Abstract
Background: Despite the high prevalence of constipation and its related public health implications, there is relatively little research available on the condition from large epidemiological studies. The aim of this study was to investigate the epidemiology of general practitioner (GP)-diagnosed constipation and the prescribing trends for laxatives in the UK, within the general population and during pregnancy.
Methods: A cohort study for the period from 2005 to 2009 was performed using the UK primary care database (General Practice Research Database), which contains information on over 3 million individuals.
Results: The prevalence of GP-diagnosed constipation ranged from 12 per 1000 persons in 2005 (0.012 per person year) to 12.8 per 1000 in 2009 (0.013 per person year). The prevalence was almost twice as high in women as in men, and was higher in older patients. In 2005 the most commonly prescribed laxatives were lactulose (37%), senna (26%), macrogol (19%), ispaghula (6%), docusate sodium (5%), bisacodyl (4%) and glycerol suppositories (2%). By 2009, this pattern had changed: macrogol (31%), lactulose (29%), senna (22%), ispaghula (5%), docusate sodium (6%), bisacodyl (3%) and glycerol suppositories (3%). In pregnancy, lactulose accounted for 81% of laxative use in 2005, falling to 64% by 2009. In contrast, macrogol use in pregnancy rose from 13% in 2005 to 32% in 2009.
Conclusions: GP-diagnosed constipation is common, accounting for a large number of consultations. Laxative prescribing trends have changed over the 5-year study period, prescriptions for macrogol becoming increasingly common and prescriptions for lactulose and senna less common. Macrogol also appears to have been replacing lactulose for treating constipation in pregnant women.
Keywords: constipation, general practice, General Practice Research Database, laxatives, pregnancy, primary care
Introduction
Constipation is one of the most prevalent gastrointestinal complaints, estimates of UK prevalence varying from 8.2% to 52% [Wald et al. 2010; National Horizon Scanning Centre, 2008; Klaschik et al. 2003]. Part of the reason for such disparate estimates is the difficulty in defining constipation, which varies between patients and healthcare professionals and between studies. The NHS Clinical Knowledge Summary on constipation defines the condition as defaecation that is unsatisfactory, because of infrequent stools, difficult stool passage or seemingly incomplete defaecation [NHS Clinical Knowledge Summaries, 2010]. A study of 1055 factory workers in the UK in 1965 [Connell et al. 1965] found that 99% of the working population maintained a bowel frequency of between three bowel movements per day and three per week. On the basis of this evidence, constipation is sometimes defined as a frequency of fewer than three bowel movements per week [Higgins and Johanson, 2004].
Another factor contributing to difficulty in estimating the prevalence of the condition is that it may occur chronically in patients, but it may also be a transient short-term condition. There are many causes of constipation: it may be due to a prolonged colon passage or defaecation disorder, another disease, medication such as opioid therapy, or factors such as diet, fluid intake, immobility and lack of exercise [Klaschik et al. 2003]. Pregnancy is also known to increase the risk of constipation. Bradley and colleagues estimated that, during pregnancy, one in four women experience constipation [Bradley et al. 2007].
Laxatives as a treatment for constipation are among the most widely used of all medications [Xing and Soffer, 2001]. These medicines are available over the counter from pharmacies as well as by prescription from a general practitioner (GP). There are four main types of laxative: bulk-forming agents; osmotic laxatives; stimulant laxatives; and lubricants. Bulk-forming agents, such as ispaghula husk, are organic polymers with various water-holding capacities: they increase the intraluminal volume by retaining water, which stimulates motility and speeds the transit of luminal contents through the colon. Stimulant laxatives, such as senna, stimulate intestinal motility and affect epithelial transport of water and electrolytes [Klaschik et al. 2003; Xing and Soffer, 2001].
Macrogol and lactulose are osmotic laxatives, although the latter also promotes bacterial fermentation. They are not absorbed during their transit through the bowel and the water that is bound to them remains within the stool, thus aiding the relief of constipation. Macrogol is the international non-proprietary name for polyethylene glycol and is available in two different types, 3350 and 4000. These numbers represent the average molecular weight of the polyethylene glycol. It can be formulated either with or without electrolytes, which are added in an attempt to rectify the electrolyte depletion that can occur in some patients. The addition of electrolytes has an adverse effect on the taste of the product, however, and this can affect patient compliance.
The laxatives most commonly prescribed by GPs are lactulose, macrogol, senna, ispaghula husk, docusate sodium, bisacodyl and glycerol suppositories. In 2009, prescriptions for these products made up over 95% of all laxative prescriptions in England [NHS Information Centre, 2010]. However, there are no definitive treatment guidelines for constipation in adults and treatment of this condition may present many challenges, especially in pregnancy, in which there is an additional need to ensure the safety of treatments [Tytgat et al. 2003]. The NICE clinical guidelines on routine antenatal care give advice on managing common problems in pregnancy; the recommended treatment for constipation is a change in diet. No recommendations are given for treating constipation that is not improved by a change in diet [NICE Clinical Guidance, 2010].
The British National Formulary (BNF) recommends that, if diet and lifestyle changes do not control constipation in pregnancy, then moderate doses of poorly absorbed laxatives may be used; bulk-forming laxatives are recommended to be tried first, followed by an osmotic laxative such as lactulose, or a stimulant laxative such as senna if necessary [British National Formulary, 2010]. However, a consensus document on the use of laxatives in pregnancy has concluded that macrogols meets the criteria for the ideal laxative for use in pregnancy [Tytgat et al. 2003].
Despite the high prevalence of constipation in the UK and the related cost implications, the condition has not been widely studied in large patient groups. This study is the first investigation of constipation in the general population and in pregnancy, and also of the prescribing trends of laxatives within these patient groups, using the UK General Practice Research Database (GPRD).
Materials and methods
Study design
This was a cohort study designed to characterize the population of patients with GP-diagnosed constipation in each year between 2005 and 2009. A cohort of patients was extracted from the GPRD for each year. Patients were included in the study if they were aged 18 or older and had a diagnosis of constipation or faecal impaction within the study year, identified using a list of relevant READ codes (Appendix). The READ codes used were those for constipation, including constipation symptoms, chronic, acute and functional constipation, and faecal impaction.
Patients were included in the study only if their medical record met the acceptable standard of quality defined by the GPRD as being suitable for inclusion in research. For example, patients were required to have a valid registration date and a viable birth year. This ensured that patients with poor-quality or noncontiguous medical records were not included in the cohort. Only patients from practices that were up to standard were selected; such practices were those that had met the data quality criteria required by GPRD at the beginning of the study period. Patients were excluded if they were registered in a practice for which the latest data collection date was before the end of the study period, or if the patient transferred out of the practice before the end of the study year. This was to ensure that patient follow up was complete.
Therapies of interest were the seven most commonly prescribed laxatives: lactulose, macrogol, senna, ispaghula husk, docusate sodium, bisacodyl and glycerol. All available brands were included for each substance, and the therapies were identified using a list of codes (Appendix). Macrogol 3350 and macrogol 4000 were both included within the macrogol prescriptions.
Pregnancies can be identified within the longitudinal record of GPRD patients [Devine et al. 2010]. An algorithm was used to identify pregnancies, which combined pregnancy codes (Appendix) within the clinical details in GPRD with codes entered into the maternity module of the database. These codes were used to define a current ongoing pregnancy, and the patient records were examined for constipation diagnoses and laxative prescribing concurrent with the pregnancy.
The size of the study population was determined by the number of patients within the GPRD database with a diagnosis of constipation. A feasibility study showed that approximately 45,000 patients would be included in each year cohort. This number is large enough to give extremely precise estimates of the proportion of patients prescribed laxatives.
Data source
The GPRD is a database of longitudinal patient primary care records, containing anonymized data on demographics, diagnoses, referrals, prescribing and health outcomes for patients from almost 500 GP practices in the UK (over 3 million currently registered patients) [Jick et al. 1991]. The database contains approximately 6% of UK patients, and the geographical distribution is representative of the UK population [Garcia Rodriguez and Gutthann, 1998]. Validation studies have confirmed the high data quality and completeness of clinical records within the GPRD [Khan et al. 2010; Jick et al. 2003; Garcia Rodriguez and Gutthann, 1998]. A recent systematic literature review of studies using the GPRD reported that the median proportion of diagnoses correctly coded was 89% [Herrett et al. 2010]. The LUCK (Laxative Usage in patients with GP-diagnosed Constipation in the UK) study received approval from the Independent Scientific Advisory Committee at the Medicines and Healthcare products Regulatory Agency (Protocol number 10_078).
Data analysis
Data were extracted using GPRD OnLine Data (GOLD) and analysed using SAS® (SAS Institute Inc, North Carolina, USA) software version 9.2.
The prevalence of GP-diagnosed constipation was calculated for each year from 2005 to 2009. The patient population was summarized for each year by comedications, comorbidities, age and sex. Prescribing trends for each product were summarized by product and by patient age and sex. Regional differences in prescribing trends were examined using the GPRD-defined regions of the UK. The number of prescriptions issued per patient was summarized by product and by patient age and sex. The prescribing trends of laxatives during pregnancy were examined similarly.
