Abstract
Objective
To evaluate the cross-sectional relationship between migraine and pre-gravid obesity; and to assess the risk of adult weight gain among women with history of a pediatric diagnosis of migraine.
Background
Obesity, comorbid with pain disorders including migraine, shares common pathophysiological characteristics including systemic inflammation, and derangements in adipose-tissue derived cytokines. Despite biochemical and epidemiological commonalities, obesity-migraine associations have been inconsistently observed.
Methods
A cohort of 3,733 women was interviewed during early pregnancy. We ascertained participants’ self-reported history of physician-diagnosed migraine and collected self-reported information about pre-gravid weight, adult height and net weight change from age 18 to the 3-monthsperiodpriorto pregnancy. Using pre-gravid body mass index, we categorized participants as follows: lean (<18.5 kg/m2); normal (18.5–24.9 kg/m2); overweight (25–29.9 kg/m2), obese (30–34.9 kg/m2), severely obese (35–39.9 kg/m2), and morbidly obese (≥ 40 kg/m2). Logistic regression procedures were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
After adjusting for confounders, relative to normal weight women, obese women had a 1.48-fold increased odds of migraine(OR=1.48; 95%CI 1.12–1.96). Severely obese (OR=2.07; 95%CI 1.27–3.39) and morbidly obese (OR=2.75; 95%CI 1.60–4.70) had the highest odds of migraines. Women with a history of diagnosed pediatric migraine had a 1.67-fold higher odds of gaining ≥10.0 kg above their weight at age 18, as compared with non-migraineurs (OR=1.67; 95%CI 1.13–2.47).
Conclusion
These data support earlier observations of migraine-obesity association among women, and extend the literature to include evidence of adult weight gain among women with a history of pediatric migraine.
Keywords: Migraine, Obesity, Adult Weight Gain, Pediatric Migraine, Body Mass Index
INTRODUCTION
Migraine, a recurrent neurovascular headache disorder, is characterized by episodes of severe throbbing, pulsatile headache associated with nausea, vomiting, photophobia, phonophobia, and aversion to physical activity.1, 2 Findings from the American Migraine Prevalence and Prevention study indicate that the prevalence of migraine rises from 4% before puberty to a peak of 25% in women during their childbearing years, with a decrease after menopause.3 Additionally, an estimated total 35 million US adults (18.2% of women and 6.5% of men) are thought to suffer from migraine.4, 5 Apart from impairing quality of life, migraine is associated with great economic costs attributable to increased medical care and loss of productivity time – totaling $20 billion in 2004.5
Several investigators have noted that obesity is comorbid with pain disorders including chronic daily headache.6 Others have noted that migraine and obesity share common pathophysiological characteristics including endothelial dysfunction,7 systemic inflammation,8 derangements in adipocytokines,7, 9, 10 and hematological mediators that favor a pro-thrombotic phenotype,8 and dyslipidemia.11 Moreover, both migraine and obesity are important risk factors of chronic cardiovascular and pregnancy-related metabolic disorders including angina, myocardial infarction,11–14 ischemic stroke,15 and preeclampsia.16, 17 However, despite the biochemical and epidemiological commonalities of migraine and obesity, results from prior studies have been inconsistent, with some demonstrating a positive association between the two conditions,5, 18, 19 whereas others have not.20–23 Notably, positive associations of obesity and migraine have been more consistently observed when classified as reproductive aged women5, 18, 19 whereas negative associations have been consistently found in all studies evaluating peri and post-menopausal women.18, 22, 23 Given the inconsistent literature and that few prior studies have sought to evaluate the extent to which, if at all, adult weight gain is elevated among subjects diagnosed with migraine as children, we conducted the present study. Using data from a large prospective cohort, we first sought to evaluate the cross-sectional relationship between migraine and pregravid obesity among reproductive aged women. We also sought to assess the risk of adult weight gain among women with history of a pediatric diagnosis of migraine (i.e., a diagnosis prior to age 18 years). We hypothesized that pediatric migraineurs, as compared with non-migraineurs counterparts, were more likely to gain weight as adults.
METHODS
Study Design and Setting
We analyzed data from the Omega Study, a prospective cohort study designed to examine risk factors of adverse pregnancy outcomes including preeclampsia. Participants were recruited from women attending prenatal care clinics affiliated with Swedish Medical Center and Tacoma General Hospital in Seattle and Tacoma, Washington, USA. Recruitment began in December 1996.24 The study protocol was approved by the Institutional Review Boards of Swedish Medical Center and Tacoma General Hospital. All participants provided informed consent.
Eligible women were those who began prenatal care before 20 weeks gestation, spoke and read English, were ≥18 years of age, and planned to carry the pregnancy to term and deliver at either of the two hospitals. During early pregnancy, participants were asked to complete a structured interviewer administered questionnaire regarding socio-demographic characteristics, lifestyle habits, and medical and reproductive histories. Pregnancy outcome information was abstracted from hospital and clinic medical records.
