenterological Association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology 133: 312–339 [DOI] [PubMed] [Google Scholar]
- Colombel J.-F., Lémann M., Bouhnik Y., Dewit O., Dupas J.L., Mross M., et al. (2010) Endoscopic mucosal improvement in patients with active Crohn’s disease treated with certolizumab pegol: week 10 and 54 results of the MUSIC trial. Gastroenterology 138(5 Suppl. 1)S-166–S-166 (Abstract) [Google Scholar]
- Colombel J.-F., Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Panaccione R., et al. (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 132: 52–65 [DOI] [PubMed] [Google Scholar]
- Cominelli F. (2004) Cytokine-based therapies for Crohn’s disease-new paradigms. N Engl J Med 351: 2045–2048 [DOI] [PubMed] [Google Scholar]
- Cosnes J., Nion-Larmurier I., Beaugerie L., Afchain P., Tiret E., Gendre J.P. (2005) Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut 54: 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Haens G., Baert F., van Assche G., Caenepeel P., Vergauwe P., Tuynman H., et al. (2008) Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 371: 660–667 [DOI] [PubMed] [Google Scholar]
- Eddleston A., Marenzana M., Marshall D., Nesbitt A. (2009) Comparison of the distribution of an IGG and a pegylated Fab' form of an anti-TNFα antibody in the inflamed gut of colitic mice. Gut 58(Suppl. II)A305, (Abstract) [Google Scholar]
- Feagan B., Sandborn W., Brown M., Brabant Y., Gerlier L. (2007) Confirmed benefits on work productivity and daily activities of certolizumab pegol in Crohn’s disease patients: data from PRECiSE 1. Gastroenterology 132(4 Suppl. 1)A507–A508 (Abstract) [Google Scholar]
- Feagan B., Wolf D.C., Tan S., Brabant Y., Brown M. (2009a) Certolizumab pegol treatment impacts hospitalizations and surgeries in Crohn’s disease patients with prior failure to infliximab: results from WELCOME trial. Gut 58(Suppl. II)A470–A470 (Abstract) [Google Scholar]
- Feagan B.G., Coteur G., Tan S., Keininger D.L., Schreiber S. (2009b) Clinically meaningful improvement in health-related quality of life in a randomized controlled trial of certolizumab pegol maintenance therapy for Crohn’s disease. Am J Gastroenterol 104: 1976–1983 [DOI] [PubMed] [Google Scholar]
- Feagan B.G., Reilly M.C., Gerlier L., Brabant Y., Brown M., Schreiber S. (2010) Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate-to-severe Crohn’s disease in the PRECiSE 2 study. Aliment Pharmacol Ther 31: 1276–1285 [DOI] [PubMed] [Google Scholar]
- Feagan B.G., Sandborn W.J., Wolf D.C., Coteur G., Purcaru O., Brabant Y., Rutgeerts P.J. (2011) Randomised clinical trial: improvement in health outcomes with certolizumab pegol in patients with active Crohn’s disease with prior loss of response to infliximab. Aliment Pharmacol Ther. [ePub 12 January 2011] [DOI] [PubMed] [Google Scholar]
- Fernandez-Blanco I., Hinojosa J. (2008) Efficacy of intensification therapy with certolizumab pegol in Crohn's disease patients included in a compassionate-use program. Am J Gastroenterol 103(Suppl. I)S427–S427 (Abstract) [Google Scholar]
- Fleischmann R., Vencovsky J., van Vollenhoven R.F., Borenstein D., Box J., Coteur G., et al. (2009) Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 68: 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer S.B., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.-F., et al. (2002) Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 359: 1541–1549 [DOI] [PubMed] [Google Scholar]
- Hanauer S.B., Panes J., Colombel J.-F., Bloomfield R., Schreiber S., Sandborn W.J. (2010) Clinical trial: impact of prior infliximab therapy on clinical response to certolizumab pegol maintenance therapy for Crohn’s disease. Aliment Pharmacol Ther 32: 384–393 [DOI] [PubMed] [Google Scholar]