Results
Diagnoses of constipation in primary care
Within a population of 3.8 million patients in the GPRD, the prevalence of GP-diagnosed constipation ranged from 12 per 1000 persons in 2005 to 12.8 per 1000 persons in 2009. Overall, the prevalence in women was almost twice that in men; in 2009 the prevalence was 9.1 per 1000 people in men and 16.5 per 1000 people in women.
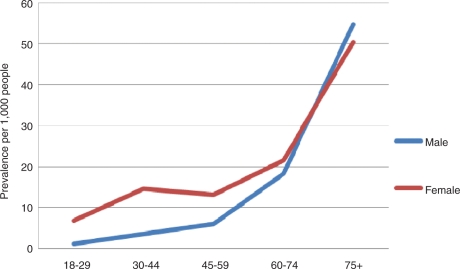
The prevalence of GP-diagnosed constipation increased with age, from 4.0 per 1000 in patients aged 18–29 years to 52.1 per 1000 in patients aged over 75 in 2009. This pattern did not change during the 5-year study period. The prevalence of constipation in 2009 was higher in women than in men in the younger age groups, but higher in men than in women in patients over the age of 75 (Figure 1). There was a peak in prevalence amongst females aged between 30 and 44, possibly reflecting an increase in the risk of constipation during pregnancy or an increase in the likelihood of consulting a GP regarding constipation during pregnancy.
Figure 1.
Prevalence of GP-diagnosed constipation in 2009 by age and sex.
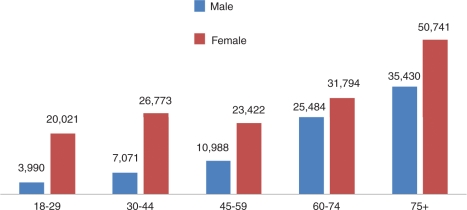
Between 2005 and 2009, the number of patients with GP-diagnosed constipation in the GPRD increased with increasing age in both male and female patients (Figure 2). In each age group there were more female patients than male. Although there were more female patients than male among patients aged over 75, the prevalence of GP-diagnosed constipation was higher in males than in females in this age group, reflecting the higher number of female patients within this age group.
Figure 2.
Number of patients with GP-diagnosed constipation by age and sex (2005–2009).
Table 1 shows the age and sex of patients with GP-diagnosed constipation. There were between 45,000 and 49,000 patients in the GPRD with a diagnosis of constipation in each study year, with an average age of between 61 and 63. A total of 65% of patients were female, and the average age and sex ratio of the patients remained constant during the 5-year study period.
Table 1.
Patients with a diagnosis of constipation between 2005 and 2009.
| Patient characteristic | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|
| Number of patients | 47,196 | 46,653 | 45,395 | 47,718 | 48,752 |
| Sex, % male | 35 | 35 | 35 | 35 | 35 |
| Age, years, mean (SD) | 62.8 (20.9) | 62.5 (21.1) | 62.3 (21.1) | 62.1 (21.1) | 61.5 (21.1) |
There are a number of diagnostic codes for constipation; there are several general constipation codes and a number of more specific codes for the type of constipation. The code used to record constipation was examined for the cohorts of patients; however, although there are READ codes available for a GP to record the type of constipation with which a patient presents, it appears that these specific codes are not often used, and a general constipation code is used more commonly.
Opiate usage and cancer diagnoses were investigated in patients with a diagnosis of constipation, as opiate usage is known to cause constipation [Klaschik et al. 2003]. The cohort was examined for any prescriptions for opioids within the year of interest. A total of 44% of patients in the 2009 cohort had a prescription for an opioid within 2009. This proportion was similar across the 5 study years.
Diagnoses of constipation during pregnancy
Within the cohorts of patients with GP-diagnosed constipation, 3296 female patients were identified as having a pregnancy during 2009, and 2291 patients were identified as having a pregnancy within 2005 (Table 2).
Table 2.
Pregnant patients with a diagnosis of constipation in 2005 and 2009.
| GP-diagnosedconstipation in pregnancy | Number of patients | % | % | Age, years, mean (SD) | Mean number of prescriptions (SD) | History of GP-diagnosed constipation prior to pregnancy (%) |
|---|---|---|---|---|---|---|
| 2009 cohort | ||||||
| All pregnant women | 1648 | – | 31.1 (7.1) | – | 29.6 | |
| Treated with laxatives | 729 | 44.2% | 31.6 (7.4) | 1.62 (1.4) | 33.5 | |
| Macrogols | 231 | 31.7% | 33.0 (7.7) | 1.46 (1.2) | – | |
| Senna | 90 | 12.3% | 33.9 (8.4) | 1.74 (2.0) | – | |
| Lactulose | 468 | 64.2% | 30.9 (6.8) | 1.45 (1.2) | – | |
| Other laxatives | ||||||
| Ispaghula | 57 | 3.5% | 31.9 (7.2) | 1.47 (1.6) | ||
| Docusate sodium | 26 | 1.6% | 35.0 (8.6) | 2.00 (2.6) | ||
| Bisacodyl | 18 | 1.1% | 34.8 (7.4) | 1.72 (1.6) | ||
| Glycerol | 61 | 3.7% | 32.1 (7.5) | 1.19 (0.5) | ||
| Not treated with laxatives | 919 | 55.8% | 31.4 (7.3) | – | 26.6 | |
| 2005 cohort | ||||||
| All pregnant women | 795 | – | 29.2 (6.0) | – | 24.9 | |
| Treated with laxatives | 263 | 33.1% | 29.3 (5.7) | 1.60 (1.1) | 28.1 | |
| Macrogols | 35 | 13.3% | 29.4 (6.2) | 1.32 (0.6) | – | |
| Senna | 39 | 14.8% | 29.7 (5.2) | 1.26 (0.6) | – | |
| Lactulose | 213 | 81.0% | 29.1 (5.7) | 1.27 (0.9) | – | |
| Other laxatives | ||||||
| Ispaghula | 24 | 3.0% | 30.0 (5.4) | 1.13 (0.4) | ||
| Docusate sodium | 7 | 0.9% | 24.3 (4.1) | 1.14 (0.4) | ||
| Bisacodyl | 7 | 0.9% | 24.1 (5.1) | 1.14 (0.4) | ||
| Glycerol | 22 | 2.8% | 28.8 (4.8) | 1.00 (0.0) | ||
| Not treated with laxatives | 532 | 66.9% | 29.2 (6.1) | – | 23.3 |
The average age of patients with GP-diagnosed constipation with a recorded pregnancy was 29.2 (SD 6.0) in 2005 and 31.1 (SD 7.1) in 2009. In 2005, 25% of these patients had a recorded history of GP-diagnosed constipation prior to their pregnancy, and 29% of patients had prior GP-diagnosed constipation in 2009.
Laxative prescribing in primary care
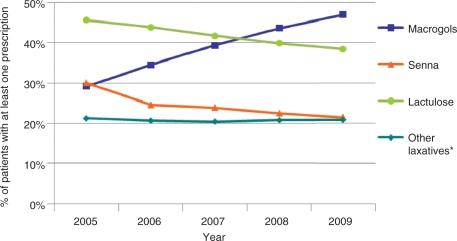
Amongst the six medications studied, lactulose was the most commonly prescribed overall. The pattern of prescribing changed over the 5-year study period, however; the percentage of patients with GP-diagnosed constipation who were prescribed lactulose and senna decreased and the proportion prescribed macrogol increased (Figure 3). Between 2005 and 2009 macrogol moved from being the least prescribed of the three most common laxatives to the most commonly prescribed; 29% of patients received a prescription for macrogol in 2005 and 47% received one in 2009.
Figure 3.
Percentage of constipation cohort prescribed each type of laxative. *Ispaghula, Docusate Sodium, Bisacodyl or Glycerol.
Table 3 summarizes the characteristics of patients with at least one prescription for macrogol, senna, lactulose, ispaghula, docusate sodium, bisacodyl or glycerol. Some patients had prescriptions for more than one substance; these patients were included in multiple groups.
Table 3.