Analytical Population
The analytical study population was derived from participants enrolled in the Omega Study between 1996 and 2008. During this period, 5,063 eligible women were approached and 4,000 (79%) agreed to participate. Women with incomplete information concerning prior history of migraine (n=267) were excluded from this analysis. Hence a cohort of 3,733 women remained for analysis.
Data Collection
From structured questionnaire and medical records, we obtained information of covariates including subject’s age, educational attainment, self-reported height, and pre-pregnancy weight, reproductive and medical histories. We also collected information on smoking status. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight in kilograms divided by height in meters squared. Subjects’ history of migraine diagnosis was determined by response to the questions “Has a doctor ever told you that you have migraine headache?” and “If so, how old were you when your doctor gave you this diagnosis?” The pediatric age range for this study was defined a priori to include children up to the age of 18 years. Subjects who reported having a physician diagnosis of migraine were classified as having pediatric diagnosed migraine.
Interviewers asked each woman to report her height without shoes, her recalled weight at age 18, and her weight during the 3-month period before her study pregnancy (pregravid weight). We used this pregravid body mass index (BMI) to estimate adiposity. Obesity was categorized using the following cut points: <18.5 (lean), 18.5–24.9 (normal), 25.0–29.9 (overweight), and ≥30.0 kg/m2 (obese). For some additional exploratory analyses, we further categorized participants pregravid BMI in the following expanded categories: <18.5 (lean), 18.5–24.9 (normal), 25.0–29.9 (overweight), 30.0–34.9 (obese), 35.0 to 39.9 (severely obese) and ≥40.0 kg/m2 (morbidly obese). We categorized height according to approximate quartiles: ≤1.60, 1.61–1.65, 1.66–1.69, and ≥1.70 meters. We calculated the net change in weight from age 18 to just before the study pregnancy and categorized it as follows: loss of 2.5 kg or more, net change of fewer than 2.5 kg, 2.6–4.9 kg gain, 5.0–9.9 kg gain, and ≥10.0 kg gain. These categorizations were chosen a priori and were similar to those used in studies of weight change in relation to chronic diseases in older women.25
Statistical Analyses
We compared the frequency distribution of socio-demographic, lifestyle, behavioral and medical history characteristics of participants according to their pregravid body mass index. We used unadjusted and multivariable-adjusted logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of the association between pre-gravid body mass index categories and migraine diagnosis. We also estimated unadjusted and multivariable adjusted ORs and 95%CIsto evaluate associations of adult weight gain with migraine diagnosis among those participants who were diagnosed with the disorder prior to their 18th birthday. We assessed confounding by entering covariates into the logistic regression model one at a time, and adjusted ORs were compared to unadjusted ORs. Final logistic regression models included covariates that altered unadjusted ORs by 10%, as well as age, race/ethnicity, educational attainment, marital status, prior history of chronic hypertension or diabetes, smoking status, and participation in leisure time physical activity. Additional adjustments for parity did not materially alter reported ORs. All reported p-values are 2-tailed with statistical significance set at 0.05.
RESULTS
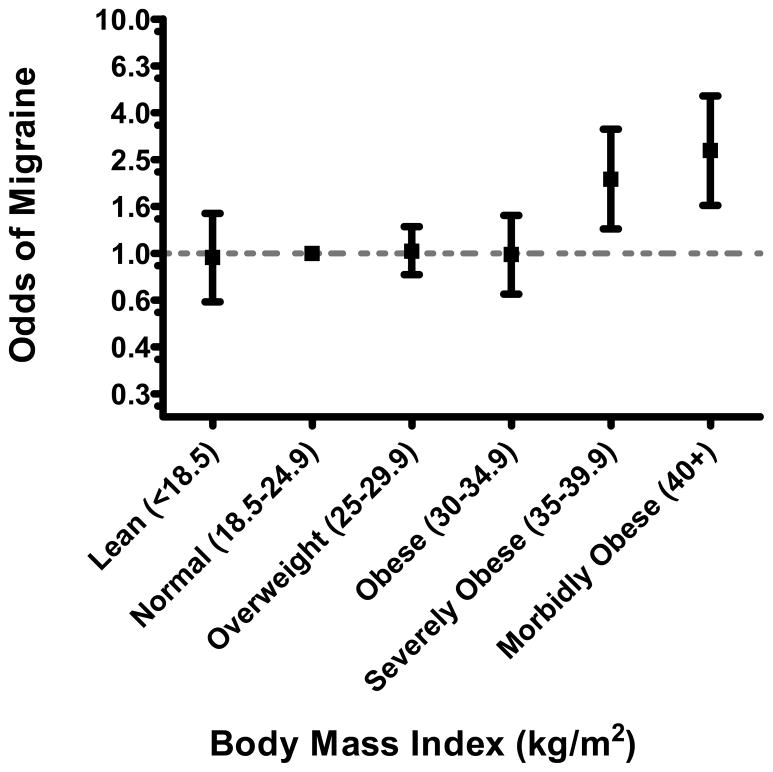
The socio-demographic, medical, and behavior characteristics of the study cohort are presented in Table 1. The frequency of current smokers, unmarried status, and positive family history of hypertension and diabetes increased across categories of pre-gravid BMI. Heavier women were less likely to have completed high school and to be nulliparous when compared with other women. Overall, 18% percent of women in the present cohort reported having a positive history of physician-diagnosed migraine. The age-specific prevalence of migraine for the entire cohort and according to participants’ pre-gravid obesity status (BMI < 30 vs. ≥30 kg/m2) is summarized in Figure 1. As shown in Table 2, obese women (BMI ≥30.0 kg/m2), as compared with women in the referent group (BMI 18.5–24.5 kg/m2), had a 1.63-fold increased odds of migraine (OR=1.63; 95%CI 1.25–2.12). After adjusting for age, race/ethnicity, educational attainment, marital status, chronic hypertension or diabetes, smoking and exercise status, the odds ratio for migraine was attenuated to 1.48, though the association remained statistically significant(OR=1.48; 95%CI 1.12–1.96). Results from analyses that further explored the odds of migraine according to extended categories of body mass index are summarized in Figure 2. The odds of migraine were 2-fold higher (OR=2.07; 95%CI 1.27–3.39) and 2.75-fold higher (OR=2.75; 95%CI 1.60–4.70) among severely obese (35–39.9 kg/m2), and morbidly obese (≥40 kg/m2) women.