- Keystone E., Heijde D., Mason D., Landewé R., Vollenhoven R.V., Combe B., et al. (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58: 3319–3329 [DOI] [PubMed] [Google Scholar]
- Krygier D.S., Ko H.H., Bressler B. (2009) How to manage difficult Crohn's disease: optimum delivery of anti-TNFs. Expert Rev Gastroenterol Hepatol 3: 407–415 [DOI] [PubMed] [Google Scholar]
- Lamour S., Bracher M., Nesbitt A. (2009) Effect of PEG component of certolizumab pegol on simulated mast cell degranulation. Gut 58(Suppl. II)A305–A305 (Abstract) [Google Scholar]
- Lichtenstein G., Thomsen O.Ø., Schreiber S., Lawrance I.C., Hanauer S.B., Bloomfield R., et al. (2010a) Long-term remission with certolizumab pegol in Crohn’s disease: efficacy over 4 years in patients with no prior TNF-α inhibitor exposure (PRECISE 3 study). Gastroenterology 138,5(Suppl.1)S-165–S-165 (Abstract) [Google Scholar]
- Lichtenstein G.R., Hanauer S.B., Sandborn W.J. (2009) Management of Crohn's disease in adults. Am J Gastroenterol 104: 465–483 [DOI] [PubMed] [Google Scholar]
- Lichtenstein G.R., Thomsen O.Ø., Schreiber S., Lawrance I.C., Hanauer S.B., Bloomfield R., et al. (2010b) Continuous therapy with certolizumab pegol maintains remission of patients with Crohn’s disease for up to 18 months. Clin Gastroenterol Hepatol 8: 600–609 [DOI] [PubMed] [Google Scholar]
- Lichtiger S., Binion D.G., Wolf D.C., Present D.H., Bensimon A.G., Wu E., et al. (2010) The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther 32: 1228–1239 [DOI] [PubMed] [Google Scholar]
- MacDonald T.T., Hutchings P., Choy M.Y., Murch S., Cooke A. (1990) Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 81: 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt A., Fossati G., Bergin M., Stephens P., Stephens S., Foulkes R., et al. (2007) Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis 13: 1323–1332 [DOI] [PubMed] [Google Scholar]
- Ng S.C., Plamondon S., Gupta A., Burling D., Kamm M.A. (2009) Prospective assessment of the effect on quality of life of anti-tumour necrosis factor therapy for perineal Crohn's fistulas. Aliment Pharmacol Ther 30: 757–766 [DOI] [PubMed] [Google Scholar]
- Porter C., Kopotsha T., Smith B.J., Nesbitt A.M., Urbaniak S.J., Armstrong-Fisher S.S. (2010) No significant transfer of certolizumab pegol with IgG in the perfused human placenta in vitro. Gastroenterology 138(Suppl. I)S-674–S-674 (Abstract) [Google Scholar]
- Reilly M., Gerlier L., Brabant Y., Brown M. (2008) Validity, reliability and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. Clin Ther 130: 393–404 [DOI] [PubMed] [Google Scholar]
- Reilly M.C., Brown M.C.J., Brabant Y., Gerlier L.C., Sandborn W.J. (2007) Defining the minimally important difference for WPAI:CD scores: what is a relevant impact on work productivity in active Crohn’s disease? Gut 56(Suppl. III)A159–A159 (Abstract) [Google Scholar]
- Reinecker H.C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R.P., Raedler A. (1993) Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 94: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgeerts P., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.-F., et al. (2004) Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology 126: 402–413 [DOI] [PubMed] [Google Scholar]
- Sadowski D.C., Bernstein C.N., Bitton A., Croitoru K., Fedorak R.N., Griffiths A., et al. (2009) Canadian Association of Gastroenterology Clinical Practice Guidelines: the use of tumour necrosis factor-alpha antagonist therapy in Crohn's disease. Can J Gastroenterol 23: 185–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W., Vermeire S., Abreu M., D’Haens G., Colombel J.-F., Mitchev K., et al. (2009) Efficacy of certolizumab pegol in Crohn’s disease patients with secondary failure to infliximab is not affected by concomitant medications. Am J Gastroenterol 104(Suppl. 3)S451–S452 (Abstract) [Google Scholar]