Characteristics of patients by prescribed laxative.
| Patients with a diagnosis of constipation between 2005 and 2009 | Macrogol | Senna | Lactulose | Other laxatives* | Not treated with laxatives | Total |
|---|---|---|---|---|---|---|
| 2009 | ||||||
| Number of patients | 22,885 | 10,477 | 18,711 | 10,172 | 7427 | 48,752 |
| Number of prescriptions | 55,860 | 39,496 | 51,632 | 31,778 | ||
| Sex, males | 37% | 38% | 34% | 36% | 34% | 35% |
| Age, years, mean (SD) | 64.5 (20.0) | 67.2 (19.6) | 62.2 (22.0) | 62.7 (20.4) | 56.6 (20.8) | 61.5 (21.1) |
| 2008 | ||||||
| Number of patients | 20,819 | 10,733 | 19,030 | 9923 | 7755 | 47,718 |
| Number of prescriptions | 50,352 | 40,151 | 54,403 | 30,188 | ||
| Sex, males | 37% | 38% | 35% | 35% | 33% | 35% |
| Age, years, mean (SD) | 65.5 (19.8) | 67.8 (19.6) | 63.0 (21.9) | 63.4 (20.4) | 55.6 (21.0) | 62.1 (21.1) |
| 2007 | ||||||
| Number of patients | 17,846 | 10,854 | 18,946 | 9273 | 7786 | 45,395 |
| Number of prescriptions | 42,648 | 39,538 | 54,094 | 27,794 | ||
| Sex, males | 37% | 38% | 36% | 36% | 33% | 35% |
| Age, years, mean (SD) | 65.8 (19.7) | 67.8 (19.5) | 63.7 (21.6) | 63.7 (20.2) | 56.0 (21.2) | 62.3 (21.1) |
| 2006 | ||||||
| Number of patients | 16,078 | 11,461 | 20,435 | 9645 | 8547 | 46,653 |
| Number of prescriptions | 39,136 | 43,050 | 61,659 | 29,240 | ||
| Sex, males | 37% | 38% | 35% | 36% | 33% | 35% |
| Age, years, mean (SD) | 66.2 (19.6) | 68.0 (19.3) | 64.2 (21.4) | 63.9 (20.2) | 55.8 (21.2) | 62.5 (21.1) |
| 2005 | ||||||
| Number of patients | 13,751 | 14,178 | 21,509 | 10,023 | 9176 | 47,196 |
| Number of prescriptions | 33,949 | 46,359 | 64,737 | 31,169 | ||
| Sex, males | 36% | 34% | 35% | 36% | 33% | 35% |
| Age, years, mean (SD) | 67.1 (19.0) | 68.3 (19.3) | 64.4 (21.3) | 69.0 (19.9) | 55.8 (21.0) | 62.8 (20.9) |
Ispaghula, docusate sodium, bisacodyl or glycerol suppositories.
Patients with a diagnosis of constipation but no prescription for a laxative were, on average, younger than patients prescribed laxatives: 56.6 years (SD 20.8) in 2009 compared with an overall average age of 61.5 (21.1). This is probably due to the fact that patients under the age of 60 pay a prescription charge in England, and laxatives bought over the counter in a pharmacy are likely to be cheaper than this charge. Patients prescribed senna tended to be older [67.2 (19.6) in 2009], and this group of patients had a slightly higher proportion of males: 38% compared with 35% overall. Patients prescribed lactulose or other laxatives were, on average, younger than patients prescribed macrogol or senna: 62.2 (22.0) and 62.7 (20.4) compared with 64.5 (20.0) for macrogol and 67.2 (19.6) for senna.
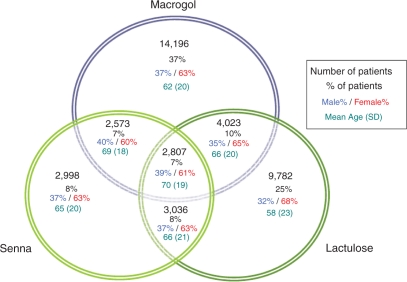
Over 76% of patients within the constipation cohort were prescribed at least one of the three most common laxatives (macrogol, senna and lactulose) within the year of diagnosis. In 2009, 32% of patients were prescribed at least two of these three types of laxative during 2009 (Figure 4). The number of prescriptions each patient received in a year increased with age.
Figure 4.
Number of patients with each combination of treatments within 2009.
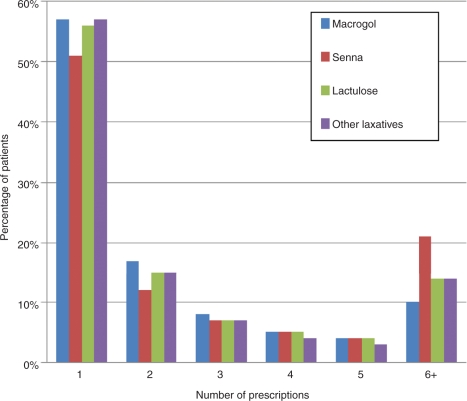
For the seven most commonly prescribed laxatives, the number of prescriptions each patient was prescribed within a 1-year period for the 2009 cohort of patients with GP-diagnosed constipation is summarized in Figure 5. Over 50% of patients who were prescribed each drug within 2009 received only one prescription. A total of 20% of patients prescribed senna were prescribed more than six prescriptions during 2009, compared with 10% of patients prescribed macrogol and 14% of those prescribed lactulose.
Figure 5.
Number of patients by number of prescriptions in 2009.
In 2009 within the GPRD, 48,752 patients werediagnosed with constipation (prevalence of 0.13 per patient year). In this group of patients the total number of GP consultations in which constipation was diagnosed was 67,493. The average number of consultations per year that included a diagnosis of constipation was 1.3 (SD 0.86) and 19% of patients consulted a GP about constipation at least twice during this 1-year period.
The prescribing trends for the three most common laxatives within the cohort of patients with GP-diagnosed constipation were examined by UK region. There was a shift in prescribing from senna and lactulose to macrogol across all regions of the UK, with the biggest shift in the south-east, south central and south-east coast of England. In these areas macrogol was the most commonly prescribed laxative by 2009.
Laxative prescribing in pregnancy
Table 2 shows laxative prescribing in pregnant patients in 2005 and 2009. In 2005, 33% of pregnant patients with GP-diagnosed constipation were prescribed laxatives, rising to 44% in 2009. Lactulose was the laxative most commonly prescribed in pregnancy, but the percentage of laxative-treated patients prescribed lactulose dropped from 81% in 2005 to 64% in 2009. The percentage of pregnant patients treated with laxatives who were prescribed macrogols rose from 13% in 2005 to 32% in 2009. Patients treated with laxatives during pregnancy were more likely to have consulted a GP regarding constipation prior to their pregnancy than patients who were not treated with laxatives during their pregnancy (in 2009, 34% of those treated compared with 27% of those not treated).
Discussion
The prevalence of GP-diagnosed constipation has remained constant over the 5-year study period. The prevalence figures produced in this study are likely to be a considerable underestimate of the actual prevalence of constipation in the UK. This is because constipation can be a relatively minor complaint for which patients can self-medicate with a change in diet or can buy over-the-counter medications from a pharmacy.
The prevalence of GP-diagnosed constipation was found to be higher in female patients in all age groups under 75, but in patients aged over 75 the prevalence was higher in males. This may reflect a lower frequency of GP visits in general amongst younger males or an actual lower prevalence of constipation in men under the age of 75.
A high proportion of patients in this cohort had a record of opioid use, suggesting the possibility of medication-induced constipation in these patients. GPs prescribing opiates are aware that these agents can cause constipation, so may coprescribe laxatives or be more likely to inquire during their consultations whether the patient is constipated. Constipation in these patients could also be more severe or chronic than in patients not being prescribed opiates, which would mean that these patients are more likely to mention it to the GP.
There are other medications that are known to cause constipation, such as anticholinergic agents, tricyclic antidepressants, calcium channel blockers, diuretic drugs and NSAIDs, which were not investigated in this study. Further studies are needed to fully explain the proportion of medication-induced constipation within the GP-diagnosed constipation cohort.
Age is another factor that affects whether a patient consults a GP or a pharmacist. In both male and female patients the prevalence of GP-diagnosed constipation in patients aged 75 and older was much higher than the prevalence in younger patients. The results of this study suggest that older patients are either more likely to suffer from constipation or more likely to consult their GP if they do suffer from constipation, as the number of diagnoses and prescriptions was higher in the older age groups. Older patients would be likely to suffer from more comorbidities than younger patients and may therefore consult their GP more, and may therefore be more likely to mention constipation symptoms within a consultation. It is also possible that younger patients may prefer to visit a pharmacy for minor illness.
It is likely that patients with more severe and more chronic cases of constipation would be those more likely to be consulting a GP, although it was not possible to conclude this from this study as the diagnostic codes for constipation do not specify severity.
The trend in prescribing of laxatives by GPs to patients diagnosed with constipation has changed during the 5-year study period. The use of senna and lactulose products has decreased, while prescriptions for macrogol have increased steadily. Macrogol is now the most commonly prescribed laxative to patients with GP-diagnosed constipation in the UK. This pattern is also reflected in pregnant women; the proportion of patients prescribed macrogols for constipation in pregnancy has increased and the proportion prescribed lactulose and other laxatives has decreased.
The results of this study indicate that the most common laxatives prescribed in pregnancy are lactulose and macrogols, with lactulose being replaced by macrogols in the 5-year study period, reflecting the trend in the general population. The database does not allow us to examine the reasons GPs may have had for preferentially prescribing macrogols, so more research is needed to establish whether this might be due to their experience suggesting that macrogol is more effective, or to a perception that macrogol it is a safer product because of its mode of action.
There are no definitive guidelines on laxative prescribing in pregnancy, but the BNF suggests that if laxatives are required in pregnancy, bulk-forming laxatives should be tried first, followed by an osmotic laxative such as lactulose, followed by a stimulant such as senna if needed. Although the BNF suggests lactulose as the osmotic laxative to be prescribed, the results of this study suggest that GPs are increasingly confident in prescribing macrogols in pregnancy, and that therefore this advice is perhaps outdated. This is in line with the Summary of Product Characteristics for Macrogol 4000, which states that the product is suitable for use in pregnancy.