Table 1.
Socio-demographic and other characteristics of the study cohort, Seattle and Tacoma, Washington, USA 1996–2008
| Characteristics | Cohort (N=3733) | Pregravid Body Mass Index (kg/m2)
|
P-value Trend test | |||
|---|---|---|---|---|---|---|
| Lean (<18.5) (N=160) | Normal (18.5–24.9) (N=2623) | Overweight (25.0–29.9) (N=612) | Obese (≥30.0) (N=338) | |||
| % | ||||||
| Age (years)* | 32.6 ± 4.5 | 32.0 ± 4.8 | 32.7 ± 4.3 | 33.0 ± 4.8 | 32.1 ± 5.4 | 0.80 |
| <30 | 22.7 | 25.6 | 22.0 | 22.2 | 28.1 | 0.02* |
| 30–34 | 43.6 | 45.7 | 44.9 | 39.7 | 39.3 | |
| 35–39 | 27.1 | 23.1 | 27.1 | 29.4 | 24.6 | |
| ≥40 | 6.6 | 5.6 | 6.0 | 8.7 | 8.0 | |
| Race/ethnicity | ||||||
| Non-Hispanic White | 86.4 | 84.4 | 87.8 | 84.6 | 80.2 | <0.001 |
| Other | 13.6 | 15.6 | 12.2 | 15.4 | 19.8 | |
| Annual Household Income ($) | ||||||
| <30,000 | 3.4 | 5.0 | 2.6 | 4.3 | 7.7 | <0.001 |
| 30,000–69,999 | 19.8 | 13.8 | 18.1 | 21.9 | 32.0 | |
| ≥ 70,000 | 73.5 | 75.0 | 76.3 | 71.2 | 54.7 | |
| Missing | 3.3 | 6.3 | 3.0 | 2.8 | 5.6 | |
| Parity | ||||||
| 0 | 62.4 | 57.5 | 64.7 | 59.2 | 53.0 | <0.001 |
| 1–2 | 36.3 | 41.9 | 34.5 | 39.0 | 42.3 | |
| ≥ 3 | 1.3 | 0.6 | 0.8 | 1.8 | 4.7 | |
| Education ≤ high school | 4.1 | 1.9 | 3.2 | 4.6 | 10.4 | <0.001 |
| Unmarried | 9.0 | 8.1 | 8.2 | 9.6 | 14.5 | 0.001 |
| History of Diabetes Mellitus | 1.3 | 0.6 | 1.0 | 1.0 | 4.7 | <0.001 |
| History of Chronic Hypertension | 4.8 | 1.9 | 2.3 | 8.7 | 18.6 | <0.001 |
| Family History of Diabetes Mellitus | 14.8 | 8.8 | 13.2 | 17.5 | 25.2 | <0.001 |
| Family History of Hypertension | 50.0 | 46.3 | 48.5 | 51.1 | 62.1 | <0.001 |
| Currently Employed | 81.5 | 76.3 | 81.6 | 83.5 | 79.6 | 0.62 |
| Current Smoker | 27.8 | 21.9 | 26.4 | 34.0 | 30.8 | 0.001 |
| Consume Multivitamin | 97.3 | 95.6 | 97.5 | 97.2 | 97.0 | 0.54 |
| No Current Exercise | 7.9 | 7.5 | 6.4 | 8.8 | 17.5 | <0.001 |
| Pre-pregnancy Body Mass Index (kg/m2)* | 23.6 ± 5.0 | 17.9 ± 0.8 | 21.6 ± 1.7 | 27.0 ± 1.4 | 35.8 ± 5.7 | <0.001 |
Mean ± standard deviation (SD)
Figure 1.