- Sandborn W.J., Abreu M.T., D’Haens G., Colombel J.-F., Vermeire S., Mitchev K., et al. (2010a) Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol 8: 696–702 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Feagan B.G., Stoinov S., Honiball P.J., Rutgeerts P., Mason D., et al. (2007) Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 357: 228–238 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Schreiber S., Hanauer S.B., Colombel J.-F., Bloomfield R., Lichtenstein G.R. (2010b) Reinduction with certolizumab pegol in patients with relapsed Crohn’s disease: results from the precise 4 study. Clin Gastroenterol Hepatol 8: 696–702 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Schreiber S., Hanauer S.B., Colombel J.-F., Bloomfield R., Lichtenstein G.R. (2010c) Patients with Crohn’s disease treated with certolizumab pegol experienced long-term remission regardless of prior TNF-α inhibitor exposure (PRECISE 4 study). Gastroenterology 138(5 Suppl. 1)S-9–S-9 (Abstract) [Google Scholar]
- Schreiber S., Colombel J.-F., Bloomfield R., Nikolaus S., Schölmerich J., Panés J., et al. (2010a) Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of precise 2 randomized maintenance trial data. Am J Gastroenterol 105: 1574–1582 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Khaliq-Kareemi M., Lawrance I., Thomsen O., Bloomfield R., Sandborn W. (2009) Rapid improvement of patient-reported CDAI diary components by day 8 in active Crohn’s disease patients treated with certolizumab pegol. Am J Gastroenterol 104(Suppl. 3)S442–S442 (Abstract) [Google Scholar]
- Schreiber S., Khaliq-Kareemi M., Lawrance I.C., Thomsen O.Ø., Hanauer S.B., Bloomfield R., et al. (2010b) Certolizumab pegol demonstrates efficacy in maintaining response and remission in patients with active Crohn’s disease regardless of their immunosuppressant treatment status at entry to the PRECISE 2 study. Gastroenterology 138(5 Suppl. 1)S-165–S-165 (Abstract) [Google Scholar]
- Schreiber S., Khaliq-Kareemi M., Lawrance I.C., Thomsen O.Ø., Hanauer S.B., McColm J., et al. (2007) Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 357: 239–250 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Lawrance I.C., Thomsen O.Ø., Hanauer S.B., Bloomfield R., Sandborn W.J. (2011) Randomized clinical trial: certolizumab pegol for fistulas in Crohn's disease—subgroup results from a placebo-controlled study. Aliment Pharmacol Ther 33: 185–193 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J., Hämling J., Koop I., Groessner B., et al. (1999) Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet 353: 459–461 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Rutgeerts P., Fedorak R.N., Khaliq-Kareemi M., Kamm M.A., Boivin M., et al. (2005) A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 129: 807–818 [DOI] [PubMed] [Google Scholar]
- Smolen J., Landewe R.B., Mease P., Brzezicki J., Mason D., Luijtens K., et al. (2009) Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 68: 797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCB (2009) Cimzia [prescribing information]. Smyrna, GA: UCB, Inc [Google Scholar]
- Van Assche G., Vermeire S., Rutgeerts P. (2010) The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol 7: 79–85 [DOI] [PubMed] [Google Scholar]
- Veronese F.M., Mero A. (2008) The impact of PEGylation on biological therapies. BioDrugs 22: 315–329 [DOI] [PubMed] [Google Scholar]