In 2009 there were approximately 67,000 GP consultations in which constipation was discussed and diagnosed. Although these consultations may also have been used to discuss otherconditions, the condition still represents a significant use of GP resources. Projecting thesefigures up to the whole UK population, itis estimated that there are over one millionGP consultations regarding constipation every year.
Acknowledgements
The authors would like to thank Dr Jas Kalsi for medical input into the writing up of the study, and Lindsay Vye and Eryl Lloyd for continued support of the study.
Funding
The study was funded by Boehringer Ingelheim Ltd.
Conflict of interest statement
SL and ACES are employees of Boehringer Ingelheim. PJW has served as an advisory board member or received research funding from the following pharmaceutical companies: Novartis Pharmaceuticals, GlaxoSmithKline, Solvay Pharmaceuticals, Rotta Research, Proctor and Gamble, Danone Research, Astellas Pharma, Ironwood Pharmaceuticals, Sucampo Pharmaceuticals, Almirall Pharma, Movetis UK, Norgine and Chr Hansen. PJW has acted as a paid consultant to Boehringer Ingelheim but received no payment for his contribution to this article. JSOD is chair of the Primary Care Society for Gastroenterology, which is funded by the following pharmaceutical companies: Danone, Norgine, Shire, Reckitt Benckiser, Warner Chilcott, Yalkult, ProBio and Puricore. JSOD has contributed to advisory boards for Astra Zeneca, Danone and Shire. He has not received payment for his contribution to this article.
Ethical approval
The protocol for this study received approval from the Independent Scientific Advisory Committee at the Medicines and Healthcare products Regulatory Agency (Protocol number 10_078).
Appendix
Medical Codes
Constipation codes.
| Medical Code | Number of clinical events | READ code | Term |
|---|---|---|---|
| 1028 | 635856 | 19C..00 | Constipation |
| 2004 | 609583 | 19C..11 | Constipation symptom |
| 5803 | 183861 | J520z00 | Constipation NOS |
| 1709 | 36286 | J520.00 | Constipation - functional |
| 10687 | 6428 | J503100 | Faecal impaction |
| 20450 | 5381 | 19CZ.00 | Constipation NOS |
| 6364 | 2352 | J520100 | Chronic constipation with overflow |
| 23641 | 819 | J520000 | Acute constipation |
| 25797 | 815 | J520200 | Chronic constipation without overflow |
| 26022 | 580 | J520300 | Drug induced constipation |
| 15939 | 213 | E264500 | Psychogenic constipation |
| 24180 | 150 | J520y00 | Other specified constipation |
Pregnancy Codes.
| Current pregnancy codes | ||
|---|---|---|
| medcode | readcode | readterm |
| 127 | 62…00 | Patient pregnant |
| 6184 | ZV22.00 | [V]Normal pregnancy |
| 5709 | 62…13 | Pregnancy care |
| 3030 | 4654 | Urine pregnancy test positive |
| 5044 | 13H7.00 | Unwanted pregnancy |
| 4536 | 621..11 | Pregnancy confirmed |
| 13165 | 621..00 | Patient currently pregnant |
| 1771 | L182.00 | Anaemia during pregnancy, childbirth and the puerperium |
| 6715 | 6219 | Patient ? pregnant |
| 10306 | Z22C314 | Weeks pregnant |
| 5778 | 67A..00 | Pregnancy advice |
| 9408 | L10y.11 | Bleeding in early pregnancy |
| 49519 | Z229.00 | Observation of position of pregnancy |
| 1668 | L182500 | Iron deficiency anaemia of pregnancy |
| 14899 | 621Z.00 | Patient pregnant NOS |
| 15567 | 6218 | Pregnant -unplanned-not wanted |
| 1130 | L210.00 | Twin pregnancy |
| 29631 | 6222 | Antenatal care: 2nd pregnancy |
| 15033 | ZV61900 | [V]Other unwanted pregnancy |
| 7517 | 621C.00 | Unplanned pregnancy |
| 1357 | L210100 | Twin pregnancy - delivered |
| 2638 | L1…00 | Pregnancy complications |
| 3766 | L10..00 | Haemorrhage in early pregnancy |
| 3191 | L16y500 | Abdominal pain in pregnancy |
| 20240 | 6216 | Pregnant - planned |
| 10185 | L13..00 | Excessive pregnancy vomiting |
| 9754 | Z22A400 | Early stage of pregnancy |
| 29593 | 6223 | Antenatal care: 3rd pregnancy |
| 14925 | L10z.00 | Early pregnancy haemorrhage NOS |
| 10775 | 8B74.00 | Iron supplement in pregnancy |
| 3421 | L12z300 | Unspecified hypertension in preg/childb/puerp - not deliv |
| 16215 | 6211 | Pregnant - urine test confirms |
| 11760 | L13z.00 | Unspecified pregnancy vomiting |
| 16611 | ZV22300 | [V]Pregnant state, incidental |
| 12890 | Z227.00 | Confirmation of pregnancy |
| 12837 | 7F2B100 | Ultrasound monitoring of early pregnancy |
| 15318 | 6214 | Pregnant - on history |
| 18500 | Z22D100 | Viable pregnancy |
| 14842 | 6217 | Pregnant - unplanned - wanted |
| 14994 | 6174 | Pregnant, sheath failure |
| 13672 | 8HHf.00 | Refer to early pregnancy unit |
| 16775 | L16E.00 | Pregnancy pruritus |
| 36903 | 67AZ.00 | Pregnancy advice NOS |
| 13968 | 584D.00 | Antenatal ultrasound confirms intra-uterine pregnancy |
| 6649 | L166800 | Urinary tract infection complicating pregnancy |
| 22193 | Z229100 | Intrauterine pregnancy |
| 35158 | Z225.00 | Normal pregnancy |
| 67975 | L166.00 | Genitourinary tract infections in pregnancy |
| 1850 | 7F06012 | Shirodkar suture in pregnancy |
| 22183 | 957..11 | Prescription exempt form-preg |
| 21119 | L182300 | Anaemia during pregnancy - baby not yet delivered |
| 10184 | 67A3.00 | Pregnancy smoking advice |
| 13759 | 445..00 | Serum pregnancy test (B-HCG) |
| 5693 | L16A.00 | Glycosuria during pregnancy |
| 23495 | L265.00 | Small-for-dates fetus in pregnancy |
| 61835 | L161z00 | Oedema or excessive weight gain in pregnancy NOS |
| 10205 | ZG9..00 | Advice relating to pregnancy and fertility |
| 15338 | 621A.00 | Pregnancy unplanned ? wanted |
| 37701 | Z22A.00 | Observation of pattern of pregnancy |
| 53685 | L410500 | Varicose veins of legs in pregnancy |
| 43140 | 67A2.00 | Diet in pregnancy advice |
| 21849 | L031.00 | Tubal pregnancy |
| 30365 | Z22AA00 | Wanted pregnancy |
| 15061 | L13..12 | Hyperemesis of pregnancy |
| 17947 | 62a..00 | Pregnancy review |
| 26286 | L18A000 | Cholestasis of pregnancy |
| 20439 | L123.00 | Transient hypertension of pregnancy |
| 9986 | Z212.11 | Pregnancy care |
| 23421 | 615C.00 | IUD failure - pregnant |
| 14644 | L166z11 | UTI - urinary tract infection in pregnancy |
| 35912 | ZV22200 | [V]Pregnancy confirmed |
| 25131 | Z22AD11 | Reported conception - pregnancy |
| 28103 | Z22A300 | Concealed pregnancy |
| 15433 | L21..00 | Multiple pregnancy |
| 30618 | Z22AB00 | Unplanned pregnancy |
| 26201 | Z22AC00 | Pregnancy with uncertain dates |
| 35859 | 67A5.00 | Pregnancy alcohol advice |
| 20197 | L211.00 | Triplet pregnancy |
| 14877 | 621B.00 | Pregnant - ? planned |
| 3029 | L166500 | Infections of kidney in pregnancy |
| 41122 | L10zz00 | Early pregnancy haemorrhage NOS |