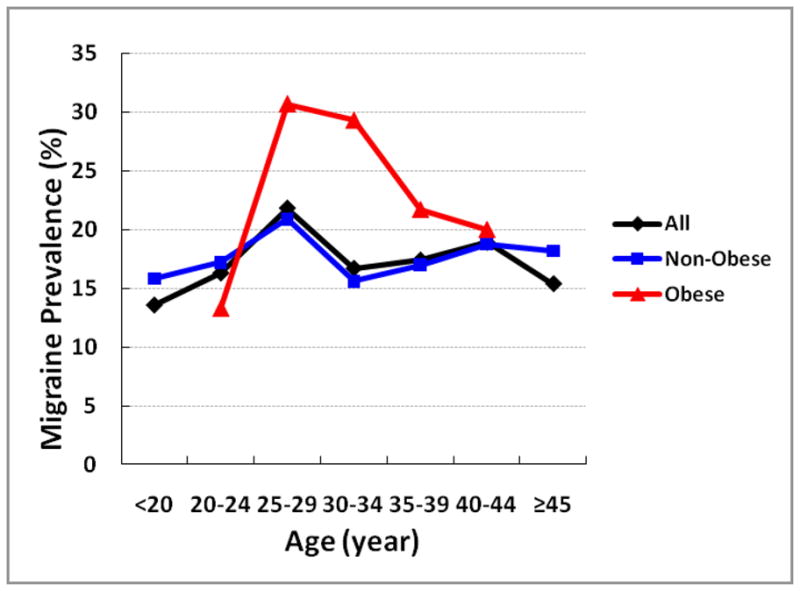
Age-specific prevalence of migraine for the entire cohort (black) and according to participants’ obesity status (BMI < 30 vs. ≥30 kg/m2)
Table 2.
Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) of migraine according to categories of participants’ body mass index Seattle and Tacoma, Washington, USA 1996–2008
| Pregravid Body Mass Index (kg/m2)
|
P-value Trend test | ||||
|---|---|---|---|---|---|
| Lean (<18.5) (N=160) | Normal (18.5–24.9) (N=2,623) | Overweight (25.0–29.9) (N=612) | Obese (≥30.0) (N=338) | ||
| Physician Diagnosed Migraine | |||||
| No, n (%) | 134 (83.8) | 2174 (82.9) | 502 (82.0) | 253 (74.8) | |
| Yes, n (%) | 26 (16.3) | 449 (17.1) | 110 (18.0) | 85 (25.2) | |
| Unadjusted OR (95%CI) | 0.94 (0.61–1.45) | 1.00 (Reference) | 1.06 (0.84–1.35) | 1.63 (1.25–2.12) | 0.004 |
| * Adjusted OR (95%CI) | 0.96(0.62–1.48) | 1.00 (Reference) | 1.02(0.81–1.29) | 1.48(1.12–1.96) | 0.04 |
Adjusted for age, race/ethnicity, educational attainment, marital status, history of chronic hypertension or diabetes mellitus, smoking and exercise status
Figure 2.
Adjusted odds ratio (OR) and 95% confidence intervals (CI) of migraine according to participants’ body mass index OR and 95% CI adjusted for participants’ age, race/ethnicity, educational attainment, marital status, history of chronic hypertension or diabetes mellitus, smoking and exercise status
We next evaluated the odds of adult weight gain among women with a history of diagnosed pediatric migraine compared with women having no history of migraine. Pediatric migraineurs were younger, more likely to be non-Hispanic white, to be single, and to have a personal history of hypertension as compared with non-migraineurs. The two study groups were similar with regards to annual household income, educational attainment, and history of exercise as a teenager (Supplementary Table 1). Pediatric migraineurs and non-migraineurs had similar mean weights at age 18 years, and similar adult height as those women without a history of migraine (Supplementary Table 2). However, on average, pediatric migraineurs had higher pre-gravid body mass index (25.0±7.1 vs. 23.4±4.7 kg/m2, p-value=0.001) and higher adult weight gain values (11.1±14.3 vs. 7.6±10.4 kg, p-value=0.001) as compared with non-migraineurs. As shown in Table 3, after adjusting for age, race/ethnicity, history of chronic hypertension, smoking status, adult height and BMI at age 18, adult weight gain was significantly associated with pediatric migraine. Pediatric migraineurs had a 1.67-fold higher odds of gaining at least 10.0 kg in adulthood than non-migraineurs (OR=1.67; 95%CI 1.13–2.47). Associations of pediatric migraine with other anthropometric measures are summarized in Table 3.
Table 3.
Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) of adult weight gain according to participants’ pediatric migraine Seattle and Tacoma, Washington, USA 1996–2008
| Anthropometric Characteristics | Pediatric Migraine (N=220) | No Migraine (N=3063) | Unadjusted OR(95%CI) | Adjusted OR(95%CI) |
|---|---|---|---|---|
| Adult Height (m) | ||||
| ≤1.60 | 31 (14.1) | 386 (12.6) | 1.01 (0.66–1.54) | 1.23 (0.79–1.92) |
| 1.61–1.65 | 46 (20.9) | 702 (22.9) | 0.82 (0.57–1.19) | 0.87 (0.59–1.26) |
| 1.66–1.69 | 54 (24.6) | 856 (28.0) | 0.79 (0.56–1.13) | 0.81 (0.57–1.16) |
| ≥1.70 | 89 (42.4) | 1119 (36.5) | 1.00 (Reference) | 1.00 (Reference)3 |
| Body Mass Index at Age 18 (kg/m2) | ||||
| Lean (<18.5) | 40 (18.2) | 542 (17.7) | 1.11 (0.78–1.60) | 1.19 (0.83–1.72) |
| Normal (18.5–24.9) | 151 (68.6) | 2281 (74.5) | 1.00 (Reference) | 1.00 (Reference)1 |
| Overweight (25.0–29.9) | 21 (9.6) | 150 (4.9) | 2.11 (1.30–3.44) | 1.69 (1.02–2.80) |
| Obese (≥30.0) | 6 (2.7) | 45 (1.5) | 2.01 (0.85–4.80) | 1.62 (0.66–3.95) |
| Unknown | 2 (0.9) | 45 (1.5) | ||
| Pregravid Body Mass Index (kg/m2) | ||||
| Lean (<18.5) | 8 (3.6) | 134 (4.4) | 0.90 (0.43–1.86) | 0.89 (0.43–1.87) |
| Normal (18.5–24.9) | 145 (65.9) | 2174 (71.0) | 1.00 (Reference) | 1.00 (Reference)2 |
| Overweight (25.0–29.9) | 34 (15.5) | 502 (16.4) | 1.02 (0.69–1.49) | 1.00 (0.68–1.48) |
| Obese (≥30.0) | 33 (15.0) | 253 (8.2) | 1.96 (1.31–2.92) | 1.72 (1.13–2.63) |
| Adult Weight Gain (kg) | ||||
| ≤ −2.5 | 12 (5.4) | 244 (8.0) | 0.82 (0.43–1.59) | 0.68 (0.33–1.39) |
| −2.5 to 2.5 | 42 (19.1) | 703 (22.9) | 1.00 (Reference) | 1.00 (Reference)4 |
| 2.6 to 4.9 | 25 (11.4) | 478 (15.6) | 0.88 (0.53–1.46) | 0.91 (0.54–1.52) |
| 5.0 to 9.9 | 51 (23.2) | 676 (22.1) | 1.26 (0.83–1.93) | 1.30 (0.85–2.00) |
| ≥ 10.0 | 88 (40.0) | 917 (29.9) | 1.61 (1.10–2.35) | 1.67 (1.13–2.47) |
| missing | 2 (0.9) | 45 (1.5) | ||
Adjustedforage, race/ethnicity, history of chronic hypertension and smoking status
Adjusted for age, race/ethnicity, marital status, history of chronic hypertension, and smoking status
Adjusted for age, race/ethnicity, history of chronic hypertension, smoking status, and body mass index at age 18
Adjusted for age, race/ethnicity, history of chronic hypertension, smoking status, adult height and body mass index at age 18
DISCUSSION
In our cohort of reproductive aged women, BMI was associated with migraine diagnosis in a linear fashion. After adjusting for confounders we found that obese women (BMI ≥30.0 kg/m2), when compared with normal weight women (BMI 18.5–24.5 kg/m2), were more likely to have had a diagnosis of migraine (OR=1.48; 95%CI 1.12–1.96). Further analyses revealed a linear trend in the prevalence and odds of migraine according to the severity of obesity. Extremely obese women (BMI ≥40.0 kg/m2) had a 2.75-fold higher odds of migraine (95%CI 1.60–4.70) when compared with the reference group. We also found that women diagnosed with migraine as children, were more likely than women without a diagnosis, to gain a substantial amount of weight as adults. Women with a diagnosis of migraine as a child had a 1.67-fold increased odds of gaining ≥10.0 kg since the age of 18 (OR=1.67; 95%CI 1.13–2.47), as compared with non-migraineurs.
Our finding of a cross-sectional association of migraine with obesity is in agreement with previous studies which evaluated the migraine-obesity relationship in predominantly reproductive age populations,5, 18 though not all,20, 21 prior studies on this topic. Ford et al5 reported a positive association between measured BMI and self-reported migraine or severe headache. The authors noted that obese (BMI ≥30 kg/m2) participants in the 1999–2002 National Health and Nutrition Examination Survey (NHANES), had a significantly higher risk of having headache including migraine (OR=1.37; 95%CI 1.09–1.72) as compared with participants who had normal BMI values (18.5–24.5 kg/m2).5 Peterlin et al18 in their analysis of 21,783 adult participants NHANES, reported that the relationship between migraine or severe headaches and obesity may vary by age, gender, and adipose tissue distribution. The authors reported that the prevalence of self-reported migraine/severe headache was increased in younger (≤55 years) men and women with obesity (categorized as measure BMI ≥30 kg/m2), independent of abdominal obesity (waist circumference ≥88 cm for women and ≥102 cm for men). After adjusting for confounders including as age, race and abdominal obesity, the ORs of migraine or severe headache for overall obesity were 1.38 (95%CI 1.1–1.7) for men and 1.20 (95%CI 1.04–1.39) for women <55 years of age. Abdominal obesity was independently associated with increased odds of migraine and severe headache among women (OR=1.26, 95%CI 1.10–1.45), but not men (OR=1.03, 95%CI 0.84–1.27). The authors observed no clear evidence of a positive association between migraine or severe headache with either overall obesity or abdominal obesity among older (>55 years) men and women.