| 15418 | L166300 | Genitourinary tract infection in pregnancy - not delivered |
| 46270 | ZV22z00 | [V]Unspecified pregnant state |
| 51298 | 6215 | Pregnant - on abdom. palpation |
| 36006 | L16z.00 | Pregnancy complication NOS |
| 10278 | L180800 | Diabetes mellitus arising in pregnancy |
| 2602 | L166.11 | Cystitis of pregnancy |
| 10261 | L2…00 | Risk factors in pregnancy |
| 37693 | 13Hd.00 | Teenage pregnancy |
| 2937 | L175.11 | Rubella contact in pregnancy |
| 97034 | 67AE.00 | Folic acid advice in first trimester of pregnancy |
| 24603 | L10y.00 | Other haemorrhage in early pregnancy |
| 15634 | L166z00 | Genitourinary tract infection in pregnancy NOS |
| 29692 | 615C.11 | Pregnant, IUD failure |
| 38882 | L123600 | Transient hypertension of pregnancy |
| 20118 | 62O..12 | Static weight gain pregnancy |
| 25254 | Z21..00 | Care relating to reproduction and pregnancy |
| 28107 | L161.00 | Oedema or excessive weight gain in pregnancy no hypertension |
| 50421 | Z22A900 | Unwanted pregnancy |
| 39117 | L126500 | Eclampsia in pregnancy |
| 14651 | 13H8.00 | Illegitimate pregnancy |
| 29205 | 4453 | Serum pregnancy test positive |
| 48552 | L010.11 | Anembryonic pregnancy |
| 42614 | Z22..00 | Pregnancy observations |
| 32975 | 6166 | Pregnant, diaphragm failure |
| 29746 | 8B75.00 | Vitamin supplement - pregnancy |
| 35592 | 6213 | Pregnant - V.E. confirms |
| 35509 | 624..00 | A/N care: precious pregnancy |
| 65834 | ZV23200 | [V]Pregnancy with history of abortion |
| 23438 | L2z..00 | Risk factors in pregnancy NOS |
| 27451 | L166000 | Genitourinary tract infection in pregnancy unspecified |
| 45965 | Z22C311 | Pregnancy duration |
| 50058 | 13SZ.00 | Pregnancy benefit NOS |
| 38346 | ZV22000 | [V]First normal pregnancy supervision |
| 33708 | L182100 | Anaemia during pregnancy - baby delivered |
| 34173 | L12B.00 | Proteinuric hypertension of pregnancy |
| 41587 | L210z00 | Twin pregnancy NOS |
| 30351 | 67A6.00 | Drugs in pregnancy advice |
| 12521 | L161.11 | Excessive weight gain in pregnancy |
| 22557 | Z22B100 | Single pregnancy |
| 31162 | L165.00 | Asymptomatic bacteriuria in pregnancy |
| 15065 | 8B7..11 | Pregnancy vitamin/iron prophyl |
| 22215 | Z22A200 | High risk pregnancy |
| 44729 | L031000 | Fallopian tube pregnancy |
| 32493 | Z22AD00 | Presentation of pregnancy |
| 26866 | L2y..00 | Other specified risk factors in pregnancy |
| 27740 | L16..00 | Other pregnancy complication NEC |
| 35646 | L123z00 | Transient hypertension of pregnancy NOS |
| 45729 | Z22D.00 | Observation of viability of pregnancy |
| 52583 | L168.00 | Fatigue during pregnancy |
| 23334 | L162.11 | Albuminuria in pregnancy without hypertension |
| 29786 | L416600 | Haemorrhoids in pregnancy |
| 42015 | L16y.00 | Other pregnancy complications |
| 30817 | 6212 | Pregnant - blood test confirms |
| 44057 | ZV23111 | [V]Pregnancy with history of hydatidiform mole |
| 23751 | L266.00 | Large-for-dates fetus in pregnancy |
| 40978 | Z22A700 | Surrogate pregnancy |
| 49884 | 6761 | Diabetic pre-pregnancy counselling |
| 56451 | L13zz00 | Unspecified pregnancy vomiting NOS |
| 6580 | ZV23800 | [V]Supervision of high-risk pregnancy due to social problems |
| 38771 | Lyu2200 | [X]Other venous complications in pregnancy |
| 18258 | L167.00 | Liver disorder in pregnancy |
| 10173 | Z23D200 | Pregnant abdomen observation |
| 33441 | L511.00 | Maternal care for viable fetus in abdominal pregnancy |
| 46577 | 66AX.00 | Diabetes: shared care in pregnancy - diabetol and obstet |
| 24063 | 62O7.00 | Pregnancy prolonged - 41 weeks |
| 23373 | L150.00 | Post-term pregnancy |
| 54942 | L123100 | Transient hypertension of pregnancy - delivered |
| 21196 | ZV23.00 | [V]High-risk pregnancy supervision |
| 27980 | L166700 | Infections of the genital tract in pregnancy |
| 36235 | 67A4.00 | Pregnancy exercise advice |
| 49855 | Q015100 | Fetus or neonate affected by twin pregnancy |
| 34639 | L180100 | Diabetes mellitus during pregnancy - baby delivered |
| 37048 | 6282 | A/N care:10yrs+since last preg |
| 36394 | L16C.00 | Pregnancy induced oedema + proteinuria without hypertension |
| 52048 | Z22AB11 | Accidental pregnancy |
| 38022 | Z22CF00 | Date symptom of pregnancy first noted |
| 50512 | L031z00 | Tubal pregnancy NOS |
| 25501 | Z22A100 | Low risk pregnancy |
| 16017 | L166100 | Genitourinary tract infection in pregnancy - delivered |
| 30302 | 7F…12 | Pregnancy operations |
| 12230 | L263900 | Maternal care for fetal tachycardia during pregnancy |
| 65555 | L132.00 | Late vomiting of pregnancy |
| 34938 | L212.00 | Quadruplet pregnancy |
| 38556 | 67A7.00 | Pregnancy dental advice |
| 45413 | L263A11 | Maternal care for reduced fetal heart rate during pregnancy |
| 35514 | 624Z.00 | A/N care: precious preg. NOS |
| 40633 | Z22A600 | Teenage pregnancy |
| 41033 | Z22C511 | EDC - Estimated date of conception |
| 23831 | ZV22y00 | [V]Other specified pregnant state |
| 40964 | L192.00 | Continuing preg after intrauterine death one fetus or more |
| 54866 | L030.00 | Abdominal pregnancy |
| 55338 | L123000 | Transient hypertension of pregnancy unspecified |
| 32950 | L03y100 | Cornual pregnancy |
| 25872 | L16yz00 | Other pregnancy complication NOS |
| 46082 | K5Cz.11 | Habitual aborter-not pregnant |
| 49559 | L180300 | Diabetes mellitus during pregnancy - baby not yet delivered |
| 70993 | L16Az00 | Glycosuria during pregnancy NOS |
| 24170 | L15..00 | Prolonged or post-term pregnancy |
| 26055 | L263A00 | Maternal care for fetal bradycardia during pregnancy |
| 39359 | L385.00 | Failed or difficult intubation during pregnancy |
| 40357 | L2D..00 | Retained intrauterine contraceptive device in pregnancy |
| 47608 | L177.00 | Infections of bladder in pregnancy |
| 53097 | Z22C313 | Duration of pregnancy |
| 61301 | L10y000 | Other haemorrhage in early pregnancy unspecified |
| 20623 | ZV4J000 | [V]Problems related to unwanted pregnancy |
| 50440 | Z22A500 | Biochemical pregnancy |
| 27833 | L13y.00 | Other pregnancy vomiting |
| 49407 | L2B..00 | Low weight gain in pregnancy |
| 53167 | L188300 | Abnormal GTT during pregnancy - baby not yet delivered |
| 54107 | L10z000 | Early pregnancy haemorrhage NOS unspecified |
| 37038 | Z23D100 | Girth of pregnant abdomen |
| 53849 | L16y000 | Other pregnancy complication unspecified |
| 37348 | Lyu2500 | [X]Other specified pregnancy-related conditions |
| 43000 | L16D.00 | Excessive weight gain in pregnancy |
| 59276 | L032.00 | Ovarian pregnancy |
| 60345 | M240500 | Alopecia of pregnancy |
| 61284 | L210200 | Twin pregnancy with antenatal problem |
| 23995 | L15..11 | Post-term pregnancy |