In our present study, we observed particularly higher odds of a life-time prevalence of self-reported physician diagnosed migraine among severely obese and morbidly obese reproductive age women. This observation is also in general agreement with results from a small clinic based study of extremely obese women. There is a very high frequency of migraine noted among the morbidly obese. Horev et al,26 in their clinic-based study of 27 morbidly obese women attending preoperative clinic for laparoscopic gastric banding, noted that 48% reported suffering from severe migraine.
Our findings, and those of others,5, 18 are inconsistent with some published reports.20, 22 Mattssonet al22 found no association between chronic migraine and obesity in their study of 684 peri and post menopausal Swedish women aged 40–74 years (OR=1.1; 95%CI 0.6–1.8). Similarly, Bigal et al,20 in their telephone interview study of 30,215 adults, aged 18–89 years of age, found no evidence suggestive of a positive association between prevalent migraine and obesity based on self-reported BMI. The authors did, however, observe significant associations of body mass index with episodic migraine of high frequency. Compared with the normal weight group (18.5–24.9 kg/m2), obese (30–34.9 kg/m2) subjects have a 1.9-foldincreased odds (OR = 1.9; 95%CI 1.9–4.4) and morbidly obese (≥ 35 kg/m2) subjects have a 5.7-foldincreased odds (OR=5.7; 95%CI 3.6–8.8) of having 10–14 headaches days per month. Attack severity was also associated with BMI; 65% of the morbidly obese (OR=1.9, 95%CI 1.2–2.4) report severe migraine attacks compared to 54% of the normal weight group.
Inconsistent findings across studies may be attributable to methodological differences in obesity and migraine classification and in participant selection. Residual confounding, and sample size may also have contributed to observed inconsistencies across studies. For example, some have argued that the obesity-migraine association may be more consistently observed among migraineurs with concurrent sleep apnea,27 whereas others note that age and gender significantly contributes to a change in disease risk.28
To the best of our knowledge, investigators have not previously evaluated risk of adult weight gain in patients diagnosed with migraine during childhood or early adolescence. However, available evidence from an observational follow-up study of 913 children across seven pediatric headache centers in the US, suggest cross-sectional associations of weight change with the prevalence and severity of headache and migraine.29 For children whom were obese or overweight at the initial visit, BMI change was positively correlated with change in headache frequency at 3 and 6 month follow-up (r=0.32, p=0.01). Our observations potentially extend the literature by indicating increased odds of adult weight gain among migraineurs diagnosed with the condition prior to age 18 years.
The biological mechanisms underlying observed migraine-obesity and migraine-adult weight gain associations are yet to be fully elucidated. However, investigators have proposed several plausible and compelling hypotheses by which obesity (or severe headache) might lead to the development, worsening and chronification of headache disorders including migraine28, 30, 31. Plasma concentrations of calcitonin gene-related peptide (CGRP), an important neurobiological mediator,32 are increased in obese versus non-obese women;33 and this has been implicated as one mechanism that accounts for associations of migraine, migrainous symptoms, and appetite with weight gain and obesity.20, 31 An alternative, or perhaps complementary, hypothesis is that the effects of obesity are mediated by a low-grade chronic inflammatory state. Adipose tissue secretes numerous cytokines, and adipocytokines known to influence immune and vascular endothelial functions as well as glucose metabolism in ways that favor chronic inflammation (including neurovascular inflammation), platelet aggregation, endothelial dysfunction and insulin resistance--conditions common to migraine and obesity.28, 34 Investigators35, 36 also speculate that common underlying pathophysiological neuro-endocrine alterations involving the hypothalamus, serotonin and melatonin synthesis and secretion may, in part, explain observations of increased odds of obesity and weight gain in patients with migraine and other primary headache disorders.
The observation that the prevalence of migraine or severe headaches varies with the distribution of adipose tissue, age and gender,18 with increased prevalence among younger men and women with central adiposity, and decreased prevalence in older women with central adiposity have led some investigators to postulate that differences in sex hormones, age, menopausal status, and the site of adipose tissue storage (e.g., peripheral vs. central fat depots) may exert different neuro-endocrinological effects across the life-course and thus may account for inconsistent findings of obesity with migraine, headaches and their clinical course across study populations. Yet other investigators31 hypothesize that migraine may increase adiposity, via eating behaviors. This biologically plausible hypothesis is, in part, supported by our observation of increased odds of weight gain among women who were diagnosed with migraine as children.