| 46009 | Q0…00 | Fetus/neonate affected by maternal problem unrelated to preg |
| 41625 | L13yz00 | Other pregnancy vomiting NOS |
| 54577 | L210000 | Twin pregnancy unspecified |
| 47080 | L162.00 | Unspecified renal disease in pregnancy |
| 48534 | ZV23100 | [V]Pregnancy with history of trophoblastic disease |
| 52221 | L03y000 | Cervical pregnancy |
| 53921 | L161000 | Oedema or excessive weight gain in pregnancy, unspecified |
| 56252 | ZV22100 | [V]Other normal pregnancy supervision |
| 52685 | ZV23000 | [V]Pregnancy with history of infertility |
| 63344 | Z22C500 | Estimated date of conception |
| 68004 | Q01..00 | Fetus/neonate affected by maternal complication of pregnancy |
| 24944 | L163300 | Pregnancy care of habitual aborter |
| 36421 | L167z00 | Liver disorder in pregnancy NOS |
| 38312 | L162.12 | Nephropathy NOS in pregnancy without hypertension |
| 55730 | ZV22.11 | [V]Supervision of normal pregnancy |
| 59634 | L411512 | Vaginal varices in pregnancy |
| 64366 | L412500 | Superficial thrombophlebitis in pregnancy |
| 26852 | ZV23400 | [V]Pregnancy with other poor obstetric history |
| 65783 | L030000 | Delivery of viable fetus in abdominal pregnancy |
| 69327 | L10yz00 | Other haemorrhage in early pregnancy NOS |
| 41504 | L13y000 | Other pregnancy vomiting unspecified |
| 48304 | L10z200 | Early pregnancy haemorrhage NOS - not delivered |
| 61408 | L120300 | Benign essential hypertension in preg/childb/puerp-not deliv |
| 64247 | Z235.00 | Observation of shape of pregnant abdomen |
| 25230 | 9Ea2.00 | Less 24 wk involv risk injury physic/mentl health preg woman |
| 58142 | L16A000 | Glycosuria during pregnancy unspecified |
| 59588 | L411500 | Genital varices in pregnancy |
| 61563 | L161300 | Oedema or excessive weight gain in pregnancy - not delivered |
| 72835 | Q01z.00 | Fetus/neonate affected by maternal complic pregnancy NOS |
| 96757 | L411513 | Vulval varices in pregnancy |
| 56953 | Z235400 | Pendulous pregnant abdomen |
| 64292 | L16y300 | Other pregnancy complication - not delivered |
| 53661 | L13z200 | Unspecified pregnancy vomiting - not delivered |
| 64125 | L09yz00 | Other specified complication NOS follow abortive pregnancy |
| 64523 | L091z00 | Delayed/excess haemorrhage NOS following abortive pregnancy |
| 65256 | L164.00 | Peripheral neuritis in pregnancy |
| 67098 | L183300 | Drug dependence during pregnancy - baby not yet delivered |
| 67863 | Z22B.00 | Observation of quantity of pregnancy |
| 69722 | ZV23z00 | [V]Unspecified high-risk pregnancy |
| 73559 | L13z000 | Unspecified pregnancy vomiting unspecified |
| 43344 | ZV61800 | [V]Illegitimate pregnancy |
| 44734 | 62O8.00 | Pregnancy prolonged - 42 weeks |
| 49502 | L228.00 | Multiple pregnancy with malpresentation |
| 53490 | L178.00 | Infections of urethra in pregnancy |
| 54677 | L167200 | Liver disorder in pregnancy - not delivered |
| 55618 | ZV23600 | [V]Supervisn/pregnancy wth history insufficnt antenatal care |
| 73917 | L10y200 | Other haemorrhage in early pregnancy - not delivered |
| 44770 | L191.00 | Continuing pregnancy after abortion of one fetus or more |
| 59313 | Z22BA00 | Contin pregnancy after intrauterine death of sibling fetus |
| 63751 | L16A300 | Glycosuria during pregnancy - not delivered |
| 64099 | L168000 | Fatigue during pregnancy unspecified |
| 66649 | L41z500 | Venous complication of pregnancy, unspecified |
| 69011 | L132000 | Late pregnancy vomiting unspecified |
| 71730 | L123300 | Transient hypertension of pregnancy - not delivered |
| 73455 | Q015200 | Fetus or neonate affected by triplet pregnancy |
| 94804 | 67AB.00 | Preg. prescription exempt adv. |
| 37573 | Z22B900 | Continuing pregnancy after abortion of sibling fetus |
| 41959 | L171300 | Maternal gonorrhoea in pregnancy - baby not yet delivered |
| 60309 | Q0y..00 | Maternal problems unrelated preg affecting fetus/neonate OS |
| 61935 | L185.11 | Congenital heart disease in pregnancy |
| 67164 | L18z300 | Medical condition NOS in pregnancy - baby not yet delivered |
| 68694 | Z22C.00 | Observation of measures of pregnancy |
| 69815 | 62H3.00 | Rh screen - 1st preg. sample |
| 72883 | Lyu2100 | [X]Other vomiting complicating pregnancy |
| 73914 | L412511 | Thrombophlebitis of legs in pregnancy |
| 91254 | Z235200 | Rounded pregnant abdomen |
| 21467 | L03y200 | Membranous pregnancy |
| 29623 | 62H4.00 | Rh screen - 2nd preg. sample |
| 35855 | L15z.00 | Prolonged pregnancy NOS |
| 40825 | Z22B800 | Undiagnosed multiple pregnancy |
| 54701 | ZV23y00 | [V]Other specified high-risk pregnancy |
| 55889 | L175300 | Maternal rubella during pregnancy - baby not yet delivered |
| 57059 | L187300 | Orthopaedic disorder in pregnancy - baby not yet delivered |
| 61466 | Z22A211 | HRP - High risk pregnancy |
| 62358 | L167000 | Liver disorder in pregnancy unspecified |
| 66390 | Q0z..00 | Maternal problem unrelated preg affecting fetus/neonate NOS |
| 69599 | L21y.00 | Other multiple pregnancy |
| 69686 | L417000 | Cerebral venous thrombosis in pregnancy |
| 91888 | L181300 | Thyroid dysfunction in pregnancy - baby not yet delivered |
| 93895 | Z22B500 | Quintuplet pregnancy |
| 94473 | L211z00 | Triplet pregnancy NOS |
| 99247 | L13y200 | Other pregnancy vomiting - not delivered |
| 49193 | 67A7.11 | Care of teeth advice -in preg. |
| 51956 | L212200 | Quadruplet pregnancy with antenatal problem |
| 54293 | Q015.00 | Fetus or neonate affected by multiple pregnancy |
| 54938 | L162000 | Unspecified renal disease in pregnancy unspecified |
| 59650 | Z235300 | Transversely enlarged pregnant abdomen |
| 60877 | L263800 | Maternal care for fetal decelerations during pregnancy |
| 61576 | L211000 | Triplet pregnancy unspecified |
| 64500 | L183.11 | Pregnancy and drug dependence |
| 66594 | L186.11 | Heart disease during pregnancy |
| 67698 | L150000 | Post-term pregnancy unspecified |
| 67893 | L2C..00 | Malnutrition in pregnancy |
| 72014 | L212z00 | Quadruplet pregnancy NOS |
| 72019 | L132200 | Late pregnancy vomiting - not delivered |
| 73727 | L168z00 | Fatigue during pregnancy NOS |
| 92579 | L162.13 | Uraemia in pregnancy without hypertension |
| 93303 | ZV22400 | [V]Supervision of other normal pregnancy |
| 96743 | L122300 | Other pre-exist hypertension in preg/childb/puerp-not deliv |
| 97349 | L121300 | Renal hypertension in preg/childbirth/puerp - not delivered |
| 99237 | L411511 | Perineal varices in pregnancy |
| 99980 | L168300 | Fatigue during pregnancy - not delivered |
| 37163 | L150z00 | Post-term pregnancy NOS |
| 67728 | L150200 | Post-term pregnancy - not delivered |
Therapy Codes
Macrogols.
| Product code | Number of events | Product | Substance |
|---|---|---|---|
| 5201 | 1132924 | MOVICOL sachets [NORGINE] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 6581 | 108523 | macrogol compound npf oral powder 13.8 g | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 6599 | 76211 | MOVICOL PAEDIATRIC PLAIN oral powder [NORGINE] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 6119 | 11695 | MOVICOL HALF oral powder [NORGINE] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 10069 | 11380 | IDROLAX powder 10 g [SCHWARZ] | macrogol 4000 |
| 38390 | 6906 | LAXIDO ORANGE oral powder [GALEN] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 10237 | 5952 | macrogol compound npf half strength sugar free oral powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 10261 | 3805 | macrogol npf oral powder 10 g | macrogol 4000 |
| 10125 | 1767 | macrogol 4000 powder 10 g | macrogol 4000 |
| 35443 | 1608 | MOVICOL PLAIN sachets [NORGINE] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 39532 | 658 | MOVICOL CHOCOLATE sachets [NORGINE] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 39660 | 283 | macrogol compound npf oral powder 13.7 g | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 40791 | 264 | macrogol compound npf sugar free oral powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 12915 | 246 | macrogol with sodium sulphate + electrolytes powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride/sodium sulphate |
| 7030 | 209 | polyethylene glycol with electrolytes oral powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 39734 | 175 | LAXIDO NATURAL oral powder [GALEN] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 39702 | 152 | macrogol compound npf oral powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 41776 | 3 | MOLAXOLE powder for oral solution [MEDA] | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride |
| 30905 | 2 | GOLYTELY powder | potassium chloride/macrogol 3350/sodium bicarbonate/sodium chloride/sodium sulphate |
Senna.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 52 | 2455687 | senna tablets 7.5 mg | sennoside | tablets | Stimulant laxatives |
| 2494 | 295499 | MANEVAC granules [HFA] | senna fruit/ispaghula husk | granules | Bulk-forming laxatives/ Stimulant laxatives |
| 1858 | 219088 | senna syrup 7.5 mg/5 ml | sennoside | syrup | Stimulant laxatives |
| 7105 | 81606 | senna oral solution 7.5 mg/5 ml | sennoside | oral solution | Stimulant laxatives |
| 3672 | 36598 | SENOKOT syrup 7.5 mg/5 ml [RECKITT B] | sennoside | syrup | Stimulant laxatives |
| 6034 | 20106 | ispaghula husk with senna fruits granules 54.2% + 12.4% | senna fruit/ispaghula husk | granules | Bulk-forming laxatives/ Stimulant laxatives |
| 5897 | 14995 | senna tablets 15 mg | sennoside | tablets | Stimulant laxatives |
| 5210 | 9743 | senna granules | sennoside | granules | Stimulant laxatives |
| 6324 | 8689 | SENOKOT granules [RECKITT B] | sennoside | granules | Stimulant laxatives |
| 14215 | 4312 | SENOKOT syrup [RECKITT B] | sennoside | syrup | Stimulant laxatives |
| 171 | 1708 | SENOKOT tablets [RECKITT B] | sennoside | tablets | Stimulant laxatives |
| 9890 | 1479 | senna tablets 12 mg | sennoside | tablets | Stimulant laxatives |
| 17587 | 666 | sennosides-total elixir | senna leaf | elixir | Stimulant laxatives |
| 16030 | 612 | senna chewable tablet 15 mg | sennoside | chewable tablet | Stimulant laxatives |
| 14292 | 507 | SENOKOT HI-FIBRE ORANGE granules [RECKITT B] | ispaghula husk | granules | Bulk-forming laxatives |
| 5987 | 378 | CALIFIG SYRUP OF FIGS elixir [MERCK CONS] | senna leaf | elixir | Stimulant laxatives |
| 28003 | 369 | SENNA tablets 7.5 mg [ACTAVIS] | sennoside | tablets | Stimulant laxatives |
| 35847 | 329 | SENOKOT DUAL RELIEF tablets [RECKITT B] | aloes (aloe)/taraxacum (dandelion root)/cascara/senna leaf/fennel seed | tablets | Stimulant laxatives/Herbal remedy |
| 28122 | 283 | SENNA tablets 7.5 mg [TEVA] | sennoside | tablets | Stimulant laxatives |
| 20452 | 116 | SENOKOT HI-FIBRE LEMON granules [RECKITT B] | ispaghula husk | granules | Bulk-forming laxatives |
| 28831 | 107 | SENOKOT MAX STRENGTH tablets 15 mg [RECKITT B] | sennoside | tablets | Stimulant laxatives |
| 16150 | 63 | SENOKOT PHARMACY syrup [RECKITT B] | sennoside | syrup | Stimulant laxatives |
| 14143 | 50 | EX-LAX SENNA pill [NOVARTIS] | sennoside | pill | Stimulant laxatives |
| 20747 | 44 | SENOKOT | Unknown | ||
| 21570 | 43 | EX-LAX SENNA tablets 15 mg [NOVARTIS] | sennoside | tablets | Stimulant laxatives |
| 23084 | 42 | senna with cascara tablets 32 mg + 130 mg | cascara/senna leaf | tablets | Stimulant laxatives |
| 41565 | 22 | SENNA tablets [FAMILY H] | sennoside | tablets | Stimulant laxatives |
| 15181 | 12 | NYLAX WITH SENNA tablets [RECKITT B] | sennoside | tablets | Stimulant laxatives |
| 33700 | 10 | SENNA tablets 7.5 mg [HILLCROSS] | sennoside | tablets | Stimulant laxatives |
| 22303 | 7 | SENNA FRUIT 12.4%/ISPAGHULA SEED 54.2% | Unknown | ||
| 10287 | 6 | X-PREP liquid [NAPP] | senna leaf | liquid | Stimulant laxatives |
| 16484 | 5 | SENOKOT DIRECT RELIEF suppository 4 g [RECKITT B] | glycerol | suppository | Stimulant laxatives |
| 30150 | 2 | SURE-LAX SENNA chewable tablet 15 mg [POTTER'S] | sennoside | chewable tablet | Stimulant laxatives |
| 28836 | 1 | AGIOLAX granules [RADIOL] | senna fruit/ispaghula husk | granules | Bulk-forming laxatives/Stimulant laxatives |
| 31988 | 1 | NYLAX tablets [CROOKES] | bisacodyl/phenolphthalein/senna | tablets | Stimulant laxatives |
| 32858 | 1 | SENNA tablets 7.5 mg [ASPAR] | sennoside | tablets | Stimulant laxatives |
| 36256 | 1 | SENNA | Unknown | ||
| 39230 | 1 | DUAL LAX EXTRA STRONG tablets [LANE] | aloin/cascara extract/senna leaf | tablets | Stimulant laxatives |
Lactulose.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 12 | 4051008 | lactulose solution 3.35 g/5 ml | lactulose | solution | Osmotic laxatives |
| 4613 | 1614999 | lactulose solution 3.1–3.7 g/5 ml | lactulose | solution | Osmotic laxatives |
| 4695 | 43057 | lactulose solution (flavoured) 3.35 g/5 ml | lactulose | solution (flavoured) | Osmotic laxatives |
| 5010 | 12193 | lactulose sachets 10 g | lactulose | sachets | Osmotic laxatives |
| 9489 | 8814 | LACTUGAL solution [INTRAPHARM] | lactulose | solution | Osmotic laxatives |
| 19524 | 8187 | LACTULOSE | Unknown | ||
| 16088 | 6669 | LACTULOSE solution 3.1–3.7 g/5 ml [IVAX] | lactulose | solution | Osmotic laxatives |
| 28877 | 1856 | LACTULOSE solution 3.1–3.7 g/5 ml [TEVA] | lactulose | solution | Osmotic laxatives |
| 9650 | 1222 | DUPHALAC DRY powder 10 g [SOLVAY] | lactulose | powder | Osmotic laxatives |
| 34015 | 1221 | LACTULOSE solution 3.1–3.7 g/5 ml [BERK] | lactulose | solution | Osmotic laxatives |
| 18423 | 488 | REGULOSE solution [NOVARTIS] | lactulose | solution | Osmotic laxatives |
| 8911 | 313 | DUPHALAC solution [SOLVAY] | lactulose | solution | Osmotic laxatives |
| 34055 | 141 | LACTULOSE solution 3.1–3.7 g/5 ml [HILLCROSS] | lactulose | solution | Osmotic laxatives |
| 27708 | 35 | LACTULOSE solution 3.1–3.7 g/5 ml [GEN (UK)] | lactulose | solution | Osmotic laxatives |
| 4559 | 12 | LEMLAX solution 3.28 g/5 ml [CO-PHARMA] | lactulose | solution | Osmotic laxatives |
| 33678 | 6 | LACTULOSE solution 3.1–3.7 g/5 ml [KENT] | lactulose | solution | Osmotic laxatives |
| 41638 | 6 | LACTULOSE solution 3.1–3.7 g/5 ml [SOLVAY] | lactulose | solution | Osmotic laxatives |
| 26590 | 5 | LAXOSE solution [BERK] | lactulose | solution | Osmotic laxatives |
| 32598 | 5 | LACTULOSE solution 3.1–3.7 g/5 ml [SANDOZ] | lactulose | solution | Osmotic laxatives |
| 34360 | 3 | LACTULOSE solution 3.1–3.7 g/5 ml [NOVARTIS] | lactulose | solution | Osmotic laxatives |
Ispaghula.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 1227 | 719146 | ispaghula husk gluten-free sugar-free effervescent granules | ispaghula husk | sugar-free effervescent granules | Bulk-forming laxatives |
| 2337 | 74909 | ispaghula husk gluten-free sugar free powder 3.4 g | ispaghula husk | sugar free powder | Bulk-forming laxatives |
| 6430 | 60013 | ispaghula husk gluten-free sugar free powder 3.5 g | ispaghula husk | sugar free powder | Bulk-forming laxatives |
| 5598 | 47783 | mebeverine hydrochloride with ispaghula husk sachets 135 mg + 3.5 g | ispaghula husk/mebeverine hydrochloride | sachets | Bulk-forming laxatives/Other antispasmodics |
| 6034 | 21204 | ispaghula husk with senna fruits granules 54.2% + 12.4% | senna fruit/ispaghula husk | granules | Bulk-forming laxatives/Stimulant laxatives |
| 2582 | 19004 | ispaghula husk gluten-free sugar free effervescent powder 3.6 g | ispaghula husk | sugar free effervescent powder | Bulk-forming laxatives |