While the strengths of this study include the large sample size, and relatively high participation rates, several limitations of our study merit discussion and consideration. First, participants’ migraine status was based on self-reports made during interviews and on medical records review. Similar questions to the ones we used in the current study to ascertain subject’s migraine status have been widely used in epidemiological studies such the Women’s Health Study37 and the National Health and Nutrition Examination Surveys (NHANES);18 and investigators have documented good agreement between migraine classification based on self-reports with information derived from medical records review.37, 38 Nevertheless, we cannot exclude the possibility of that migraine status was under-reported in our study. This concern is particularly important given that underreporting is likely related to participants’ social class, educational status and other socio-demographic characteristics. Studies that systematically use screening and confirmatory diagnostic evaluations will likely attenuate concerns about misclassification of migraine diagnoses in epidemiological studies. We were also unable to differentiate migraineurs on the basis of features such as the frequency and severity of attacks and efficacy and type of therapies used to manage symptoms. Moreover, we did not have information concerning the age of migraine onset. Hence, our use of age of migraine diagnosis can only be viewed as a proxy for migraine onset. Second, errors in reporting of anthropometric characteristics are likely to have occurred.39 Weights reported by participants in other studies, however, have been shown to be valid.40 Troy et al40 reported that women’s self-reported recalled weight at 18 years was highly correlated (r=0.87) with weight at ages 17–21 abstracted from nursing school records. Third, although we adjusted for several potential confounders, we cannot exclude the possibility of residual confounding due to misclassification of adjusted variables or confounding by other unmeasured variables (e.g., the severity and frequency of migraine and medications used to control symptoms). Lastly, the generalizability of our study may also be limited as our cohort was primarily comprised of Non-Hispanic White and well-educated women. The concordance of our results with those from other studies that have included racially, ethnically and geographically diverse populations, however, serve to attenuate these concerns.
In summary, we found the positive association between pre-gravid body mass index and prevalent migraine. Our data support some earlier observations that the odds of migraine are increased in reproductive-aged women who are obese; and extend the literature to include evidence of adult weight gain among women with a history of pediatric migraine. If confirmed, our findings add to the call for further studies designed to comprehensively evaluate the effects of migraine (and its treatment) on appetite, energy expenditure, and dietary and sleep behaviors. Information from such studies may motivate the development and promotion of public health campaigns that target young adults with migraine, chronic headache and other pain disorders who may be more attuned to making healthful behavioral changes that can positively impact their reproductive outcomes and long-term health.
Supplementary Material
Acknowledgments
Study funding: This research was supported by awards from the National Institutes of Health (R01 HD-032562, R01HD-055566, and T37-MD001449).
This research was completed while Ms. Michelle Vo and Ms. Abinnet Ainalem were research training fellows with the Multidisciplinary International Research Training (MIRT) Program of the Department of Epidemiology, University of Washington, and School of Public Health. The MIRT Program is supported by an award from the National Institutes of Health, National Center on Minority Health and Health Disparities (T37-MD001449). This research was also supported by awards from the National Institutes of Health (R01 HD-032562 and R01HD-055566). The authors wish to thank the staff of the Center for Perinatal Studies, Swedish Medical Center, and Seattle, WA, USA for their technical assistance with this research.
Footnotes
COMPETING INTERESTS
None to declare
AUTHOR CONTRIBUTIONS
MAW had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the decision to submit for publication. MAW conceived, designed and obtained funding for the study. MV, AA, and CQ analyzed the data. CZ, JO, BLP, SKA and MAW drafted the manuscript. All authors interpreted the data, critically revised the draft for important intellectual content, and gave final approval of the manuscript to be published.
References
- 1.Menon R, Bushnell CD. Headache and pregnancy. Neurologist. 2008;14:108–119. doi: 10.1097/NRL.0b013e3181663555. [DOI] [PubMed] [Google Scholar]
- 2.Machado RB, Pereira AP, Coelho GP, Neri L, Martins L, Luminoso D. Epidemiological and clinical aspects of migraine in users of combined oral contraceptives. Contraception. 2010;81:202–208. doi: 10.1016/j.contraception.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME. Ten lessons on the epidemiology of migraine. Headache. 2007;47 (Suppl 1):S2–9. doi: 10.1111/j.1526-4610.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Pearson WS, Zhao G, Strine TW, Mokdad AH. Body mass index and headaches: findings from a national sample of US adults. Cephalalgia. 2008;28:1270–1276. doi: 10.1111/j.1468-2982.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 6.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106:81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 7.Peterlin BL, Bigal ME, Tepper SJ, Urakaze M, Sheftell FD, Rapoport AM. Migraine and adiponectin: is there a connection? Cephalalgia. 2007;27:435–446. doi: 10.1111/j.1468-2982.2007.01306.x. Erratum in Cephalalgia. 427(436):570. [DOI] [PubMed] [Google Scholar]
- 8.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 9.Peterlin BL, Alexander G, Tabby D, Reichenberger E. Oligomerization state-dependent elevations of adiponectin in chronic daily headache. Neurology. 2008;70:1905–1911. doi: 10.1212/01.wnl.0000312278.40250.6e. [DOI] [PubMed] [Google Scholar]
- 10.Guldiken B, Guldiken S, Demir M, Turgut N, Tugrul A. Low leptin levels in migraine: a case control study. Headache. 2008;48:1103–1107. doi: 10.1111/j.1526-4610.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 11.Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864–1871. doi: 10.1212/WNL.0b013e3181a71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64:1573–1577. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- 13.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. Erratum: JAMA 296(283):292, JAMA 296(286):654. [DOI] [PubMed] [Google Scholar]
- 14.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. Br Med J. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeney KL, Williams MA. Migraine headachesand preeclampsia: an epidemiologic review. Headache. 2006;46:794–803. doi: 10.1111/j.1526-4610.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez SE, Qiu C, Williams MA, Lam N, Sorensen TK. Headaches and migraines are associated with an increased risk of preeclampsia in Peruvian women. Am J Hypertens. 2008;21:360–364. doi: 10.1038/ajh.2007.46. [DOI] [PubMed] [Google Scholar]
- 18.Peterlin BL, Rosso AL, Rapoport AM, Scher AI. Obesity and migraine: the effect of age, gender and adipose tissue distribution. Headache. 2010;50:52–62. doi: 10.1111/j.1526-4610.2009.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peres MF, Lerario DD, Garrido AB, Zukerman E. Primary headaches in obese patients. Arq Neuropsiquiatr. 2005;63:931–933. doi: 10.1590/s0004-282x2005000600005. [DOI] [PubMed] [Google Scholar]
- 20.Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 21.Bigal ME, Tsang A, Loder E, Serrano D, Reed ML, Lipton RB. Body mass index and episodic headaches: a population-based study. Arch Intern Med. 2007;167:1964–1970. doi: 10.1001/archinte.167.18.1964. [DOI] [PubMed] [Google Scholar]
- 22.Mattsson P. Migraine headache and obesity in women aged 40–74 years: a population-based study. Cephalalgia. 2007;27:877–880. doi: 10.1111/j.1468-2982.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 23.Winter AC, Berger K, Buring JE, Kurth T. Body mass index, migraine, migraine frequency and migraine features in women. Cephalalgia. 2009;29:269–278. doi: 10.1111/j.1468-2982.2008.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens. 2004;17:154–160. doi: 10.1016/j.amjhyper.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Rudra CB, Sorensen TK, Leisenring WM, Dashow E, Williams MA. Weight characteristics and height in relation to risk of gestational diabetes mellitus. Am J Epidemiol. 2007;165:302–308. doi: 10.1093/aje/kwk007. [DOI] [PubMed] [Google Scholar]
- 26.Horev A, Wirguin I, Lantsberg L, Ifergane G. A high incidence of migraine with aura among morbidly obese women. Headache. 2005;45:936–938. doi: 10.1111/j.1526-4610.2005.05162.x. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert GJ. Obesity and migraine: a population study. Neurology. 2007;68:241. author reply 241. [PubMed] [Google Scholar]
- 28.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. 2010;50:631–648. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershey AD, Powers SW, Nelson TD, et al. Obesity in the pediatric headache population: a multicenter study. Headache. 2009;49:170–177. doi: 10.1111/j.1526-4610.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 30.Bigal ME, Lipton RB. What predicts the change from episodic to chronic migraine? Curr Opin Neurol. 2009;22:269–276. doi: 10.1097/WCO.0b013e32832b2387. [DOI] [PubMed] [Google Scholar]
- 31.Ray ST, Kumar R. Migraine and obesity: cause or effect? Headache. 2010;50:326–328. doi: 10.1111/j.1526-4610.2009.01539.x. [DOI] [PubMed] [Google Scholar]
- 32.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH. Calcitonin gene-related peptide in human obesity. Peptides. 1991;12:861–863. doi: 10.1016/0196-9781(91)90147-h. [DOI] [PubMed] [Google Scholar]
- 34.Guldiken B, Guldiken S, Taskiran B, et al. Migraine in metabolic syndrome. Neurologist. 2009;15:55–58. doi: 10.1097/NRL.0b013e31817781b6. [DOI] [PubMed] [Google Scholar]
- 35.Alberti A. Headache and sleep. Sleep Med Rev. 2006;10:431–437. doi: 10.1016/j.smrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Dodick DW, Eross EJ, Parish JM, Silber M. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. 2003;43:282–292. doi: 10.1046/j.1526-4610.2003.03055.x. Erratum in: Headache 244:384. [DOI] [PubMed] [Google Scholar]
- 37.Schurks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women’s Health Study. Cephalalgia. 2009;29:1086–1090. doi: 10.1111/j.1468-2982.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards WS, Winn DM, Collins JG. Evaluation of 2-week doctor visit reporting in the national health interview survey. Vital Health Stat. 1996;2:1–46. [PubMed] [Google Scholar]
- 39.Katsnelson MJ, Peterlin BL, Rosso AL, Alexander GM, Erwin KL. Self-reported vs measured body mass indices in migraineurs. Headache. 2009;49:663–668. doi: 10.1111/j.1526-4610.2009.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.