| 6851 | 15952 | ispaghula husk gluten-free sugar free granules | ispaghula husk | sugar free granules | Bulk-forming laxatives |
| 8559 | 8400 | ISPAGHULA HUSK 90 % GRA | Unknown | ||
| 14618 | 3254 | ispaghula husk gluten-free sugar-free effervescent granules | ispaghula husk | sugar-free effervescent granules | Bulk-forming laxatives |
| 1655 | 2649 | ISPAGHULA HUSK 66 % GRA | Unknown | ||
| 13171 | 1949 | ispaghula husk gluten-free granules | ispaghula husk | granules | Bulk-forming laxatives |
| 11124 | 851 | ispaghula husk gluten-free powder 3.4 g | ispaghula husk | powder | Bulk-forming laxatives |
| 11243 | 308 | ispaghula husk gluten-free sugar free effervescent powder 6 g | ispaghula husk | sugar free effervescent powder | Bulk-forming laxatives |
| 25032 | 34 | ISPAGHULA HUSK EFFERVESCENT SACHET | Unknown | ||
| 34800 | 32 | ISPAGHULA HUSK GLUTEN-FREE sugar free effervescent powder 3.5 g [HILLCROSS] | ispaghula husk | sugar free effervescent powder | Bulk-forming laxatives |
| 22303 | 7 | SENNA FRUIT 12.4%/ISPAGHULA SEED 54.2% | Unknown | ||
| 37647 | 7 | ispaghula husk with lactobacillus and bifidobacteria oral powder | ispaghula husk/lactobacillus acidophilus/ | ||
| bifidobacterium bifidum | oral powder | Bulk-forming laxatives/Unlicensed product | |||
| 24523 | 6 | ISPAGHULA HUSK ORANGE SACHET | Unknown | ||
| 20683 | 4 | ISPAGHULA HUSK SACHET | Unknown | ||
| 25637 | 4 | ISPAGHULA HUSK | Unknown | ||
| 29829 | 1 | ISPAGHULA HUSK MICRONISED + DEXTROSE | Unknown | ||
Docusate Sodium.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 2468 | 417366 | docusate sodium capsules 100 mg | docusate sodium | capsules | Stimulant laxatives/Faecal softeners |
| 5215 | 16627 | docusate sodium sugar free oral solution 50 mg/5 ml | docusate sodium | sugar free oral solution | Stimulant laxatives/Faecal softeners |
| 3558 | 14562 | docusate sodium sugar free paediatric oral solution 12.5 mg/5 ml | docusate sodium | sugar free paediatric oral solution | Stimulant laxatives/Faecal softeners |
| 2699 | 7880 | docusate sodium tablets 100 mg | docusate sodium | tablets | Stimulant laxatives |
| 9510 | 3942 | docusate sodium ear drops 0.5% | docusate sodium | ear drops | Removal of ear wax |
| 17989 | 408 | docusate sodium enema 120 mg | docusate sodium | enema | Stimulant laxatives |
| 13999 | 214 | docusate sodium with glycerol enema 90 mg + 3.78 g/5 ml | docusate sodium | enema | Stimulant laxatives |
| 24073 | 69 | docusate sodium ear drops 5% | docusate sodium | ear drops | Removal of ear wax |
| 15002 | 37 | docusate sodium with glycerol ear drops | docusate sodium/glycerol | ear drops | Removal of ear wax |
| 24109 | 36 | docusate sodium with sorbitol enema | docusate sodium/sorbitol | enema | Faecal softeners |
| 23668 | 34 | DOCUSATE SODIUM | Unknown | ||
| 21613 | 8 | DOCUSATE SODIUM | Unknown | ||
| 22260 | 4 | docusate sodium and bisacodyl tablets | bisacodyl/docusate sodium | tablets | Stimulant laxatives |
| 28791 | 3 | DOCUSATE SODIUM S/F ORAL | Unknown | ||
Bisacodyl.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 2451 | 400750 | bisacodyl enteric coated tablets 5 mg | bisacodyl | Oral | Stimulant laxatives |
| 2771 | 99721 | bisacodyl suppository 10 mg | bisacodyl | Rectal | Stimulant laxatives |
| 2770 | 22219 | bisacodyl paediatric suppository 5 mg | bisacodyl | Rectal | Stimulant laxatives |
| 11565 | 1300 | bisacodyl rectal solution 2.74 mg/ml | bisacodyl | Rectal | Stimulant laxatives/Unlicensed medicinal product (specials) |
| 12012 | 954 | BISACODYL 10 MG TAB | Unknown | ||
| 34016 | 219 | BISACODYL tablets 5 mg [CELLTECH] | bisacodyl | Oral | Stimulant laxatives |
| 33799 | 155 | BISACODYL tablets 5 mg [HILLCROSS] | bisacodyl | Oral | Stimulant laxatives |
| 12079 | 66 | bisacodyl with dioctyl sodium sulphosuccinate tablets | bisacodyl/docusate sodium | Oral | Stimulant laxatives |
| 36071 | 27 | BISACODYL suppository 10 mg [DANIEL] | bisacodyl | Rectal | Stimulant laxatives |
| 34772 | 16 | BISACODYL tablets 5 mg [APS] | bisacodyl | Oral | Stimulant laxatives |
| 32322 | 6 | BISACODYL suppository 10 mg [HILLCROSS] | bisacodyl | Rectal | Stimulant laxatives |
| 34027 | 6 | BISACODYL tablets 5 mg [IVAX] | bisacodyl | Oral | Stimulant laxatives |
| 33798 | 5 | BISACODYL tablets 5 mg [ACTAVIS] | bisacodyl | Oral | Stimulant laxatives |
| 22260 | 4 | docusate sodium and bisacodyl tablets | bisacodyl/docusate sodium | Oral | Stimulant laxatives |
| 38425 | 3 | BISACODYL suppository 10 mg [CELLTECH] | bisacodyl | Rectal | Stimulant laxatives |
| 27532 | 2 | BISACODYL | Unknown | ||
| 34352 | 1 | BISACODYL enteric coated tablets 5 mg [SOVEREIGN] | bisacodyl | Oral | Stimulant laxatives |
| 41685 | 1 | BISACODYL suppository 10 mg [MARTINDALE] | bisacodyl | Rectal | Stimulant laxatives |
Glycerol.
| Product code | Number of events | Product | Substance | Formulation | BNF |
|---|---|---|---|---|---|
| 4364 | 131673 | glycerol suppository 4 g | glycerol | Rectal | Stimulant laxatives |
| 2989 | 43410 | glycerol suppository 1 g | glycerol | Rectal | Stimulant laxatives |
| 3160 | 17494 | glycerol suppository 2 g | glycerol | Rectal | Stimulant laxatives |
| 13999 | 214 | docusate sodium with glycerol enema 90 mg + 3.78 g/5 ml | docusate sodium | Rectal | Stimulant laxatives |
| 41730 | 15 | GLYCEROL suppository 4 g [MARTINDALE] | glycerol | Rectal | Stimulant laxatives |
References
- Bradley C.S., Kennedy C.M., Turcea A.M., Roa S.S.C., Nygaard I.E. (2007) Constipation in pregnancy: prevalence, symptoms and risk factors. Obstet Gynecol 110: 1351–1357 [DOI] [PubMed] [Google Scholar]
- British National Formulary (2010) Edition 60 BMJ Group & Pharmaceutical Press London.
- Connell A.M., Hilton C., Irvine G., Lennard-Jones J.E., Misiewicz J.J. (1965) Variation of bowel habit in two population samples. BMJ 2: 1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S., West S., Andrew E., Tennis P., Hammad T.A., Eaton S., et al. (2010) The identification of pregnancies within the general practice research database. Pharmacoepidemiol Drug Saf 19: 45–50 [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez L.A., Gutthann S.P. (1998) Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol 45: 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrett E., Thomas S.L., Schoonen W.M., Smeeth L., Hall A.J. (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69: 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P.D.R., Johanson J.F. (2004) Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 99: 750–759 [DOI] [PubMed] [Google Scholar]
- Jick H., Jick S.S., Derby L.E. (1991) Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 302: 766–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick S.S., Kaye J.A., Vasilakis-Scaramozza C., Garcia Rodriguez L.A., Ruigomez A., Meier C.R., et al. (2003) Validity of the general practice research database. Pharmacotherapy 23: 686–689 [DOI] [PubMed] [Google Scholar]
- Khan N.F., Harrison S.E., Rose P.W. (2010) Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 60: e128–e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaschik E., Nauck F., Ostgathe C. (2003) Constipation – modern laxative therapy. Support Care Cancer 11: 679–685 [DOI] [PubMed] [Google Scholar]
- National Horizon Scanning Centre (2008) Prucalopride (Resolor) for chronic constipation. Available at: http://www.haps.bham.ac.uk/publichealth/horizon/outputs/documents/2008/sept-dec/Prucalopride.pdf.
- NICE Clinical Guidance (2010) Routine antenatal care for health pregnant women. Available at: http://www.nice.org.uk/nicemedia/pdf/CG062PublicInfo.pdf.
- NHS Clinical Knowledge Summaries (2010) Constipation. Available at: http://www.cks.nhs.uk/constipation/management/quick_answers/scenario_constipation_in _adults#-320506.
- NHS Information Centre (2010) Prescription Cost Analysis Data 2009. Available at: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescription.
- Tytgat G.N., Heading R.C., Muller-Lissner S., Kamm M.A., Scholmerich J., Berstad A., et al. (2003) Contemporary understanding and management of reflux and constipation in the general population and pregnancy: a consensus meeting. Aliment Pharmacol Ther 18: 291–301 [DOI] [PubMed] [Google Scholar]
- Wald, A., Kamm, M., Mueller-Lissner, S., Scarpignato, C., Marx, W. and Schiojt, C. (2010) An international survey of community prevalence of constipation and laxative use in adults. Available at: http://www.dulcolaxo.es/es/Main/Notas_de_Prensa/Poster_Epi_data_FINAL_06.05.08.pdf.
- Xing J.H., Soffer E.E. (2001) Adverse effects of laxatives. Dis Colon Rectum 44: 1201–1209 [DOI] [PubMed] [Google Scholar]